Field-Measured Hydraulic Traits and Remotely Sensed NDVI of Four Subtropical Tree Species Showed Transient Declines during the Drought–Heatwave Event
Abstract
1. Introduction
2. Materials and Methods
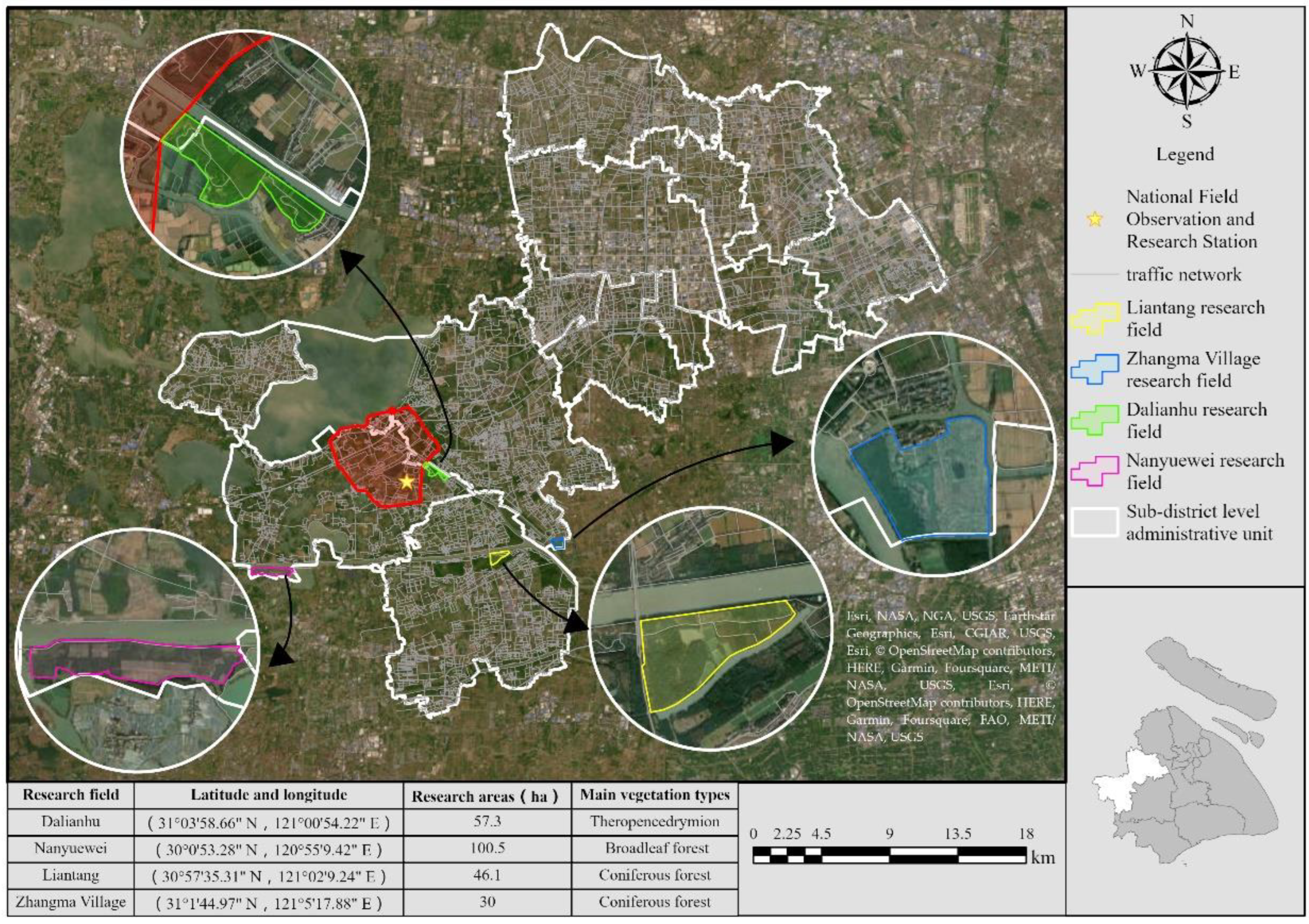
2.1. Study Site and Plant Species
2.2. Meteorological Data Collection and Tidying
2.3. Remote Sensing Monitoring
2.4. Water Potential Measurements
2.5. Hydraulic Conductivity
2.6. Measurement of Native Embolism
2.7. Measurement of P50 and Safety Margin
2.8. Leaf Gas Exchange
2.9. Leaf SPAD Value and Relative Water Content of Leaves
3. Results
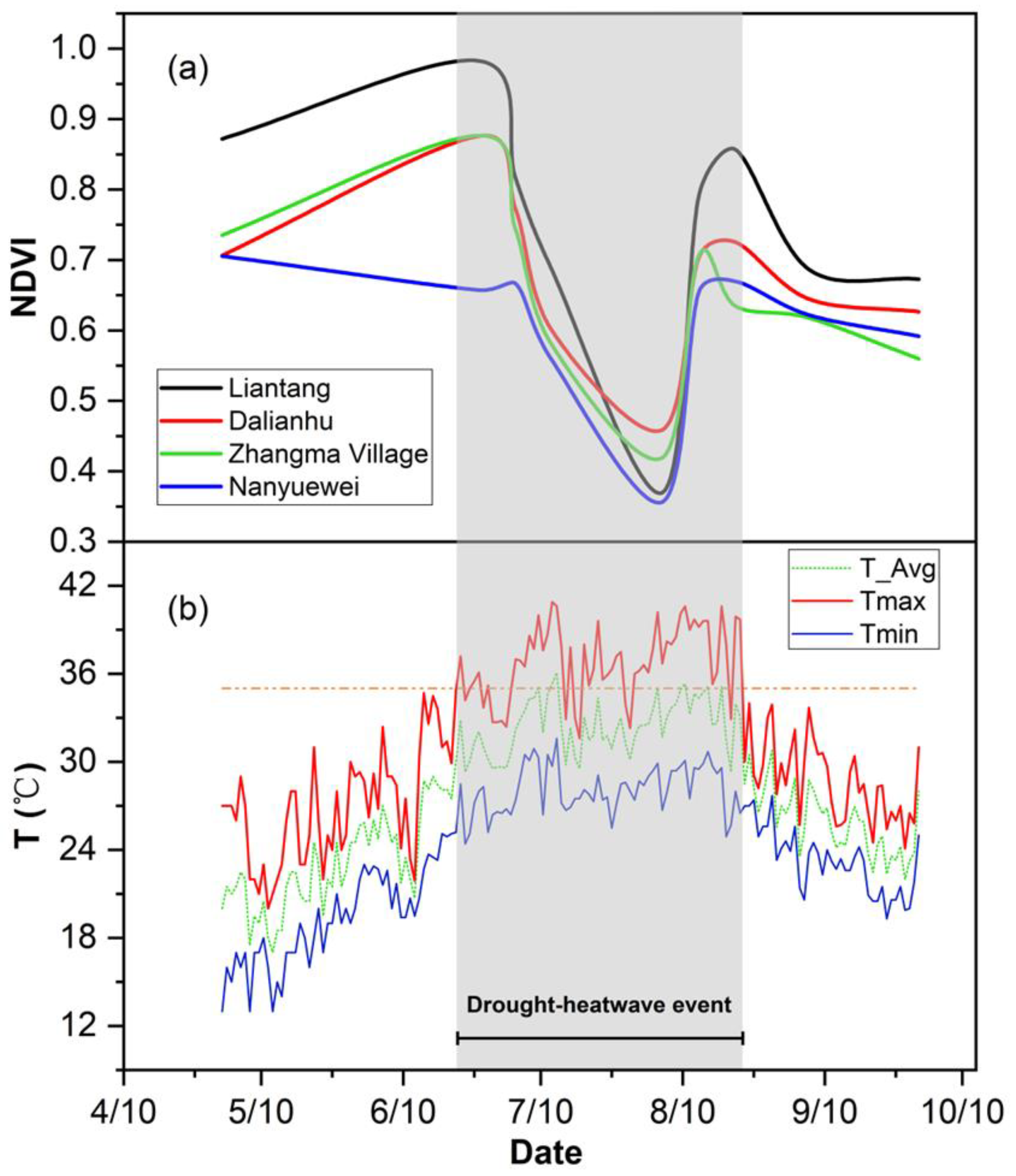
3.1. Meteorology Conditions during the Drought–Heatwave Event in Summer 2022

3.2. Remote Sensing Data and Temperature Changes Are Closely Related
3.3. Instantaneous Changes in Physiological Traits of the Four Tree Species during and after the Drought–Heatwave Event
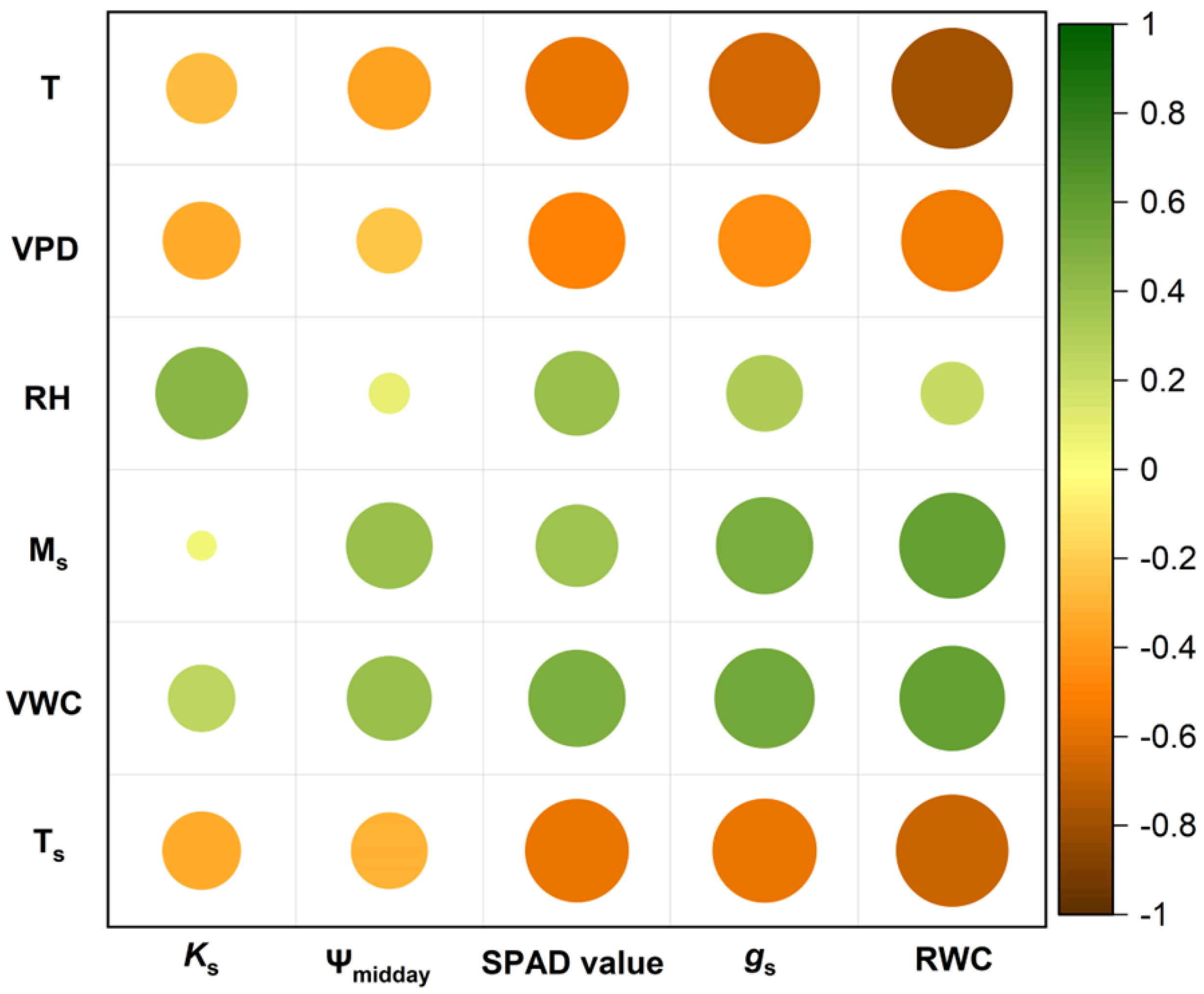
3.4. Relationship between Physiological Characteristics and Meteorological Factors
4. Discussion
4.1. Response of NDVI Variation from Remote Sensing to Drought and Heatwave
4.2. Responses of Hydraulics and Photosynthesis in the Field
4.3. Relationship between Hydraulic Functioning in the Field and NDVI
4.4. Trade-Off between Resistance and Resilience of Hydraulic Traits to Drought–Heatwave
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryan, M.G. Tree responses to drought. Tree Physiol. 2011, 31, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; López, R.; Medlyn, B.E. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G.; Michaletz, S.T.; Bennett, K.E.; Solander, K.C.; Xu, C.; Maxwell, R.M.; Middleton, R.S. Predicting chronic climate-driven disturbances and their mitigation. Trends Ecol. Evol. 2018, 33, 15–27. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate change. The physical science basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013; pp. 3–29. [Google Scholar]
- Yuan, X.; Wang, Y.; Ji, P.; Wu, P.; Sheffield, J.; Otkin, J.A. A global transition to flash droughts under climate change. Science 2023, 380, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Yuan, W.; Cai, W.; Chen, Y.; Liu, S.; Dong, W.; Zhang, H.; Yu, G.; Chen, Z.; He, H.; Guo, W.; et al. Severe summer heatwave and drought strongly reduced carbon uptake in Southern China. Sci. Rep. 2016, 6, 18813. [Google Scholar] [CrossRef]
- Birami, B.; Gattmann, M.; Heyer, A.G.; Grote, R.; Arneth, A.; Ruehr, N.K. Heat waves alter carbon allocation and increase mortality of aleppo pine under dry conditions. Front. For. Glob. Chang. 2018, 1, 8. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Kane, J.L.; Anderegg, L.D.L. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Chang. 2013, 3, 30–36. [Google Scholar] [CrossRef]
- Luber, G.; McGeehin, M. Climate change and extreme heat events. Am. J. Prev. Med. 2008, 35, 429–435. [Google Scholar] [CrossRef]
- Kurz, W.A.; Dymond, C.C.; Stinson, G.; Rampley, G.J.; Neilson, E.T.; Carroll, A.L.; Ebata, T.; Safranyik, L. Mountain pine beetle and forest carbon feedback to climate change. Nature 2008, 452, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Schuldt, B.; Buras, A.; Arend, M.; Vitasse, Y.; Beierkuhnlein, C.; Damm, A.; Gharun, M.; Grams, T.E.E.; Hauck, M.; Hajek, P.; et al. A first assessment of the impact of the extreme 2018 summer drought on Central European forests. Basic Appl. Ecol. 2020, 45, 86–103. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 129. [Google Scholar] [CrossRef]
- Shu, Z.K.; Li, W.X.; Zhang, J.Y.; Jin, J.L.; Xue, Q.; Wang, Y.T.; Wang, G.Q. Historical changes and future trends of extreme precipitation and high temperature in China. Eng. Sci. 2022, 24, 116–125. (In Chinese) [Google Scholar] [CrossRef]
- Breshears, D.D.; Cobb, N.S.; Rich, P.M.; Price, K.P.; Allen, C.D.; Balice, R.G.; Romme, W.H.; Kastens, J.H.; Floyd, M.L.; Belnap, J.; et al. Regional vegetation die-off in response to global-change-type drought. Proc. Natl. Acad. Sci. USA 2005, 102, 15144–15148. [Google Scholar] [CrossRef]
- Smith, M.D. An ecological perspective on extreme climatic events: A synthetic definition and framework to guide future research. J. Ecol. 2011, 99, 656–663. [Google Scholar] [CrossRef]
- Walker, X.; Johnstone, J.F. Widespread negative correlations between black spruce growth and temperature across topographic moisture gradients in the boreal forest. Environ. Res. Lett. 2014, 9, 064016. [Google Scholar] [CrossRef]
- De Micco, V.; Carrer, M.; Rathgeber, C.B.K.; Camarero, J.J.; Voltass, J.; Cherubini, P.; Battipaglia, G. From xylogenesis to tree rings: Wood traits to investigate tree response to environmental changes. Iawa J. 2019, 40, 155–182. [Google Scholar] [CrossRef]
- Lu, M.T. Effects of Drought and Heat Wave on Vegetation Greenness and Productivity in the Yangtze River Basin. Ph.D. Dissertation, Zhejiang University, Hangzhou, China, 2022. (In Chinese). [Google Scholar]
- Amoroso, M.M.; Daniels, L.D.; Larson, B.C. Temporal patterns of radial growth in declining Austrocedrus chilensis forests in Northern Patagonia: The use of tree-rings as an indicator of forest decline. For. Ecol. Manag. 2012, 265, 62–70. [Google Scholar] [CrossRef]
- Bhandari, A.K.; Kumar, A.; Singh, G.K. Feature Extraction using normalized difference vegetation index (NDVI): A case study of jabalpur city. Procedia Technol. 2012, 6, 612–621. [Google Scholar] [CrossRef]
- Field, C.B. Climate Change 2014: Impacts, Adaptation and Vulnerability: Regional Aspects; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Chen, L.; Song, W.L.; Yang, Y.M.; Li, X.T.; Xin, J.F.; Lin, S.J.; Xu, J.X. Remote sensing monitoring of drought in the Yangtze River basin in 2022. China Flood Drought Manag. 2023, 33, 26–30. (In Chinese) [Google Scholar]
- Li, Y.; Ye, D.X.; Gao, G.; Mei, M.; Wang, Y.M.; Wang, G.F.; Wang, L.; Cui, T. Climate characteristics and major meteorological events in China during the summer of 2022. J. Atmos. Sci. 2023, 46, 110–118. (In Chinese) [Google Scholar]
- Mei, M.; Gao, G.; Li, Y.; Wang, G.F.; Dai, T.L.; Chen, Y.X. Change characteristics in compound high temperature and drought extreme events over Yangtze River Basin from 1961 to 2022. Yangtze River 2023, 54, 1001–4179. (In Chinese) [Google Scholar]
- Lin, S.; Li, H.Y.; Huang, P.C.; Duan, X.Y. Characteristics of high temperature, drought and circulation situation in summer 2022 in China. J. Arid. Meteorol. 2022, 40, 748–763. (In Chinese) [Google Scholar]
- Teskey, R.; Wertin, T.; Bauweraerts, I.; Ameye, M.; McGuire, M.A.; Steppe, K. Responses of tree species to heat waves and extreme heat events. Plant Cell Environ. 2015, 38, 1699–1712. [Google Scholar] [CrossRef]
- Hopkins, A.J.M.; Ruthrof, K.X.; Fontaine, J.B.; Matusick, G.; Dundas, S.J.; Hardy, G.E. Forest die-off following global-change-type drought alters rhizosphere fungal communities. Environ. Res. Lett. 2018, 13, 095006. [Google Scholar] [CrossRef]
- Xu, L.; Chen, N.C.; Zhang, X. Global drought trends under 1.5 and 2 degrees C warming. Int. J. Climatol. 2019, 39, 2375–2385. [Google Scholar] [CrossRef]
- Perkins-KirKPatrick, S.E.; Lewis, S.C. Increasing trends in regional heatwaves. Nat. Commun. 2020, 11, 3357. [Google Scholar] [CrossRef]
- Rohner, B.; Kumar, S.; Liechti, K.; Gessler, A.; Ferretti, M. Tree vitality indicators revealed a rapid response of beech forests to the 2018 drought. Ecol. Indic. 2021, 120, 106903. [Google Scholar] [CrossRef]
- Brun, P.; Psomas, A.; Ginzler, C.; Thuiller, W.; Zappa, M.; Zimmermann, N.E. Large-scale early-wilting response of Central European forests to the 2018 extreme drought. Glob. Chang. Biol. 2020, 26, 7021–7035. [Google Scholar] [CrossRef]
- Salomón, R.L.; Peters, R.L.; Zweifel, R.; Sass-Klaassen, U.G.W.; Stegehuis, A.I.; Smiljanic, M.; Poyatos, R.; Babst, F.; Cienciala, E.; Fonti, P.; et al. The 2018 European heatwave led to stem dehydration but not to consistent growth reductions in forests. Nat. Commun. 2022, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Arend, M.; Link, R.M.; Patthey, R.; Hoch, G.; Schuldt, B.; Kahmen, A. Rapid hydraulic collapse as cause of drought-induced mortality in conifers. Proc. Natl. Acad. Sci. USA 2021, 118, e2025251118. [Google Scholar] [CrossRef] [PubMed]
- Obladen, N.; Dechering, P.; Skiadaresis, G.; Tegel, W.; Kessler, J.; Hoellerl, S.; Kaps, S.; Hertel, M.; Dulamsuren, C.; Seifert, T.; et al. Tree mortality of European beech and Norway spruce induced by 2018-2019 hot droughts in central Germany. Agric. For. Meteorol. 2021, 307, 108482. [Google Scholar] [CrossRef]
- Marusig, D.; Petruzzellis, F.; Tomasella, M.; Napolitano, R.; Altobelli, A.; Nardini, A. Correlation of field-measured and remotely sensed plant water status as a tool to monitor the risk of drought-induced forest decline. Forests 2020, 11, 77. [Google Scholar] [CrossRef]
- Duan, H.L.; Wu, J.P.; Liu, W.F.; Liao, Y.C.; Li, W.; Fan, H.B. Main and interactive effects of drought and warming on plant physiology. J. Nanchang Inst. Technol. 2017, 36, 28–32. [Google Scholar]
- Wu, G.; Liu, H.; Hua, L.; Luo, Q.; Lin, Y.; He, P.; Feng, S.; Liu, J.; Ye, Q. Differential responses of stomata and photosynthesis to elevated temperature in two co-oCCurring subtropical forest tree species. Front. Plant Sci. 2018, 9, 467. [Google Scholar] [CrossRef]
- Anderegg, W.R.; Berry, J.A.; Smith, D.D.; Sperry, J.S.; Anderegg, L.D.; Field, C.B. The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proc. Natl. Acad. Sci. USA 2012, 109, 233–237. [Google Scholar] [CrossRef]
- Way, D.A.; Oren, R.; Kroner, Y. The space-time continuum: The effects of elevated CO2 and temperature on trees and the importance of scaling. Plant Cell Environ. 2015, 38, 991–1007. [Google Scholar] [CrossRef]
- Blackman, C.J.; Brodribb, T.J.; Jordan, G.J. Leaf hydraulics and drought stress: Response, recovery and survivorship in four woody temperate plant species. Plant Cell Environ. 2009, 32, 1584–1595. [Google Scholar] [CrossRef]
- McDowell, N.G.; Sapes, G.; Pivovaroff, A.; Adams, H.D.; Allen, C.D.; Anderegg, W.R.L.; Arend, M.; Breshears, D.D.; Brodribb, T.; Choat, B.; et al. Mechanisms of woody-plant mortality under rising drought, CO2 and vapour pressure deficit. Nat. Rev. Earth Environ. 2022, 3, 294–308. [Google Scholar] [CrossRef]
- Vodnik, D.; Gricar, J.; Lavric, M.; Ferlan, M.; Hafner, P.; Eler, K. Anatomical and physiological adjustments of pubescent oak (Quercus pubescens Willd.) from two adjacent sub-Mediterranean ecosites. Environ. Exp. Bot. 2019, 165, 208–218. [Google Scholar] [CrossRef]
- Flo, V.; Martínez-Vilalta, J.; Mencuccini, M.; Granda, V.; Anderegg, W.R.L.; Poyatos, R. Climate and functional traits jointly mediate tree water-use strategies. New Phytol. 2021, 231, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, P.R.L.; Oliveira, R.S.; da Costa, A.C.L.; Giles, A.L.; Coughlin, I.; Costa, P.B.; Bartholomew, D.C.; Ferreira, L.V.; Vasconcelos, S.S.; Barros, F.V.; et al. Amazonia trees have limited capacity to aCClimate plant hydraulic properties in response to long-term drought. Glob. Chang. Biol. 2020, 26, 3569–3584. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.F.; Duan, H.L.; Huang, G.M.; Zhou, J.H.; Li, W.; Huang, R.Z.; Fan, H.B. Effects of high temperature and drought stress on growth, nutrient concentration, and nutrient use efficiency of tomato seedlings. Acta Ecol. Sin. 2019, 39, 3199–3209. (In Chinese) [Google Scholar]
- Dong, Y.C.; Liu, Y.H. Changes in the response of leaf traits in Pinus koraiensis (Korean pine) seedlings of different ages to controlled temperatures and light conditions. Acta Ecol. Sin. 2017, 37, 5662–5672. (In Chinese) [Google Scholar]
- Han, R.Y.; Gong, X.W.; Li, M.Y.; Leng, Q.N.; Zhou, Y.J.; Ning, Q.R.; Hao, G.Y. Combined tree-ring width and wood anatomy chronologies provide insights into the radial growth and hydraulic strategies in response to an extreme drought in plantation-grown Mongolian pine trees. Environ. Exp. Bot. 2023, 208, 105259. [Google Scholar] [CrossRef]
- Berry, Z.C.; Espejel, X.; Williams-Linera, G.; Asbjornsen, H. Linking coordinated hydraulic traits to drought and recovery responses in a tropical montane cloud forest. Am. J. Bot. 2019, 106, 1316–1326. [Google Scholar] [CrossRef]
- Castellaneta, M.; Rita, A.; Camarero, J.J.; Colangelo, M.; Ripullone, F. Declines in canopy greenness and tree growth are caused by combined climate extremes during drought-induced dieback. Sci. Total Environ. 2022, 813, 152666. [Google Scholar] [CrossRef]
- Kerr, J.T.; Ostrovsky, M. From space to species: Ecological applications for remote sensing. Trends Ecol. Evol. 2003, 18, 299–305. [Google Scholar] [CrossRef]
- Turner, W.; Spector, S.; Gardiner, N.; Fladeland, M.; Sterling, E.; Steininger, M. Remote sensing for biodiversity science and conservation. Trends Ecol. Evol. 2003, 18, 306–314. [Google Scholar] [CrossRef]
- Powers, J.S.; Vargas, G.G.; Brodribb, T.J.; Schwartz, N.B.; Pérez-Aviles, D.; Smith-Martin, C.M.; Becknell, J.M.; Aureli, F.; Blanco, R.; Calderón-Morales, E.; et al. A catastrophic tropical drought kills hydraulically vulnerable tree species. Glob. Chang. Biol. 2020, 26, 3122–3133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, X.; Zhou, S.; Ciais, P.; Mccarthy, H.; Luo, Y. Canopy and physiological controls of GPP during drought and heat wave. Geophys. Res. Lett. 2016, 43, 3325–3333. [Google Scholar] [CrossRef]
- D’Andrea, G.; Simunek, V.; Castellaneta, M.; Vacek, Z.; Vacek, S.; Pericolo, O.; Zito, R.G.; Ripullone, F. Mismatch between annual tree-ring width growth and NDVI index in Norway Spruce Stands of Central Europe. Forests 2022, 13, 1417. [Google Scholar] [CrossRef]
- De Souza, R.D.; Moura, V.; Paloschi, R.A.; Aguiar, R.G.; Webler, A.D.; Borma, L.D. Assessing drought response in the southwestern Amazon forest by remote sensing and in situ measurements. Remote Sens. 2022, 14, 1733. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolstrom, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wu, B.F.; Yan, N.N. Remote sensing estimates of vapor pressure deficit: An overview. Adv. Earth Sci. 2014, 29, 559–568. (In Chinese) [Google Scholar]
- Wang, A.Y.; Wang, M.; Yang, D.; Song, J.; Zhang, W.W.; Han, S.-J.; Hao, G.Y. Responses of hydraulics at the whole-plant level to simulated nitrogen deposition of different levels in Fraxinus mandshurica. Tree Physiol. 2016, 36, 1045–1055. [Google Scholar] [CrossRef]
- Song, J.; Yang, D.; Niu, C.Y.; Zhang, W.W.; Wang, M.; Hao, G.Y. Correlation between leaf size and hydraulic architecture in five compound-leaved tree species of a temperate forest in NE China. For. Ecol. Manag. 2018, 418, 63–72. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Song, J.; Wang, M.; Li, N.; Niu, C.Y.; Hao, G.Y. Coordination of xylem hydraulics and stomatal regulation in keeping the integrity of xylem water transport in shoots of two compound-leaved tree species. Tree Physiol. 2015, 35, 1333–1342. [Google Scholar] [CrossRef]
- Niu, C.Y.; Meinzer, F.C.; Hao, G.Y. Divergence in strategies for coping with winter embolism among co-oCCurring temperate tree species: The role of positive xylem pressure, wood type and tree stature. Funct. Ecol. 2017, 31, 1550–1560. [Google Scholar] [CrossRef]
- Jia, P.; Luo, S.K.; Wang, F. Distribution characteristics of leaf SPAD value and its relationship with chlorophyll content in leaves of three Magnoliaceae species. Landsc. Archit. Plant 2022, 44, 207. (In Chinese) [Google Scholar]
- Padilla, F.M.; de Souza, R.; Peña-Fleitas, M.T.; Gallardo, M.; Giménez, C.; Thompson, R.B. Different Responses of Various Chlorophyll Meters to Increasing Nitrogen Supply in Sweet Pepper. Front. Plant Sci. 2018, 9, 1752. [Google Scholar] [CrossRef] [PubMed]
- Haberstroh, S.; Werner, C.; Grün, M.; Kreuzwieser, J.; Seifert, T.; Schindler, D.; Christen, A. Central European 2018 hot drought shifts scots pine forest to its tipping point. Plant Biol. 2022, 24, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, G.E.; Maccaferri, M.; Newcomb, M.; Andrade-Sanchez, P.; White, J.W.; French, A.N.; Sciara, G.; Ward, R.; Tuberosa, R. Comparative Aerial and Ground Based High Throughput Phenotyping for the Genetic Dissection of NDVI as a Proxy for Drought Adaptive Traits in Durum Wheat. Front. Plant Sci. 2018, 9, 893. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.W.; Henebry, G.M.; Kimball, J.S.; Vanroekel-Patton, D.L.; Hildreth, M.B.; Wimberly, M.C. Satellite Microwave Remote Sensing for Environmental Modeling of Mosquito Population Dynamics. Remote Sens. Environ. 2012, 125, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Haworth, M.; Catola, S.; Marino, G.; Brunetti, C.; Michelozzi, M.; Riggi, E.; Avola, G.; Cosentino, S.L.; Loreto, F.; Centritto, M. Moderate Drought Stress Induces Increased Foliar Dimethylsulphoniopropionate (DMSP) Concentration and Isoprene Emission in Two Contrasting Ecotypes of Arundo donax. Front. Plant Sci. 2017, 8, 1016. [Google Scholar] [CrossRef] [PubMed]
- Reichstein, M.; Tenhunen, J.D.; Roupsard, O.; Ourcival, J.M.; Rambal, S.; Miglietta, F.; Peressotti, A.; Pecchiari, M.; Tirone, G.; Valentini, R. Severe drought effects on ecosystem CO2 and H2O fluxes at three Mediterranean evergreen sites: Revision of current hypotheses? Glob. Chang. Biol. 2002, 8, 999–1017. [Google Scholar] [CrossRef]
- Wang, X.; Lu, H.; Yuan, W. Inter-Annual Variations of Precipitation Modulate the Dry Spell Length. Geohealth 2022, 6, e2022GH000611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Guo, R.; Chen, K.; Wang, Y.; Niu, J.; Guo, Y.; Zhang, Y.; Yin, Z.; Xia, K.; Zhou, B.; et al. Microribbons composed of directionally self-assembled nanoflakes as highly stretchable ionic neural electrodes. Proc. Natl. Acad. Sci. USA 2020, 117, 14667–14675. [Google Scholar] [CrossRef]
- Jin, H.; Lin, J.; Zhu, Z. PIF4 and HOOKLESS1 Impinge on Common Transcriptome and Isoform Regulation in Thermomorphogenesis. Plant Commun. 2020, 1, 100034. [Google Scholar] [CrossRef]
- Rodríguez, V.M.; Soengas, P.; Alonso-Villaverde, V.; Sotelo, T.; Cartea, M.E.; Velasco, P. Effect of temperature stress on the early vegetative development of Brassica oleracea L. BMC Plant Biol. 2015, 15, 145. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Hu, S.; Xu, J.; Nian, J.; Cao, X.; Chen, M.; Cen, J.; Liu, X.; Zhang, Z.; et al. LEAF TIP RUMPLED 1 Regulates Leaf Morphology and Salt Tolerance in Rice. Int. J. Mol. Sci. 2022, 23, 8818. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-K.; Takahashi, Y.; Merilo, E.; Costa, A.; Zhang, L.; Kernig, K.; Lee, K.H.; Schroeder, J.I. Raf-like kinases and receptor-like (pseudo) kinase GHR1 are required for stomatal vapor pressure difference response. Proc. Natl. Acad. Sci. USA 2021, 118, e2107280118. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Sperry, J.S.; Tyree, M.T. Mechanism of water stress-induced xylem embolism. Plant Physiol. 1988, 88, 581–587. [Google Scholar] [CrossRef]
- Van der Molen, M.K.; Dolman, A.J.; Ciais, P.; Eglin, T.; Gobron, N.; Law, B.E.; Meir, P.; Peters, W.; Phillips, O.L.; Reichstein, M.; et al. Drought and ecosystem carbon cycling. Agric. For. Meteorol. 2011, 151, 765–773. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Powers, J.; Cochard, H.; Choat, B. Hanging by a thread? Forests and drought. Science 2020, 368, 261–266. [Google Scholar] [CrossRef]
- Hammond, W.M.; Williams, A.P.; Abatzoglou, J.T.; Adams, H.D.; Klein, T.; López, R.; Sáenz-Romero, C.; Hartmann, H.; Breshears, D.D.; Allen, C.D. Global field observations of tree die-off reveal hotter-drought fingerprint for Earth’s forests. Nat. Commun. 2022, 13, 1761. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Cochard, H. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol. 2009, 149, 575–584. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.M.; Cochard, H.; MencuCCini, M.; Delzon, S.; Badel, E. Direct observation and modelling of embolism spread between xylem conduits: A case study in Scots pine. Plant Cell Environ. 2016, 39, 2774–2785. [Google Scholar] [CrossRef] [PubMed]
- Rehschuh, R.; Ruehr, N.K. Diverging responses of water and carbon relations during and after heat and hot drought stress in Pinus sylvestris. Tree Physiol. 2022, 42, 1532–1548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, C.; Li, W.; Fang, X.; Zhang, T.; Zhu, Q.; Chen, H.; Zhao, P. Monitoring and estimating drought-induced impacts on forest structure, growth, function, and ecosystem services using remote-sensing data: Recent progress and future challenges. Environ. Rev. 2013, 21, 103–115. [Google Scholar] [CrossRef]
- Fan, K.; Ai, X.; Yao, L.; Huang, J.; Xu, Y.; Lu, X.; Ding, Y.; Zang, R. Do climate and human disturbance determine the sizes of endangered Metasequoia glyptostroboides trees in their native range? Glob. Ecol. Conserv. 2020, 21, e00850. [Google Scholar] [CrossRef]
- Zheng, R.P.; Zhou, L.L.; Zheng, D.; Huang, X.Y.; Wu, Y.L. The hydraulic architecture characteristics of different drought-tolerant Chinese fir plantlets. Chin. J. Appl. Environ. Biol. 2022, 28, 1571–1577. (In Chinese) [Google Scholar]


| Abbreviation | Full Name/Scientific Name | Unit | |
|---|---|---|---|
| Climate variables | T | Air temperature | (°C) |
| RH | Relative air humidity | (%) | |
| VPD | Vapor pressure deficit | (kPa) | |
| Ts | Soil temperature | (°C) | |
| Ms | Soil moisture content | (%) | |
| VWC | Volumetric water content | (m3/m3) | |
| Ppt | Precipitation | (mm) | |
| Leaf traits | RWC | Leaf relative water content | (%) |
| SPAD value | Relative value of chlorophyll content | - | |
| gs | Stomatal conductance | (mol−1 m−2 s−1) | |
| Hydraulic traits | Ks | Stem hydraulic conductivity | (kg m s−1 MPa−1) |
| Ψmidday | Midday water potential | (MPa) | |
| PLC | Percent loss of hydraulic conductivity | (%) | |
| Safety margin | The difference between water potential and P50 | (MPa) | |
| P50 | Water potential at 50% ofmaximum hydraulic conductivity | (MPa) | |
| Remote sensing | NDVI | Normalized difference vegetation index | - |
| Species | KP | Koelreuteria paniculata Laxm. | - |
| CC | Cinnamomum camphora (L.) Presl | - | |
| MG | Metasequoia glyptostroboides Hu and W. C. Cheng | - | |
| TD | Taxodium distichum var. imbricatum (Nuttall) Croom | - |
| Tree Species | Distributed Research Field(s) | Dieback Percentage |
|---|---|---|
| Koelreuteria paniculata Laxm. | Dalianhu | 60% |
| Cinnamomum camphora (L.) Presl | Nanyuewei/Dalinhu | 40% |
| Metasequoia glyptostroboides Hu and W. C. Cheng | Liantang/Zhangma Village | 50% |
| Taxodium distichum var. imbricatum (Nuttall) Croom | Liantang/Zhangma Village | 40% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Song, J. Field-Measured Hydraulic Traits and Remotely Sensed NDVI of Four Subtropical Tree Species Showed Transient Declines during the Drought–Heatwave Event. Forests 2023, 14, 1420. https://doi.org/10.3390/f14071420
Wang Y, Song J. Field-Measured Hydraulic Traits and Remotely Sensed NDVI of Four Subtropical Tree Species Showed Transient Declines during the Drought–Heatwave Event. Forests. 2023; 14(7):1420. https://doi.org/10.3390/f14071420
Chicago/Turabian StyleWang, Yongkang, and Jia Song. 2023. "Field-Measured Hydraulic Traits and Remotely Sensed NDVI of Four Subtropical Tree Species Showed Transient Declines during the Drought–Heatwave Event" Forests 14, no. 7: 1420. https://doi.org/10.3390/f14071420
APA StyleWang, Y., & Song, J. (2023). Field-Measured Hydraulic Traits and Remotely Sensed NDVI of Four Subtropical Tree Species Showed Transient Declines during the Drought–Heatwave Event. Forests, 14(7), 1420. https://doi.org/10.3390/f14071420






