Infrared Thermal Profiles in Silvopastoral and Full-Sun Pastures in the Eastern Amazon, Brazil
Abstract
:1. Introduction
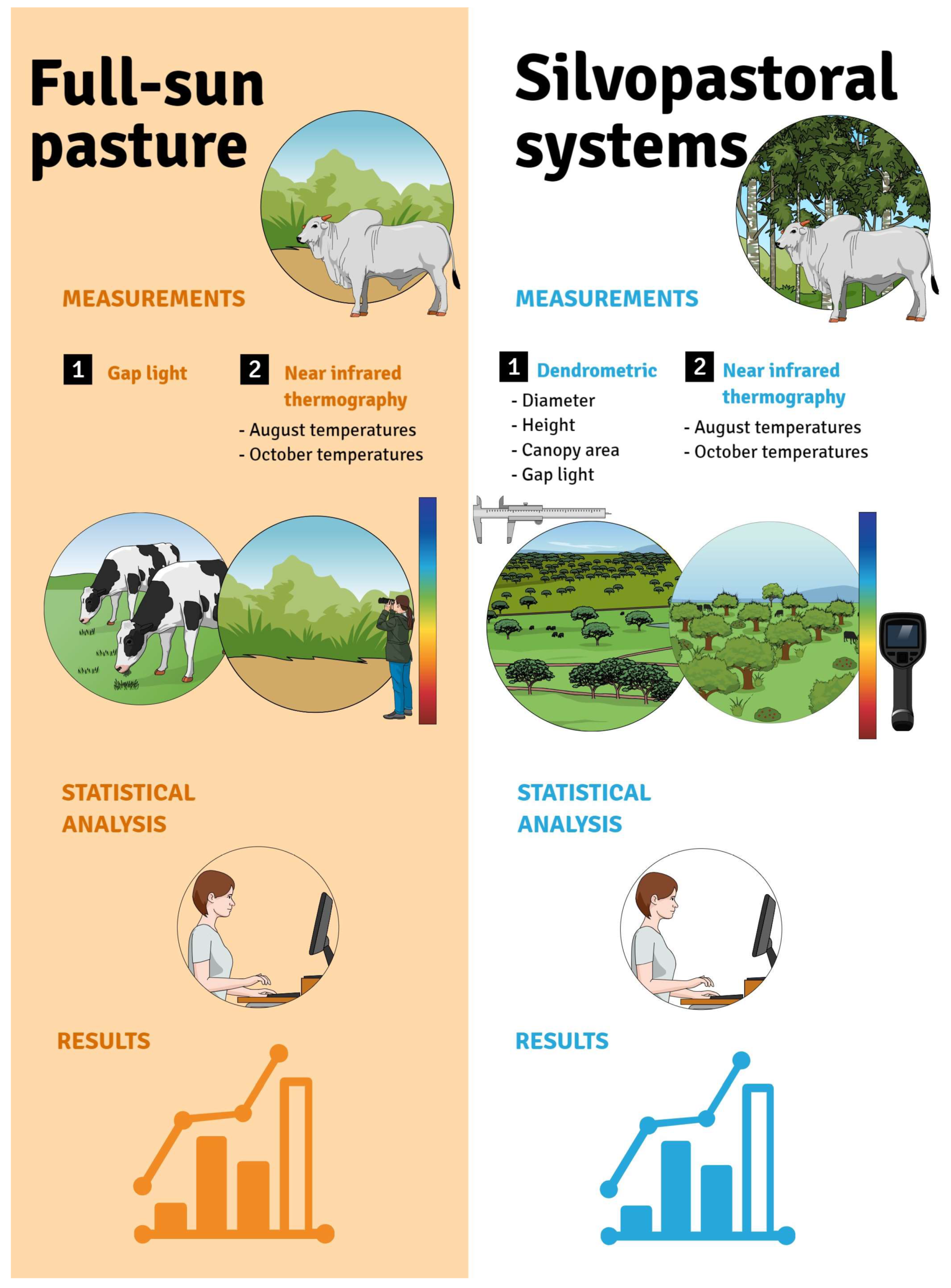
2. Materials and Methods
2.1. Field Site
2.2. Field Site History
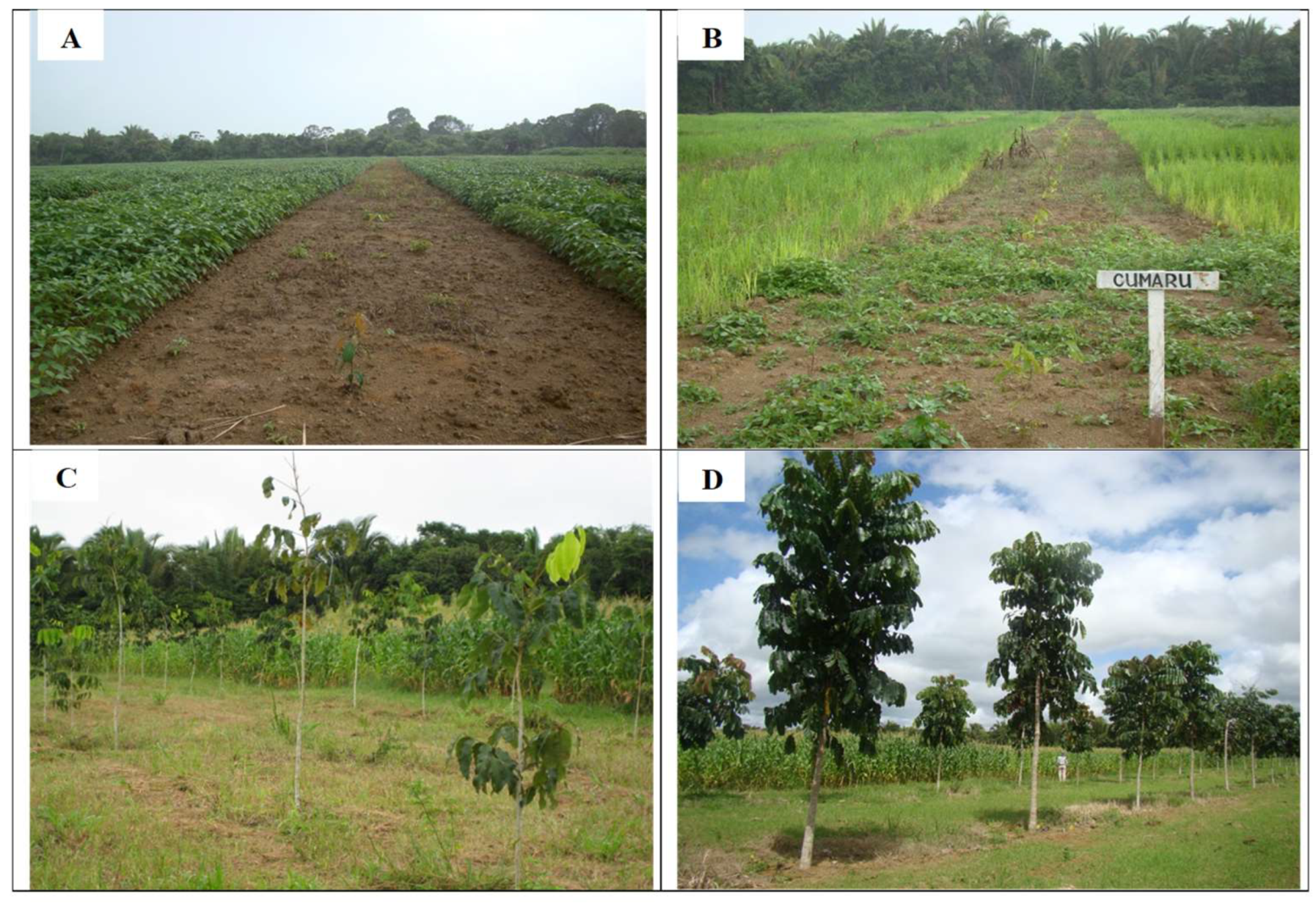
2.3. Experimental Design
2.4. Sampling of the Study Sites
2.5. Dendrometric Measurements
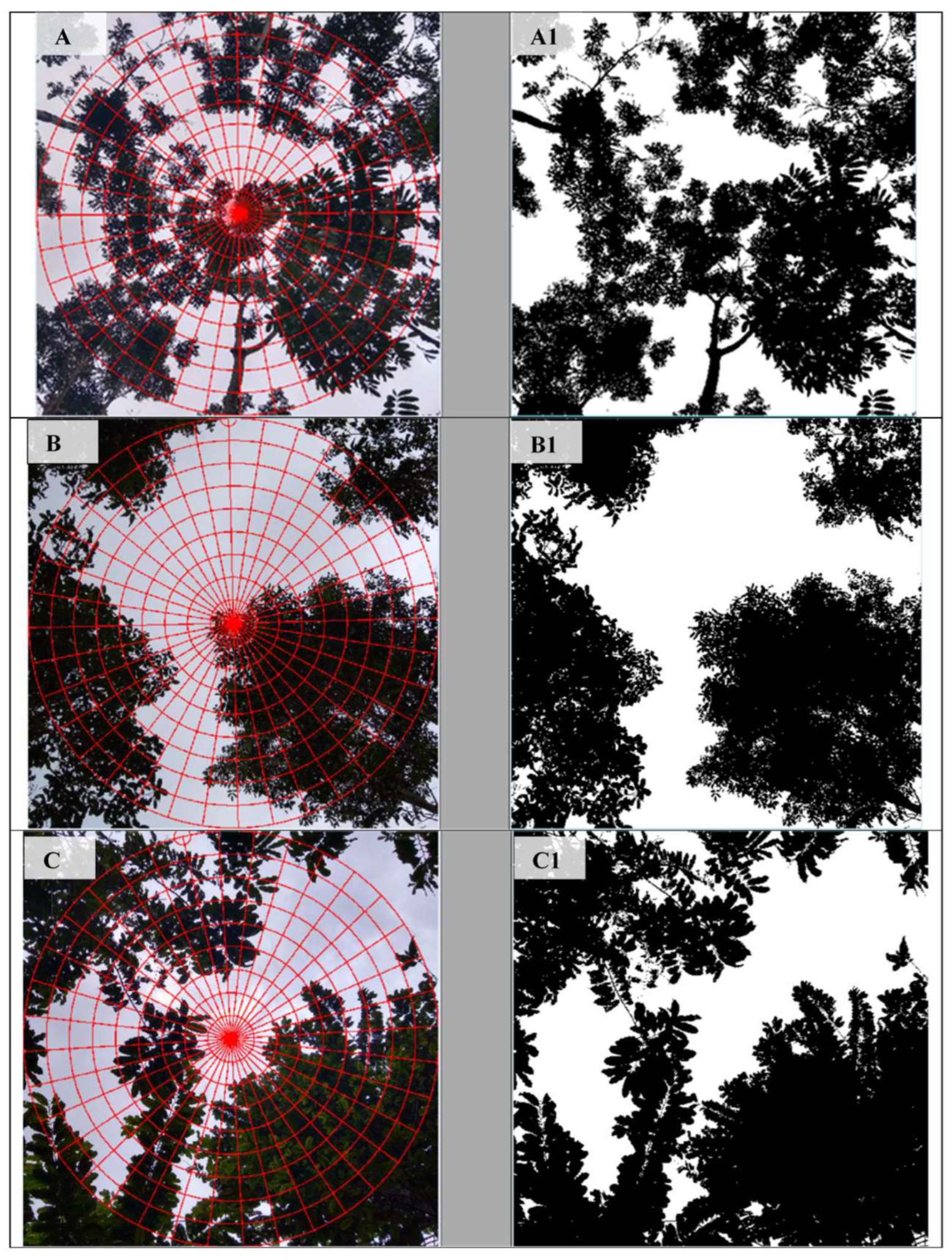
2.6. Near-Infrared Thermography
2.7. Statistical Analysis
3. Results
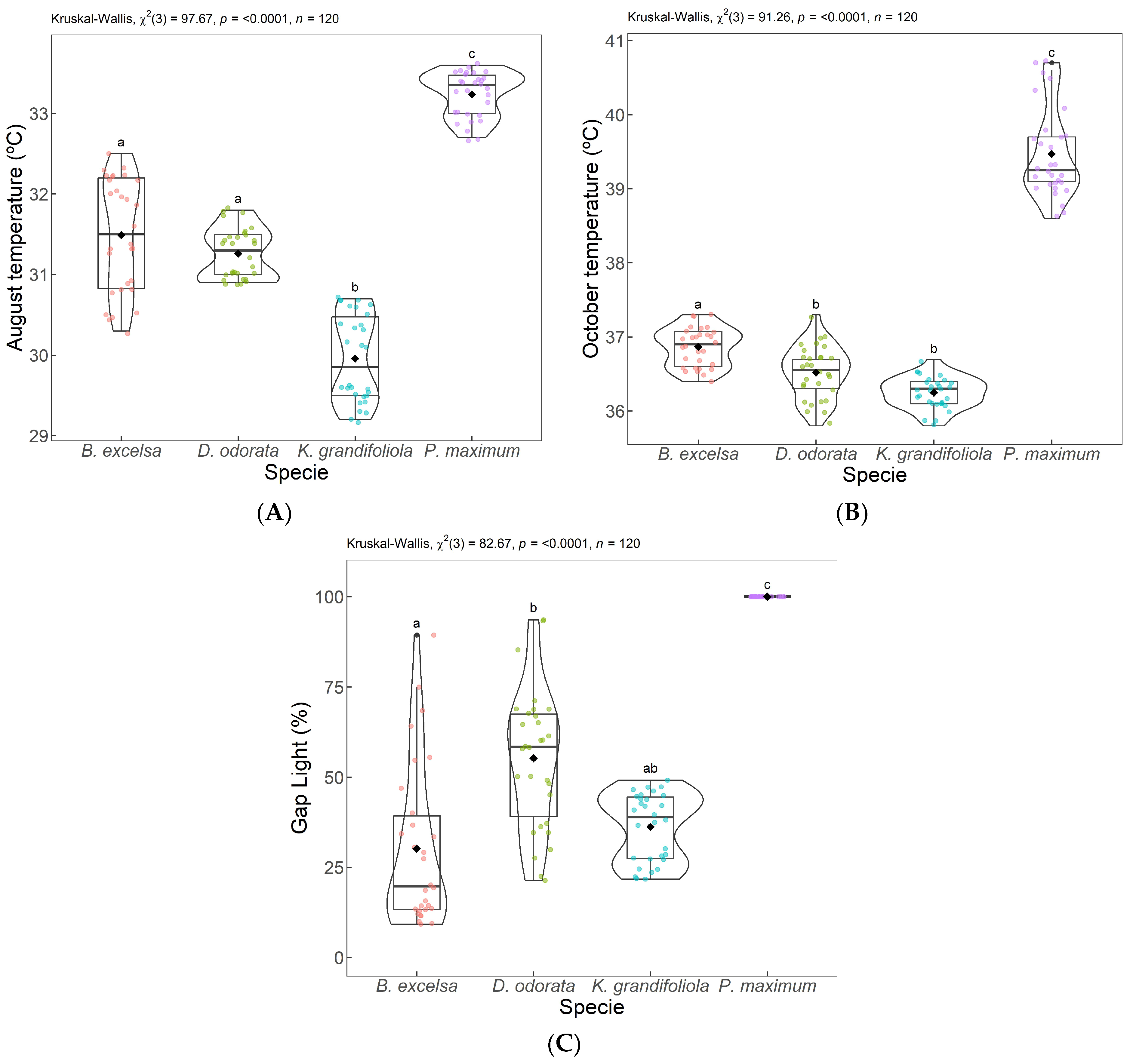
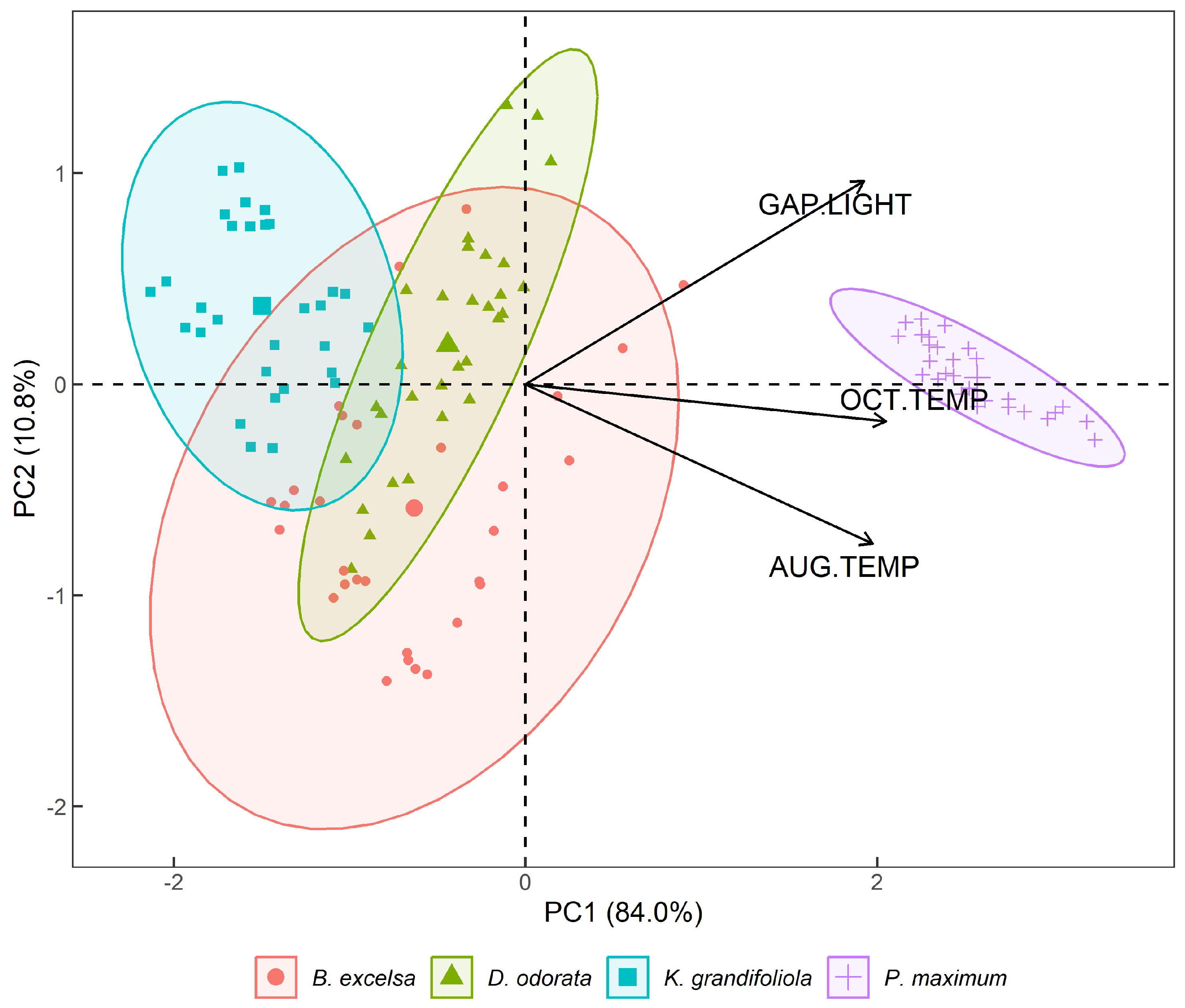
3.1. Results from PCA 1—Gap Light and Thermographic Data
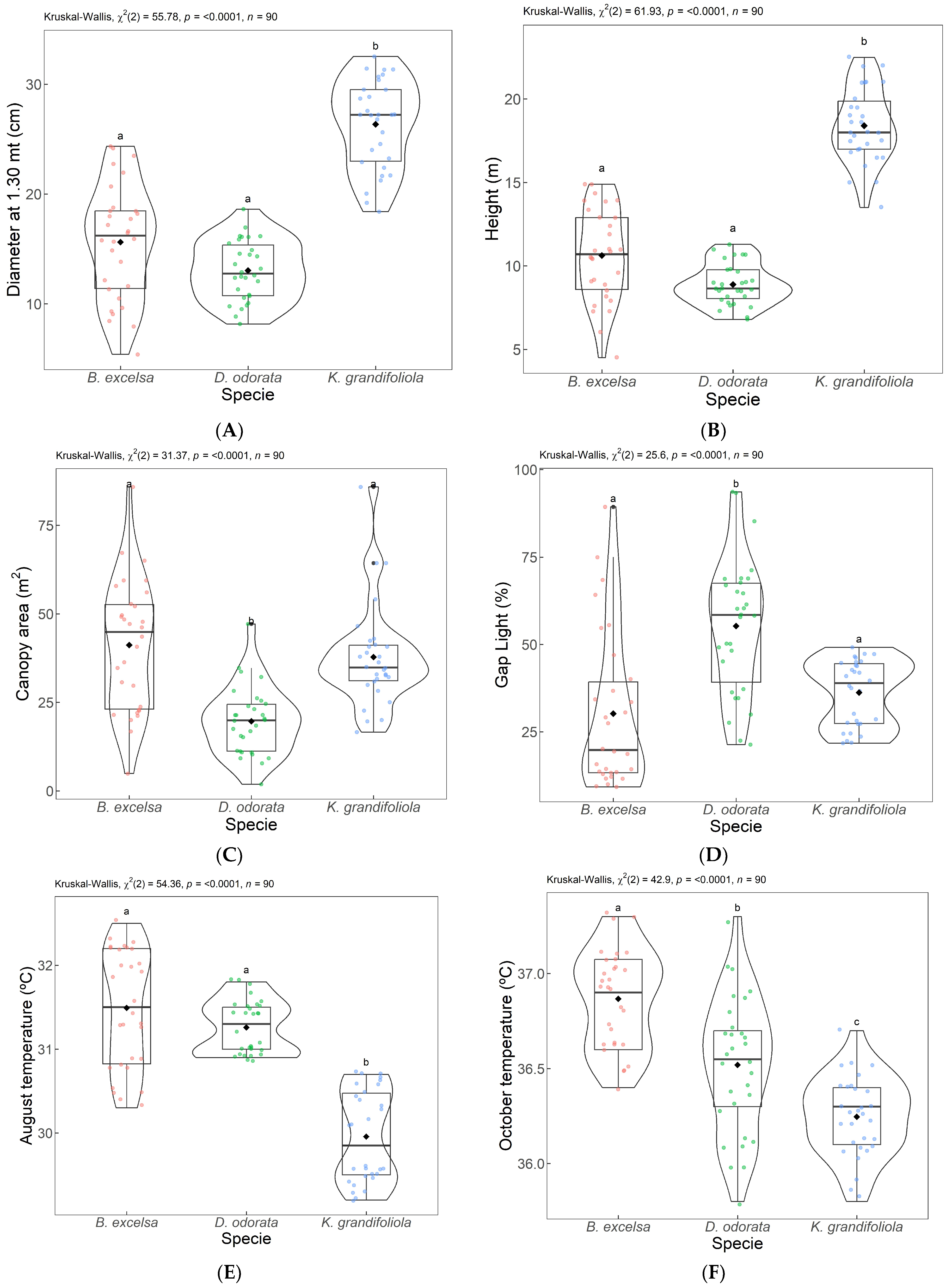
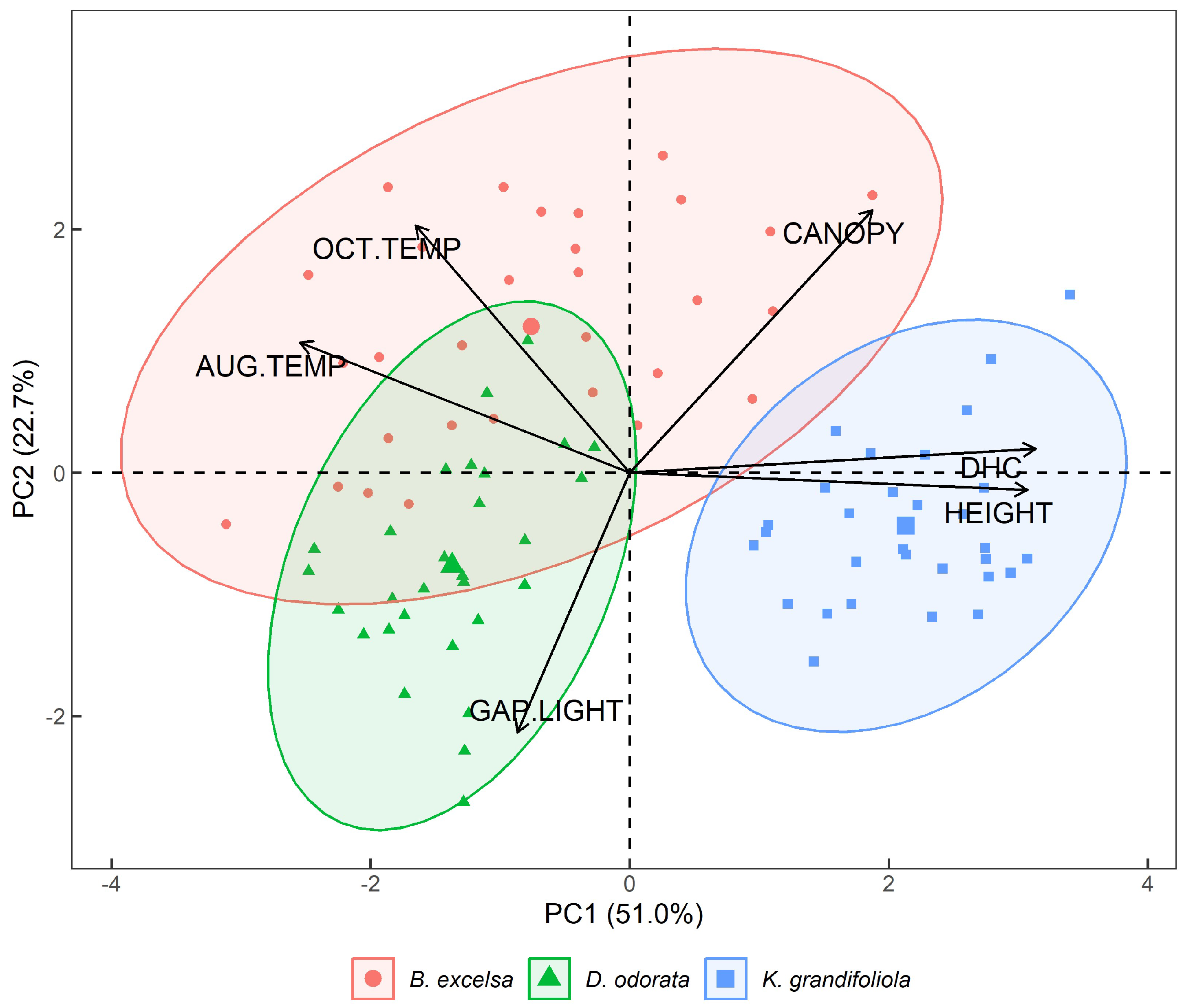
3.2. Results from ACP 2—Dendrometric Variables and Temperature
4. Discussion
4.1. ACP 1—Gap Light and Temperature of the Analyzed Targets
4.2. ACP 2—Dendrometric Variables and Thermography Data
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manaye, A.; Tesfamariam, B.; Tesfaye, M.; Worku, A.; Gufi, Y. Tree diversity and carbon stocks in agroforestry systems in northern Ethiopia. Carbon Balance Manag. 2021, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Karvatte, N.; Klosowski, E.S.; de Almeida, R.G.; Mesquita, E.E.; Oliveira, C.C.; Alves, F.V. Shading effect on microclimate and thermal comfort indexes in integrated crop-livestock-forest systems in the Brazilian Midwest. Int. J. Biometeorol. 2016, 60, 1933–1941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, C.C.; Alves, F.V.; de Almeida, R.G.; Gamarra, É.L.; Villela, S.D.J.; de Almeida Martins, P.G.M. Thermal comfort indices assessed in integrated production systems in the Brazilian savannah. Agrofor. Syst. 2017, 92, 1659–1672. [Google Scholar] [CrossRef]
- Kim, Y.; Still, C.J.; Hanson, C.V.; Kwon, H.; Greer, B.T.; Law, B.E. Canopy skin temperature variations in relation to climate, soil temperature, and carbon flux at a ponderosa pine forest in central Oregon. Agric. For. Meteorol. 2016, 226–227, 161–173. [Google Scholar] [CrossRef]
- Kovács, B.; Tinya, F.; Ódor, P. Stand structural drivers of microclimate in mature temperate mixed forests. Agric. For. Meteorol. 2017, 234–235, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Organização das Nações Unidas. Transformando o Nosso Mundo: A Agenda 2030 Para o Desenvolvimento Sustentável. Resolução A/RES/70/1. Nova Iorque: UN. 2015. Available online: https://nacoesunidas.org/wp-content/uploads/2015/10/agenda2030-pt-br.pdf (accessed on 30 March 2023).
- Renaud, V.; Innes, J.L.; Dobbertin, M.; Rebetez, M. Comparison between open-site and below-canopy climatic conditions in Switzerland for different types of forests over 10 years (1998–2007). Theor. Appl. Climatol. 2011, 105, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Maes, W.H.; Baert, A.; Huete, A.R.; Minchin, P.E.H.; Snelgar, W.P.; Steppe, K. A new wet reference target method for continuous infrared thermography of vegetations. Agric. For. Meteorol. 2016, 226–227, 119–131. [Google Scholar] [CrossRef]
- Lopes, L.B.; Eckstein, C.; Pina, D.S.; Carnevalli, R.A. The influence of trees on the thermal environment and behaviour of grazing heifers in Brazilian Midwest. Trop. Anim. Health Prod. 2016, 48, 755–761. [Google Scholar] [CrossRef] [Green Version]
- Giro, A.; Pezzopane, J.R.M.; Barioni, W., Jr.; Pedroso, A.d.F.; Lemes, A.P.; Botta, D.; Romanello, N.; Barreto, A.d.N.; Garcia, A.R. Behavior and body surface temperature of beef cattle in integrated crop-livestock systems with or without tree shading. Sci. Total Environ. 2019, 684, 587–596. [Google Scholar] [CrossRef]
- Pezzopane, J.R.M.; Bernardi, A.C.C.; Bosi, C.; Oliveira, P.P.A.; Marconato, M.H.; Pedroso, A.F.; Esteves, S.N. Forage productivity and nutritive value during pasture renovation in integrated systems. Agrofor. Syst. 2019, 93, 39–49. [Google Scholar] [CrossRef]
- Gersony, J.T.; Prager, C.M.; Boelman, N.T.; Eitel, J.U.H.; Gough, L.; Greaves, H.E.; Griffin, K.L.; Magney, T.S.; Sweet, S.K.; Vierling, L.A.; et al. Scaling thermal properties from the leaf to the canopy in the Alaskan Arctic tundra. Arctic. Antarct. Alp. Res. 2016, 48, 739–754. [Google Scholar] [CrossRef] [Green Version]
- Hammerle, A.; Meier, F.; Heinl, M.; Egger, A.; Leitinger, G. Implications of atmospheric conditions for analysis of surface temperature variability derived from landscape-scale thermography. Int. J. Biometeorol. 2017, 61, 575–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngao, J.; Adam, B.; Saudreau, M. Intra-crown spatial variability of leaf temperature and stomatal conductance enhanced by drought in apple tree as assessed by the RATP model. Agric. For. Meteorol. 2017, 237–238, 340–354. [Google Scholar] [CrossRef]
- Abreu-Harbich, L.V.; Labaki, L.C.; Matzarakis, A. Effect of tree planting design and tree species on human thermal comfort in the tropics. Landscape. Urban. Plan. 2015, 138, 99–109. [Google Scholar] [CrossRef]
- Karvatte, N., Jr.; Miyagi, E.S.; de Oliveira, C.C.; Barreto, C.D.; Mastelaro, A.P.; Bungenstab, D.J.; Alves, F.V. Infrared thermography for microclimate assessment in agroforestry systems. Sci. Total Environ. 2020, 731, 1–10. [Google Scholar] [CrossRef]
- Barreto, C.D.; Alves, F.V.; de Oliveira Ramos, C.E.C.; de Paula Leite, M.C.; Leite, L.C.; Junior, N.K. Infrared thermography for evaluation of the environmental thermal comfort for livestock. Int. J. Biometeorol. 2020, 64, 881–888. [Google Scholar] [CrossRef]
- Alves, F.V.; Karvatte, N., Jr.; Miyagi, E.S.; de Oliveira, C.C. Aplicações da Termografia por Infravermelho (TIV) na bovinocultura de corte. Série Doc. Embrapa. 2020, 276, 20–36. Available online: www.embrapa.br/fale-conosco/sac (accessed on 16 April 2023).
- De Oliveira, C.C.; Alves, F.V.; Martins, P.G.M.d.A.; Karvatte, N., Jr.; Alves, G.F.; de Almeida, R.G.; Mastelaro, A.P.; Costa e Silva, E. Vaginal temperature as indicative of thermoregulatory response in Nellore heifers under different microclimatic conditions. PloS ONE 2019, 14, 190–205. [Google Scholar] [CrossRef] [Green Version]
- Silanikove, N. Effects of heat stress on the welfare of extensively managed domestic ruminants. Liv. Prod. Sci. 2000, 67, 1–18. [Google Scholar] [CrossRef]
- Schütz, K.E.; Rogers, A.R.; Poulouin, Y.A.; Cox, N.R.; Tucker, C.B. The amount of shade influences the behavior and physiology of dairy cattle. J. Dairy Sci. 2010, 93, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Neves, E.S.; Guedes, M.C.; Rodrigues, E.G. Relação da produção de frutos de castanha-da-amazônia (Bertholletia excelsa bonpl.) com variáveis das próprias castanheiras, em capoeira e floresta da Resex Cajari. Biota Amaz. 2015, 5, 31–37. [Google Scholar] [CrossRef]
- Silva, A.A.; Santos, M.K.V.; Gama, J.R.V.; Noce, R.; Leão, S. Potencial do extrativismo da castanha-do-pará na geração de renda em comunidades da mesorregião baixo Amazonas, Pará. Floresta E Ambiente 2013, 20, 500–509. [Google Scholar] [CrossRef] [Green Version]
- Scoles, R. Ecologia e Extrativismo da Castanheira (Bertholletia excelsa, Lecythidaceae) em duas regiões da Amazônia Brasileira. In Tese de Doutorado em Biologia (Ecologia); Instituto Nacional de Pesquisas da Amazônia: Manaus, Brazil, 2010; p. 193. [Google Scholar]
- Lopes, C.L.; Nardi, J.; Chiavari, J. Reposição florestal:Panorama da regulamentação nos EstadosDiário da Justiça do Brasil, Decreto nº 23.296 de 06 de agosto de 2007. Available online: https://www.climatepolicyinitiative.org/wp-content/uploads/2021/08/Relatorio-Reposicao-Florestal.pdf (accessed on 18 April 2023).
- Fábio, F.G.; Câncio, I.A.P. Manejo florestal do cumaru: Um experimento rentável e sustentável em Óbidos, Estado do Pará. In Ensaios nas Ciências Agrárias e Ambientais, 8; Atena Editora: Ponta Grossa, Brazil, 2019; pp. 38–50. [Google Scholar]
- Sousa, B.C.M.; Barata, L.E.S.; Macêdo, C.G.; Fraga, S.S.; Kasper, A.A.M.; Lourido, K.A.; Paulino, G.S.; Almeida, E.C.; Sartoratto, A.; Lustosa, D.C. Avaliação do teor de Cumarina e atividade antifúngica de frações de Óleo de Cumaru. Rev. Ibero-Am. De Ciências Ambient. 2018, 9, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Silva, T.M.; Jardim, F.C.S.; Silva, M.S.; Shanley, P. O mercado das amêndoas de Dipteryx odorada (cumaru) no estado do Pará. Floresta 2010, 40, 603–614. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.S. Seed and seedling survival of African mahogany (Entandrophragma spp.) in the Central African Republic: Implications for forest management. Ecol. Manag. 2008, 255, 292–299. [Google Scholar] [CrossRef]
- Kasongo Yakusu, E.; Monthe, F.S.; Bourland, N.; Hardy, O.J.; Louppe, D.; Bola Mbele Lokanda, F.; Hubau, W.; Kahindo Muhongya, J.-M.; van den Bulcke, J.; van Acker, J.; et al. Entandrophragma: Taxonomy and ecology of a genus of African tree species with economic importance. A review. Biotechnol. Agron. Soc. Environ. 2021, 25, 140–153. [Google Scholar] [CrossRef]
- Bouka, U.G.D.; Doumenge, C.; Loumeto, J.J.; Florence, J.; Gonmadje, C.; Mckey, D. Des confusions entre espèces prèjudicables à la gestion durable des essences forestières: L’exemple des acajous d’Afrique (Khaya, Meliaceae). Bois Forêts Des Trop. 2019, 339, 17–32. [Google Scholar] [CrossRef]
- Poorter, L.; Bongers, F.; Kouamé, F.N.; Hawthorne, W.D. Biodiversity of West African Forests: An Ecological Atlas of Woody Plant Species; CABI Publishing: Oxford, UK, 2004; p. 528. [Google Scholar]
- Armstrong, M.; Reilly, D.F.; Lelievre, T.; Hopewell, A.; Redman, L.; Francis, R.M.R. African Mahogany Grown in Australia-Wood Quality and Potential Uses; Rural Industries Research and Development Corporation: Kingston, Australia, 2007.
- Pinheiro, L.P.; Couto, L.; Pinheiro, D.T.; Brunetta, J.M.F.C. Ecologia, Silvicultura e Tecnologia de Utilização dos Mognos Africanos (Khaya spp.); Sociedade Brasileira de Agrossilvicultura (SBAG): Viçosa, Brazil, 2011; p. 102. [Google Scholar]
- de Azevedo, M.L.; Titon, M.; Machado, E.L.M.; de Assis Júnior, S.L.; de Freitas, E.C.S. Influence of indolbutiric acid on rooting of stem and leaf minicuttings of african mahogany (Khaya grandifoliola c. dc.). Cienc. Florest. 2021, 31, 898–919. [Google Scholar] [CrossRef]
- Pennington, T.D.; Cheek, M. Khaya Grandifoliola C. DC. Pará. 2015. Available online: http://floradobrasil.jbrj.gov.br/reflora/herbarioVirtual/ConsultaPublicoHVUC/ConsultaPublicoHVUC.do?idTestemunho=4658185 (accessed on 17 April 2023).
- Silva MG, d.a.; Zaidan, Ú.R.; Borges, C.T.; da Silva, J.A.; Resende, R.T.; Venturoli, F.; Pires, P.P. Indolbutyric Acid (IBA) in the African Mahogany (Khaya grandifoliola C. DC.) cuttings and mini-cuttings development. Adv. For. Sci. 2020, 7, 1009–1016. [Google Scholar] [CrossRef]
- Lemmens, R.H.M.J.; Louppe, D.; Oteng-Amoako, A.A. Plant Resources of Tropical Africa 7. In Timbers 2, 2nd ed.; PROTA Foundation: Wageningen, The Netherlands, 2012. [Google Scholar]
- Hall, J.S.; Harris, D.J.; Medjibe, V.; Ashton, P.M.S. The efects of selective logging on forest structure and tree species composition in a Central African forest: Implications for management of conservation areas. Ecol. Manag. 2003, 183, 249–264. [Google Scholar] [CrossRef]
- Danquah, J.A.; Sarpong, D.B.; Pappinen, A. Causal relationships between African mahoganies exports and deforestation in Ghana: Policy implications. Environ. Dev. Sustain. 2013, 15, 51–66. [Google Scholar] [CrossRef]
- Altieri, M. Agroecologia: Bases Científicas Para uma Agricultura Sustentável, 3rd ed.; Expressão Popular: Rio de Janeiro, Brazil, 2012. [Google Scholar]
- Montagnini, F.; Metzel, R. Integrando Paisagens: Agroflorestas para Conservação da Biodiversidade e Soberania Alimentar; Springer: Cham, Switzerland, 2017; Available online: https://scholar.google.com/scholar_lookup?title=Integrating+Landscapes:+Agroforestry+for+Biodiversity+Conservation+and+Food+Sovereignty&author=Montagnini,+F.&author=Metzel,+R.&publication_year=2017 (accessed on 26 March 2023).
- Pinto, J.; Encinas, I.; Silva, G. Age and growth of trees. Technol. For. Commun. 2005, 7, 43. Available online: http://repositorio.unb.br/handle/10482/10018 (accessed on 2 March 2023).
- Martorano, L.G.; Nechet, D.; Pereira, L.C. Tipologia climática do Estado do Pará: Adaptação do método de Köppen. Bol. De Geogr. Teorética 1993, 23, 45–46. [Google Scholar]
- Martorano, L.G.; Vitorino, M.I.; da Silva, B.P.P.C.; de Moraes, J.R.S.C.; Lisboa, L.S.; Sotta, E.D.; Reichardt, K. Climate conditions in the eastern amazon: Rainfall variability in Belem and indicative of soil water deficit. Afr. J. Agric. Res. 2017, 12, 1801–1810. [Google Scholar]
- Empresa Brasileira de Pesquisa Agropecuária–EMBRAPA; Centro Nacional de Pesquisa de Solos. Sistema Brasileiro de Classificação de Solos; Embrapa Solos: Rio de Janeiro, Brazil, 2006; p. 306. [Google Scholar]
- Silva, J.C.N.; Silva, A.R.; Veloso, C.A.C.; Dantas, E.F.; do Sacramento, J.A.A.S. Aggregation, carbon, and total soil nitrogen in crop-livestock-forest integration in the Eastern Amazon. Rev. Bras. Eng. Agric. E Ambient. 2018, 22, 837–842. [Google Scholar] [CrossRef]
- Silva, A.R.; Sales, A.; Alberto, C.; Veloso, C.; Jorge, E.; Carvalho, M.; Pinheiro, E.; Marco, B. Propriedades físicas e teor de matéria orgânica de um latossolo amarelo sob sistemas integração lavoura-pecuária-floresta. J. Agron. Sci. 2015, 4, 144–157. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/125909/1/13.pdf (accessed on 15 March 2023).
- EMBRAPA-Empresa Brasileira de Pesquisa Agropecuária. Manual de Métodos de Análise de Solo; Embrapa Solos: Rio de Janeiro, Brazil, 2011; p. 212. [Google Scholar]
- Lameira, M.K.S.; Silva, H.K.M.; Gomes, K.M.A.; Ferreira, A.C.T.; Martorano, L.G.; Ribeiro, R.B.S.; Silva, A.R.; Gama, J.R.V. Capacidade produtiva de Dipteryx odorata (Aubl.) Willd. em sistema de produção de pecuária integrada no Baixo Amazonas. Rev. For. Mesoam. Kuru-Rfmk 2022, 19, 56–65. Available online: https://revistas.tec.ac.cr/index.php/kuru/article/view/6102 (accessed on 15 March 2023).
- Harder, I.C.F.; Ribeiro, R.C.S.; Tavares, A.R. Índices de área verde e cobertura vegetal para as praças do município de Vinhedo, SP. Rev. Árvore 2006, 30, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.C.; Gama, J.R.V.; Rode, R.; Coelho, A.L.; Coelho, M. Tree species growth in a silvopastoral system in Amazon. Afr. J. Agric. Res. 2018, 13, 95–103. Available online: https://academicjournals.org/journal/AJAR/article-full-text-pdf/D8BA09155677 (accessed on 14 March 2023).
- Hu, L.; Zhu, J. Improving gap light index (GLI) to quickly calculate gap coordinates. Can. J. For. Res. 2008, 38, 2337–2347. [Google Scholar] [CrossRef]
- Kim, J.; Song, I.; Kim, C.; Gho, H.; An, S.; Song, D.; Joung, D.; Kang, S.; Yi, Y.; Park, B.J.; et al. Thermal Comfort and Human Responses according to Tree Density in Forest Environments during and after Physical Activities in the Summer. Forests 2023, 14, 120. [Google Scholar] [CrossRef]
- Manly, B.F.J.; Alberto, J.A.N. Métodos Estatísticos Multivariados: Uma Introdução, 4th ed.; Bookman: Porto Alegre, Brazil, 2019; p. 254. [Google Scholar]
- Johnson, R.A.; Wichern, D.W. Applied Multivariate Statistical Analysis, 4th ed.; Prentice Hall: Hoboken, NJ, USA, 1998. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 20 February 2023).
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. Available online: https://core.ac.uk/download/pdf/6303121.pdf (accessed on 21 February 2023). [CrossRef] [Green Version]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses, R package version 1.0.7; DataCamp: Vienna, Austria, 2020; Available online: https://CRAN.R-project.org/package=factoextra (accessed on 21 February 2023).
- Rencher, A.C. Methods of Multivariate Analysis, 2nd ed.; A John Wiley & Sons, Inc. Publication: Hoboken, NJ, USA, 2002; p. 727. [Google Scholar]
- Baêta, F.C.; Souza, C.F. Ambiência em Edificações Rurais: Conforto Animal; Editora UFV: Viçosa, Brazil, 2010. [Google Scholar]
- Baluja, J.; Diago, M.P.; Balda, P.; Zorer, R.; Meggio, F.; Morales, F.; Tardaguila, J. Assessment of vineyard water status variability by thermal and multispectral imagery using an unmanned aerial vehicle (UAV). Irrig. Sci. 2012, 30, 511–522. [Google Scholar] [CrossRef]
- Meier, F.; Scherer, D. Spatial and temporal variability of urban tree canopy temperature during summer 2010 in Berlin, Germany. Theor. Appl. Climatol. 2012, 110, 373–384. [Google Scholar] [CrossRef]
- Silva, R.G. Predição da configuração de sombras de árvores em pastagens para bovinos. Eng. Agric. 2006, 26, 36–281. Available online: https://repositorio.unesp.br/bitstream/handle/11449/27779/S0100-69162006000100029.pdf?sequence=1&isAllowed=y (accessed on 30 March 2023). [CrossRef] [Green Version]
- Baliscei, M.A.; Barbosa, O.R.; Souza, W.; Costa, M.A.T.; Krutzmann, A.; Queiroz, E.O. Microclimate without shade and silvopastoral system during summer and winter. Acta Scientiarum. Anim. Sci. 2013, 35, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Lebart, L.; Piron, M.; Morineau, A. Statistique Exploratoire Multidimensionnelle; Dunod: Paris, France, 2000; p. 439. [Google Scholar]
- Pereira, L.M.R.; Fischer, V.; Moreno, C.B.; Pardo, M.P.; Gomes, J.F.; Monks, P.L. Diurnal ingestive behavior of Jersey heifers fed diferente supplements. Rev. Bras. Agrocienc. 2005, 11, 453–459. [Google Scholar] [CrossRef]
- Abreu, L.V.; Labaki, L.C. Conforto térmico propiciado por algumas espécies: Avaliação do raio de influência através de diferentes índices de conforto. Ambiente Constr. 2010, 10, 103–117. [Google Scholar] [CrossRef]
- Baliscei, M.A.; Souza, W.; Barbosa, O.R.; Cecato, U.; Krutzmann, A.; Edicarlos, O.Q. Behaviour of beef cattle and the microclimate with and without shade. Acta Sci. Anim. Sci. 2012, 34, 409–415. [Google Scholar] [CrossRef]
- Lees, A.M.; Lees, J.C.; Sejian, V.; Sullivan, M.L.; Gaughan, J.B. Influence of shade on panting score and behavioural responses of Bos taurus and Bos indicus feedlot cattle to heat load. Anim. Prod. Sci. 2020, 60, 305–315. [Google Scholar] [CrossRef]
- de Souza, E.C.; Salman, A.K.D.; da Cruz, P.G.; Veit, H.M.; de Carvalho, G.A.; da Silva, F.R.F.; Schmitt, E. Thermal comfort and grazing behaviour of Girolando heifers in integrated crop-livestock (ICL) and crop-livestock-forest (ICLF) systems. Acta Sci. Anim. Sci. 2019, 41, 1–10. [Google Scholar] [CrossRef]
- Lemaire, G.; Franzluebbers, A.; Carvalho, P.C.d.F.; Dedieu, B. Integrated crop-livestock systems: Strategies to achieve synergy between agricultural production and environmental quality. Agric. Ecosyst. Environ. 2014, 190, 4–8. [Google Scholar] [CrossRef]
- Garcia, A. Thermal comfort on reproductive processes of water buffaloes raised under tropical conditions. Rev. Bras. Reprod. Anim. 2013, 37, 121–130. [Google Scholar]
- Figueiredo, E.B.; Jayasundara, S.; Bordonal, R.O.; Reis, R.A.; Wagner-Riddle, C.; La Scala, N. Greenhouse gas balance and carbon footprint of beef cattle in three contrasting pasture-management systems in Brazil. J. Clean. Prod. 2017, 142, 420–431. [Google Scholar] [CrossRef] [Green Version]
- Dube, F.; Couto, L.; Silva, M.L.; Leite, H.G.; García, R.; Araújo, G.A.A. A simulation model for evaluating technical and economic aspects of an industrial eucalyptusbased agroforestry system in Minas Gerais, Brazil. Agrofor. Syst. 2002, 55, 73–80. [Google Scholar] [CrossRef]
- Garcia, A.R.; Silva, L.K.X.; Barros, D.V.; Lourenço Junior, J.B.; Martorano, L.G.; Lisboa, L.S.S.; Silva, J.A.R.; Sousa, J.S.; Silva, A.O.A. Key points for the thermal comfort of water buffaloes in Eastern Amazon. Ciência Rural 2023, 53, 1–18. [Google Scholar] [CrossRef]
- Silva, A.R.; Veloso, C.A.C.; Carvalho, E.J.M.; Alves, L.W.R.; Azevedo, C.M.B.C.; Fernandes, P.C.C. Comportamento da espécie mogno africano (Khaya ivorensis) em sistema de integração lavoura-pecuária-floresta no município de Paragominas-PA. In Proceedings of the Congresso Brasileiro de Sistemas Agroflorestais. SBSAF, Embrapa Amazônia Oriental, Belém, Brasil, 25–30 May 2011. [Google Scholar]
- Oliveira, C.C.; Almeida, R.G.; Karvatte Junior, N.; Villela, S.D.J.; Bungenstab, D.J.; Alves, F.V. Daytime ingestive behaviour of grazing heifers under tropical silvopastoral systems: Responses to shade and grazing management. Appl. Anim. Behav. Sci. 2021, 240, 1–7. [Google Scholar] [CrossRef]
- Tourne, D.C.M.; Ballester, M.V.R.; James, P.M.A.; Martorano, L.G.; Guedes, M.C.; Thomas, E. Strategies to optimize modeling habitat suitability of Bertholletia excelsa in the Pan-Amazonia. Ecol. Evol. 2019, 9, 12623–12638. [Google Scholar] [CrossRef] [Green Version]
- Martínez, G.B.; Mourão Junior, M.; Brienza Junior, S. Selection of multipurpose forest species ideotypes of the floodplains in the Low Amazon Basin, Pará. Acta Amazôn. 2010, 40, 65–74. Available online: www.researchgate.net/publication/262514205 (accessed on 21 March 2023). [CrossRef] [Green Version]
- Tonini, H.; Sartori, W.; Anjos, A.; Morales, M.; da Silva, V. Performance and quality of wood in a clone of Eucalyptus in monoculture and silvopastoral system. Nativa 2016, 4, 77–81. [Google Scholar]
- Maneschy, R.; da Veiga, J.; de Santana, A. Evaluation of growth of parica (Schizolobium amazonicum Huber) and teca (Tectona grandis l.f.) in silvopastoral systems in northeastern Pará. Luziânia, Brasil. In Dialogue and Integration of Knowledge in Agroforestry Systems for Sustainable Societies; SBSAF: Manaus, Brazil, 2009. [Google Scholar]
- Tucker, C.B.; Rogers, A.R.; Schütz, K.E. Effect of solar radiation on dairy cattle behaviour, use of shade and body temperature in a pasture-based system. Appl. Anim. Behav. Sci. 2008, 109, 141–154. [Google Scholar] [CrossRef]
- Nadin, L.; Chopa, F.S.; Agnelli, M.L.; da Trindade, J.K.; Gonda, H. Effect of sward height on short-term intake by steers grazing winter oat pastures. Livest. Sci. 2019, 225, 8–14. [Google Scholar] [CrossRef]
- de Souza, W.; Barbosa, O.R.; Marques, J.d.A.; Gasparino, E.; Cecato, U.; Barbero, L.M. Behaviour of beef cattle in silvopastoral systems with eucalyptus. R. Bras. Zootec. 2010, 39, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Van Iear, E.; Moons, C.P.H.; Ampe, B.; Sonck, B.; Vandaele, L.; de Campeneere, S.; Tuyttens, F.A.M. Effect of summer conditions and shade on behavioural indicators of thermal discomfort in Holstein dairy and Belgian Blue beef cattle on pasture. Animal 2015, 9, 1536–1546. [Google Scholar] [CrossRef] [Green Version]
- Domiciano, L.F.; Mombach, M.A.; Carvalho, P.; Silva, N.M.F.; Pereira, D.H.; Cabral, L.S.; Lopes, L.B.; Pedreira, B.C. Performance and behaviour of Nellore steers on integrated systems. Anim. Prod. Sci. 2016, 58, 920–929. [Google Scholar] [CrossRef]
- Vizzotto, E.F.; Fischer, V.; Thaler Neto, A.; Abreu, A.S.; Stumpf, M.T.; Werncke, D.; Schmidt, F.A.; McManus, C.M. Access to shade changes behavioural and physiological attributes of dairy cows during the hot season in the subtropics. Animal 2015, 9, 1559–1566. [Google Scholar] [CrossRef] [Green Version]


| Surface Temperature (°C) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | August 2018 | October 2018 | ||||||||
| Min | Mean | Max | SD | ΔT | Min | Mean | Max | SD | ΔT | |
| B. excelsa | 30.3 | 31.5 | 32.5 | 0.7 | 2.2 | 36.4 | 36.9 | 37.3 | 0.2 | 0.9 |
| D. odorata | 30.9 | 31.3 | 31.8 | 0.3 | 0.9 | 35.8 | 36.5 | 37.3 | 0.3 | 1.5 |
| K. grandifoliola | 29.2 | 30.0 | 30.7 | 0.5 | 1.5 | 35.8 | 36.3 | 36.7 | 0.2 | 0.9 |
| P. Maximum | 32.7 | 33.2 | 33.6 | 0.2 | 0.9 | 38.6 | 39.5 | 40.7 | 0.6 | 2.1 |
| Species | DBH | SD DHC | Ht | SD Ht | AC | SD AC | MAI DHC | MAI Ht |
|---|---|---|---|---|---|---|---|---|
| (cm) | (cm) | (m) | (m) | (m2) | (m2) | (cm year−1) | (m year−1) | |
| B. excelsa | 15.63 | 5.2 | 10.62 | 2.77 | 41.20 | 18,34 | 1.74 | 1.18 |
| D. odorata | 13.04 | 2.72 | 8.89 | 1.24 | 19.68 | 9.58 | 1.45 | 0.99 |
| K. grandifoliola | 26.35 | 4.05 | 18.40 | 2.42 | 37.80 | 14.37 | 2.93 | 2.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cândido, A.C.T.F.; Guerreiro Martorano, L.; Cândido, B.U.F.; Nascimento, W.; Dias, C.T.d.S.; Lisboa, L.S.S.; Fernandes, P.C.C.; Silva, A.R.; Dias-Filho, M.B.; Beldini, T.P. Infrared Thermal Profiles in Silvopastoral and Full-Sun Pastures in the Eastern Amazon, Brazil. Forests 2023, 14, 1463. https://doi.org/10.3390/f14071463
Cândido ACTF, Guerreiro Martorano L, Cândido BUF, Nascimento W, Dias CTdS, Lisboa LSS, Fernandes PCC, Silva AR, Dias-Filho MB, Beldini TP. Infrared Thermal Profiles in Silvopastoral and Full-Sun Pastures in the Eastern Amazon, Brazil. Forests. 2023; 14(7):1463. https://doi.org/10.3390/f14071463
Chicago/Turabian StyleCândido, Aureane Cristina Teixeira Ferreira, Lucieta Guerreiro Martorano, Bruno Uéslei Ferreira Cândido, Werlleson Nascimento, Carlos Tadeu dos Santos Dias, Leila Sheila Silva Lisboa, Paulo Campos Christo Fernandes, Arystides Resende Silva, Moacyr Bernardino Dias-Filho, and Troy Patrick Beldini. 2023. "Infrared Thermal Profiles in Silvopastoral and Full-Sun Pastures in the Eastern Amazon, Brazil" Forests 14, no. 7: 1463. https://doi.org/10.3390/f14071463








