The Role of Bedrock Geochemistry and Climate in Soil Organic Matter Stability in Subtropical Karst Forests of Southwest China
Abstract
:1. Introduction
2. Materials and Methods
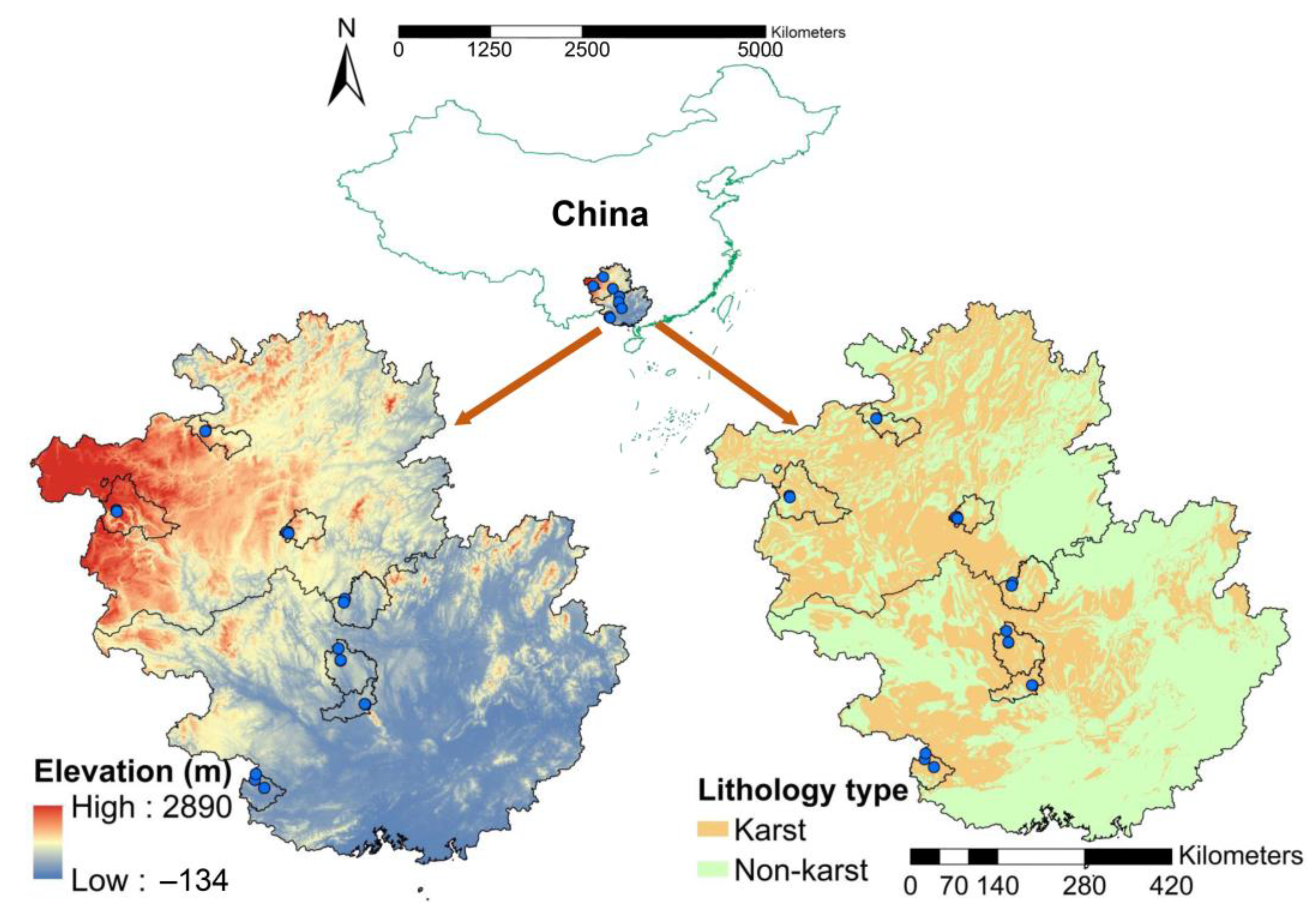
2.1. Site Description
2.2. Experimental Design and Sample Collection
2.3. SOM Stability
2.4. Soil Properties
2.5. Bedrock Geochemistry
2.6. Statistical Analyses
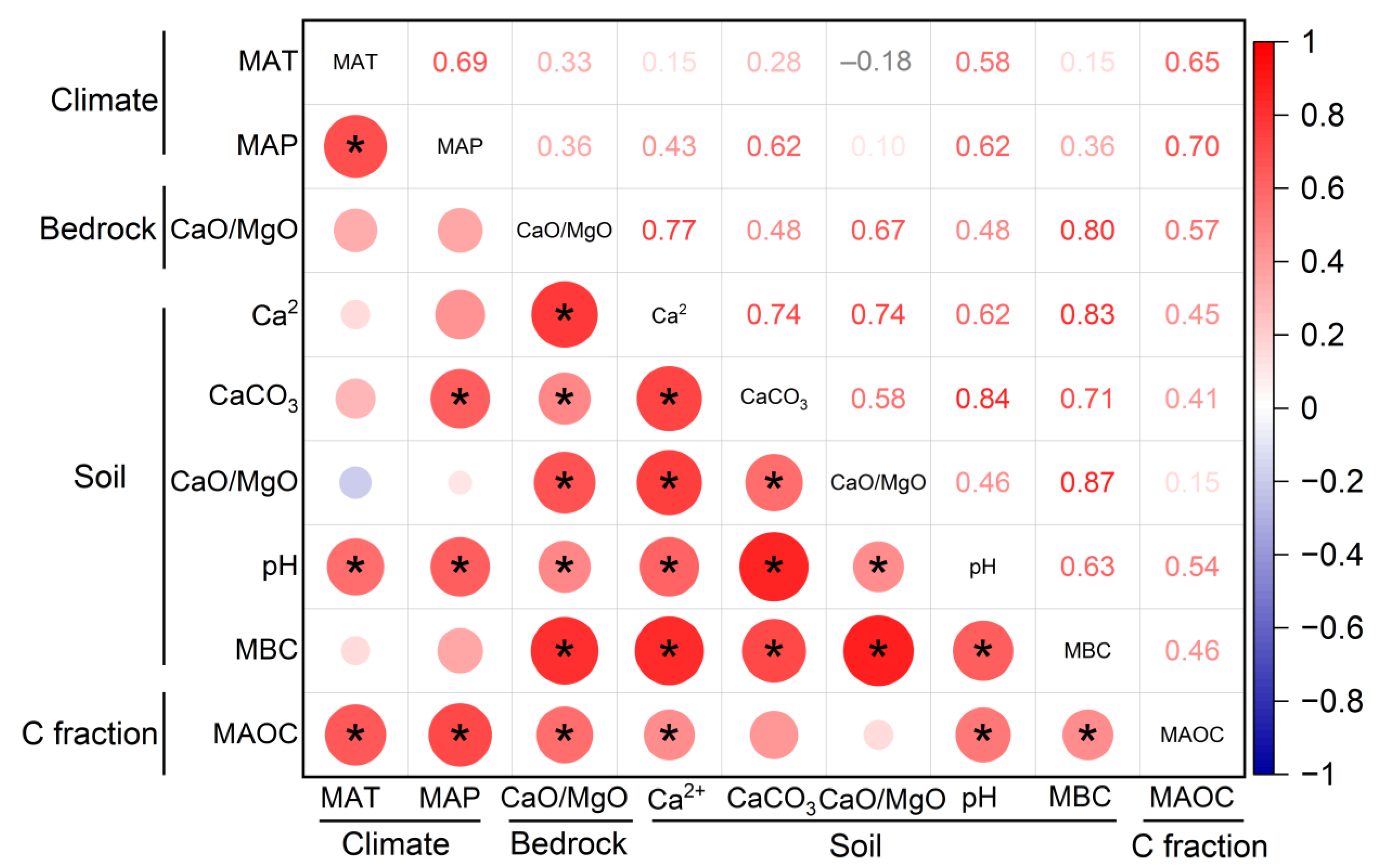
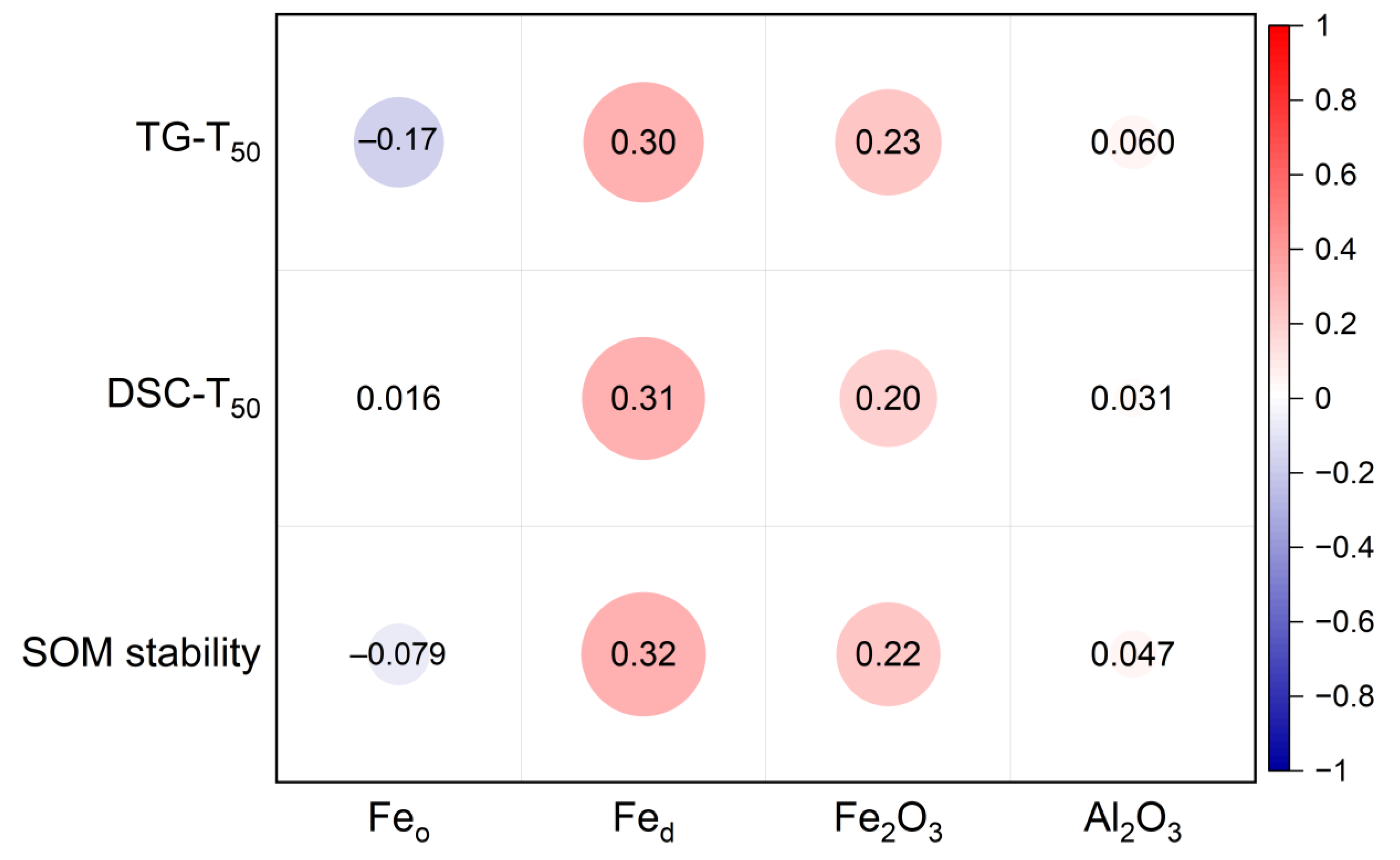
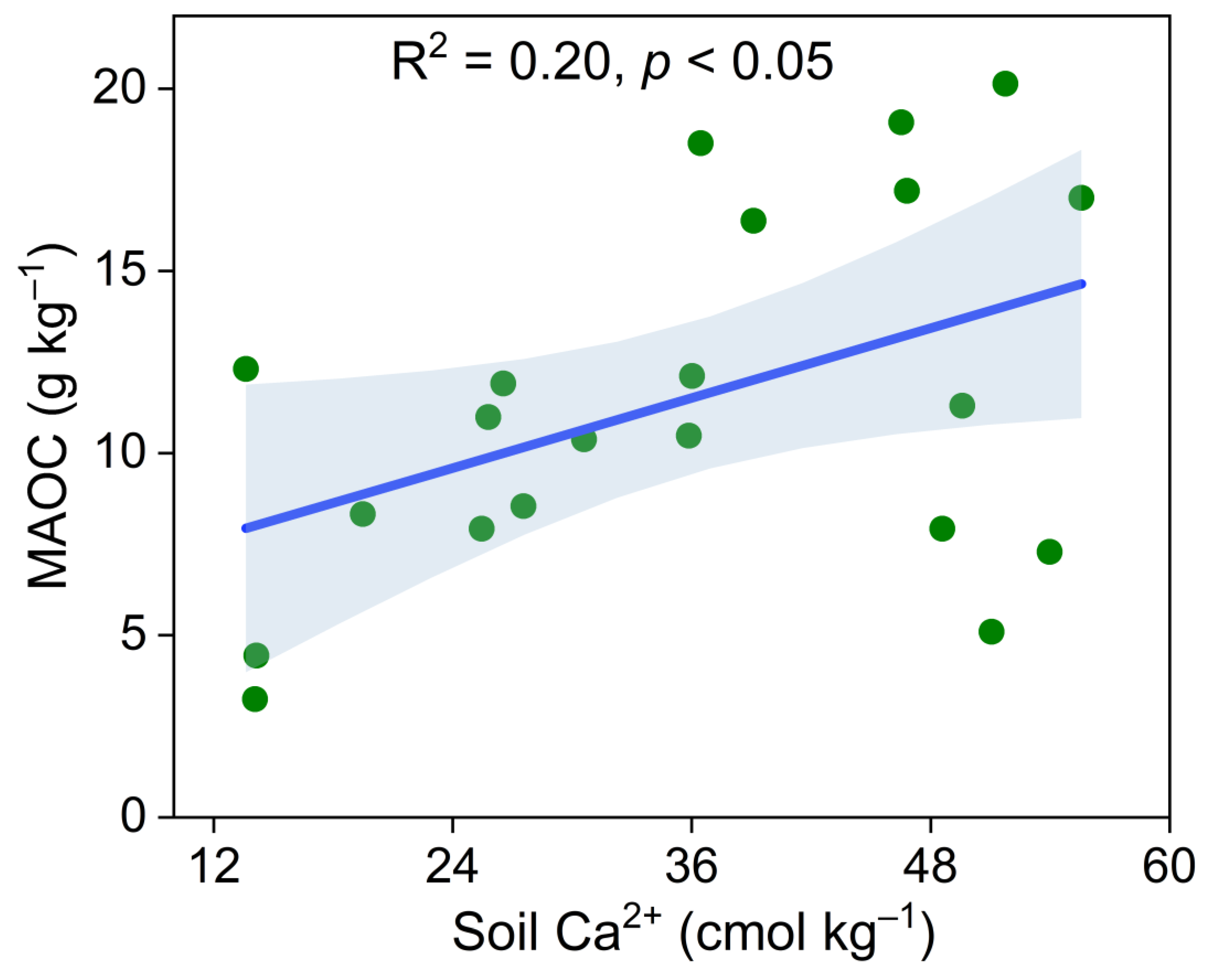
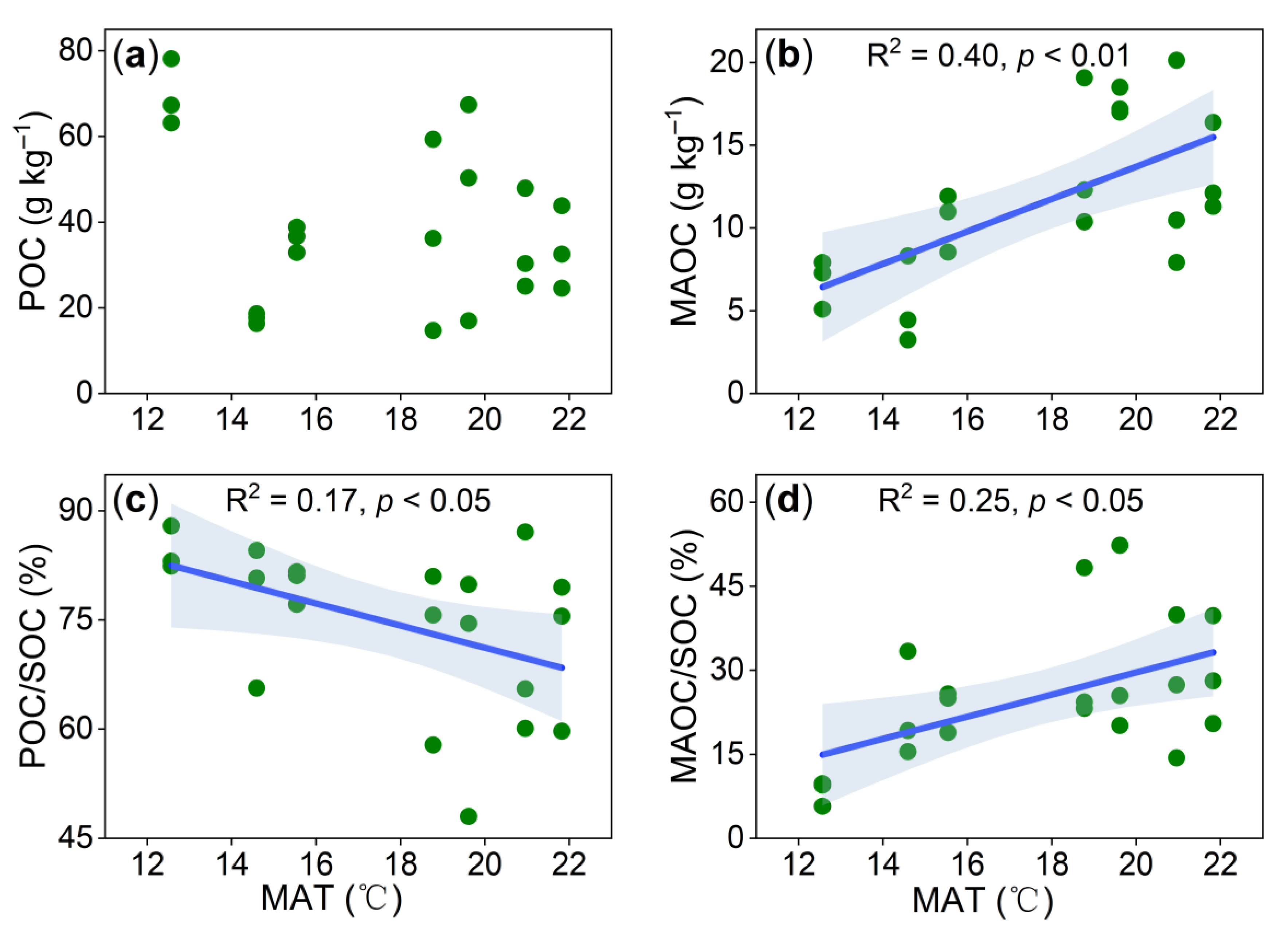
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Bossio, D.A.; Cook-Patton, S.C.; Ellis, P.W.; Fargione, J.; Sanderman, J.; Smith, P.; Wood, S.; Zomer, R.J.; von Unger, M.; Emmer, I.M.; et al. The Role of Soil Carbon in Natural Climate Solutions. Nat. Sustain. 2020, 3, 391–398. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Berhe, A.A.; Carrillo, Y.; Cavagnaro, T.R.; Chen, D.; Chen, Q.-L.; Román Dobarco, M.; Dijkstra, F.A.; Field, D.J.; Grundy, M.J.; et al. Ensuring Planetary Survival: The Centrality of Organic Carbon in Balancing the Multifunctional Nature of Soils. Crit. Rev. Environ. Sci. Technol. 2022, 52, 4308–4324. [Google Scholar] [CrossRef]
- Bailey, V.L.; Pries, C.H.; Lajtha, K. What Do We Know about Soil Carbon Destabilization? Environ. Res. Lett. 2019, 14, 083004. [Google Scholar] [CrossRef] [Green Version]
- Dungait, J.A.J.; Hopkins, D.W.; Gregory, A.S.; Whitmore, A.P. Soil Organic Matter Turnover Is Governed by Accessibility Not Recalcitrance. Glob. Chang. Biol. 2012, 18, 1781–1796. [Google Scholar] [CrossRef]
- Hemingway, J.D.; Rothman, D.H.; Grant, K.E.; Rosengard, S.Z.; Eglinton, T.I.; Derry, L.A.; Galy, V.V. Mineral Protection Regulates Long-Term Global Preservation of Natural Organic Carbon. Nature 2019, 570, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.F.; Lavallee, J.M. Chapter One—Soil Organic Matter Formation, Persistence, and Functioning: A Synthesis of Current Understanding to Inform Its Conservation and Regeneration. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 172, pp. 1–66. [Google Scholar]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Lützow, M.; Marin-Spiotta, E.; van Wesemael, B.; Rabot, E.; Ließ, M.; Garcia-Franco, N.; et al. Soil Organic Carbon Storage as a Key Function of Soils—A Review of Drivers and Indicators at Various Scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Francesca Cotrufo, M.; Lavallee, J.M.; Zhang, Y.; Hansen, P.M.; Paustian, K.H.; Schipanski, M.; Wallenstein, M.D. In-N-Out: A Hierarchical Framework to Understand and Predict Soil Carbon Storage and Nitrogen Recycling. Glob. Chang. Biol. 2021, 27, 4465–4468. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ranalli, M.G.; Haddix, M.L.; Six, J.; Lugato, E. Soil Carbon Storage Informed by Particulate and Mineral-Associated Organic Matter. Nat. Geosci. 2019, 12, 989–994. [Google Scholar] [CrossRef]
- Wang, C.; Qu, L.; Yang, L.; Liu, D.; Morrissey, E.; Miao, R.; Liu, Z.; Wang, Q.; Fang, Y.; Bai, E. Large-Scale Importance of Microbial Carbon Use Efficiency and Necromass to Soil Organic Carbon. Glob. Chang. Biol. 2021, 27, 2039–2048. [Google Scholar] [CrossRef]
- San-Emeterio, L.M.; Jiménez-Morillo, N.T.; Pérez-Ramos, I.M.; Domínguez, M.T.; González-Pérez, J.A. Changes in Soil Organic Matter Molecular Structure after Five-Years Mimicking Climate Change Scenarios in a Mediterranean Savannah. Sci. Total Environ. 2023, 857, 159288. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Xu, W.; Bongers, F.J.; Bao, W.; Chen, B.; Chen, G.; Guo, K.; Lai, J.; Lin, D.; et al. Effects of Diversity, Climate and Litter on Soil Organic Carbon Storage in Subtropical Forests. For. Ecol. Manag. 2020, 476, 118479. [Google Scholar] [CrossRef]
- Hartley, I.P.; Hill, T.C.; Chadburn, S.E.; Hugelius, G. Temperature Effects on Carbon Storage Are Controlled by Soil Stabilisation Capacities. Nat. Commun. 2021, 12, 6713. [Google Scholar] [CrossRef] [PubMed]
- Doetterl, S.; Stevens, A.; Six, J.; Merckx, R.; Van Oost, K.; Casanova Pinto, M.; Casanova-Katny, A.; Muñoz, C.; Boudin, M.; Zagal Venegas, E.; et al. Soil Carbon Storage Controlled by Interactions between Geochemistry and Climate. Nat. Geosci. 2015, 8, 780–783. [Google Scholar] [CrossRef]
- Juhos, K.; Madarász, B.; Kotroczó, Z.; Béni, Á.; Makádi, M.; Fekete, I. Carbon Sequestration of Forest Soils Is Reflected by Changes in Physicochemical Soil Indicators—A Comprehensive Discussion of a Long-Term Experiment on a Detritus Manipulation. Geoderma 2021, 385, 114918. [Google Scholar] [CrossRef]
- Fang, K.; Chen, L.; Qin, S.; Zhang, Q.; Liu, X.; Chen, P.; Yang, Y. Mineral and Climatic Controls Over Soil Organic Matter Stability Across the Tibetan Alpine Permafrost Region. Glob. Biogeochem. Cycles 2021, 35, e2021GB007118. [Google Scholar] [CrossRef]
- Kopp, M.; Alving, D.; Blackman, T.; Kaye, M.; Duncan, J.; Kaye, J. Perspectives: Critical Zone Perspectives for Managing Changing Forests. For. Ecol. Manag. 2023, 528, 120627. [Google Scholar] [CrossRef]
- Weemstra, M.; Peay, K.G.; Davies, S.J.; Mohamad, M.; Itoh, A.; Tan, S.; Russo, S.E. Lithological Constraints on Resource Economies Shape the Mycorrhizal Composition of a Bornean Rain Forest. New Phytol. 2020, 228, 253–268. [Google Scholar] [CrossRef]
- Doetterl, S.; Berhe, A.A.; Arnold, C.; Bodé, S.; Fiener, P.; Finke, P.; Fuchslueger, L.; Griepentrog, M.; Harden, J.W.; Nadeu, E.; et al. Links among Warming, Carbon and Microbial Dynamics Mediated by Soil Mineral Weathering. Nat. Geosci. 2018, 11, 589–593. [Google Scholar] [CrossRef]
- Dass, P.; Houlton, B.Z.; Wang, Y.; Wårlind, D.; Morford, S. Bedrock Weathering Controls on Terrestrial Carbon-Nitrogen-Climate Interactions. Glob. Biogeochem. Cycles 2021, 35, e2020GB006933. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, H.; Wang, H.; Peng, J.; Meersmans, J.; Green, S.M.; Quine, T.A.; Wu, X.; Song, Z. Bedrock Geochemistry Influences Vegetation Growth by Regulating the Regolith Water Holding Capacity. Nat. Commun. 2020, 11, 2392. [Google Scholar] [CrossRef]
- Bhople, P.; Keiblinger, K.; Djukic, I.; Liu, D.; Zehetner, F.; Zechmeister-Boltenstern, S.; Georg Joergensen, R.; Murugan, R. Microbial Necromass Formation, Enzyme Activities and Community Structure in Two Alpine Elevation Gradients with Different Bedrock Types. Geoderma 2021, 386, 114922. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Chen, H.; Xu, L.; Xiao, J.; Luo, Y.; Wang, K. Lithologic Control of Microbial-Derived Carbon in Forest Soils. Soil Biol. Biochem. 2022, 167, 108600. [Google Scholar] [CrossRef]
- Goldscheider, N.; Chen, Z.; Auler, A.S.; Bakalowicz, M.; Broda, S.; Drew, D.; Hartmann, J.; Jiang, G.; Moosdorf, N.; Stevanovic, Z.; et al. Global Distribution of Carbonate Rocks and Karst Water Resources. Hydrogeol. J. 2020, 28, 1661–1677. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Lian, Y.; Qin, X. Rocky Desertification in Southwest China: Impacts, Causes, and Restoration. Earth-Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, C.; Chen, H.; Yue, Y.; Zhang, W.; Zhang, M.; Qi, X.; Fu, Z. Karst Landscapes of China: Patterns, Ecosystem Processes and Services. Landsc. Ecol. 2019, 34, 2743–2763. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Wen, L.; Yang, L.; Luo, P.; Xiao, K.; Chen, H.; Zhang, W.; He, X.; Chen, H.; Wang, K. Dynamics of Soil Organic Carbon and Nitrogen Following Agricultural Abandonment in a Karst Region. J. Geophys. Res. Biogeosci. 2017, 122, 230–242. [Google Scholar] [CrossRef]
- Marin-Spiotta, E.; Chaopricha, N.T.; Plante, A.F.; Diefendorf, A.F.; Mueller, C.W.; Grandy, A.S.; Mason, J.A. Long-Term Stabilization of Deep Soil Carbon by Fire and Burial during Early Holocene Climate Change. Nat. Geosci. 2014, 7, 428–432. [Google Scholar] [CrossRef]
- Hou, Y.; He, K.; Chen, Y.; Zhao, J.; Hu, H.; Zhu, B. Changes of Soil Organic Matter Stability along Altitudinal Gradients in Tibetan Alpine Grassland. Plant Soil 2021, 458, 21–40. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Kuzyakov, Y.; Xiao, L.; Xiao, D.; Xu, L.; Chen, H.; Zhao, J.; Wang, K. Linking Bacterial Life Strategies with Soil Organic Matter Accrual by Karst Vegetation Restoration. Soil Biol. Biochem. 2023, 177, 108925. [Google Scholar] [CrossRef]
- Merino, A.; Fonturbel, M.T.; Fernández, C.; Chávez-Vergara, B.; García-Oliva, F.; Vega, J.A. Inferring Changes in Soil Organic Matter in Post-Wildfire Soil Burn Severity Levels in a Temperate Climate. Sci. Total Environ. 2018, 627, 622–632. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1983; pp. 539–579. ISBN 978-0-89118-977-0. [Google Scholar]
- Wu, J.; Joergensen, R.G.; Pommerening, B.; Chaussod, R.; Brookes, P.C. Measurement of Soil Microbial Biomass C by Fumigation-Extraction-an Automated Procedure. Soil Biol. Amp Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Hong, S.; Piao, S.; Chen, A.; Liu, Y.; Liu, L.; Peng, S.; Sardans, J.; Sun, Y.; Peñuelas, J.; Zeng, H. Afforestation Neutralizes Soil PH. Nat. Commun. 2018, 9, 520. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Jia, Z.; Guo, J.; Li, T.; Sun, D.; Meng, H.; Yu, G.; He, X.; Ran, W.; Zhang, S.; et al. Ten-Year Long-Term Organic Fertilization Enhances Carbon Sequestration and Calcium-Mediated Stabilization of Aggregate-Associated Organic Carbon in a Reclaimed Cambisol. Geoderma 2019, 355, 113880. [Google Scholar] [CrossRef]
- Lai, J.; Zou, Y.; Zhang, J.; Peres-Neto, P.R. Generalizing Hierarchical and Variation Partitioning in Multiple Regression and Canonical Analyses Using the Rdacca.Hp R Package. Methods Ecol. Evol. 2022, 13, 782–788. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing Soil Organic Matter into Particulate and Mineral-Associated Forms to Address Global Change in the 21st Century. Glob. Chang. Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buss, H.L.; Chapela Lara, M.; Moore, O.W.; Kurtz, A.C.; Schulz, M.S.; White, A.F. Lithological Influences on Contemporary and Long-Term Regolith Weathering at the Luquillo Critical Zone Observatory. Geochim. Cosmochim. Acta 2017, 196, 224–251. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-J.; Li, R.-L.; Sun, C.-X.; Zhang, D.-F.; Li, F.-Q.; Zhou, D.-Q.; Xiong, K.-N.; Zhou, Z.-F. How Types of Carbonate Rock Assemblages Constrain the Distribution of Karst Rocky Desertified Land in Guizhou Province, PR China: Phenomena and Mechanisms. Land Degrad. Dev. 2004, 15, 123–131. [Google Scholar] [CrossRef]
- Pastore, G.; Weig, A.R.; Vazquez, E.; Spohn, M. Weathering of Calcareous Bedrocks Is Strongly Affected by the Activity of Soil Microorganisms. Geoderma 2022, 405, 115408. [Google Scholar] [CrossRef]
- Taylor, L.L.; Leake, J.R.; Quirk, J.; Hardy, K.; Banwart, S.A.; Beerling, D.J. Biological Weathering and the Long-Term Carbon Cycle: Integrating Mycorrhizal Evolution and Function into the Current Paradigm. Geobiology 2009, 7, 171–191. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Qin, S.; Yang, G.; Fang, K.; Zhu, B.; Kuzyakov, Y.; Chen, P.; Xu, Y.; Yang, Y. Regulation of Priming Effect by Soil Organic Matter Stability over a Broad Geographic Scale. Nat. Commun. 2019, 10, 5112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowley, M.C.; Grand, S.; Verrecchia, É.P. Calcium-Mediated Stabilisation of Soil Organic Carbon. Biogeochemistry 2018, 137, 27–49. [Google Scholar] [CrossRef] [Green Version]
- Martí-Roura, M.; Hagedorn, F.; Rovira, P.; Romanyà, J. Effect of Land Use and Carbonates on Organic Matter Stabilization and Microbial Communities in Mediterranean Soils. Geoderma 2019, 351, 103–115. [Google Scholar] [CrossRef]
- Yang, L.; Lyu, M.; Li, X.; Xiong, X.; Lin, W.; Yang, Y.; Xie, J. Decline in the Contribution of Microbial Residues to Soil Organic Carbon along a Subtropical Elevation Gradient. Sci. Total Environ. 2020, 749, 141583. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative Assessment of Microbial Necromass Contribution to Soil Organic Matter. Glob. Chang. Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef]
- Qin, S.; Chen, L.; Fang, K.; Zhang, Q.; Wang, J.; Liu, F.; Yu, J.; Yang, Y. Temperature Sensitivity of SOM Decomposition Governed by Aggregate Protection and Microbial Communities. Sci. Adv. 2019, 5, eaau1218. [Google Scholar] [CrossRef] [Green Version]
- Lugato, E.; Lavallee, J.M.; Haddix, M.L.; Panagos, P.; Cotrufo, M.F. Different Climate Sensitivity of Particulate and Mineral-Associated Soil Organic Matter. Nat. Geosci. 2021, 14, 295–300. [Google Scholar] [CrossRef]
- Soong, J.L.; Castanha, C.; Hicks Pries, C.E.; Ofiti, N.; Porras, R.C.; Riley, W.J.; Schmidt, M.W.I.; Torn, M.S. Five Years of Whole-Soil Warming Led to Loss of Subsoil Carbon Stocks and Increased CO2 Efflux. Sci. Adv. 2021, 7, eabd1343. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Chapter One—Mineral–Organic Associations: Formation, Properties, and Relevance in Soil Environments. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 130, pp. 1–140. [Google Scholar]
- Slessarev, E.W.; Lin, Y.; Bingham, N.L.; Johnson, J.E.; Dai, Y.; Schimel, J.P.; Chadwick, O.A. Water Balance Creates a Threshold in Soil PH at the Global Scale. Nature 2016, 540, 567–569. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The Importance of Anabolism in Microbial Control over Soil Carbon Storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Zhu, X.; Jackson, R.D.; DeLucia, E.H.; Tiedje, J.M.; Liang, C. The Soil Microbial Carbon Pump: From Conceptual Insights to Empirical Assessments. Glob. Chang. Biol. 2020, 26, 6032–6039. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, T.; Hu, P.; Zhang, W.; Xiao, D.; Tang, L.; Xiao, J.; Zhao, J.; Wang, K. The Role of Bedrock Geochemistry and Climate in Soil Organic Matter Stability in Subtropical Karst Forests of Southwest China. Forests 2023, 14, 1467. https://doi.org/10.3390/f14071467
Tang T, Hu P, Zhang W, Xiao D, Tang L, Xiao J, Zhao J, Wang K. The Role of Bedrock Geochemistry and Climate in Soil Organic Matter Stability in Subtropical Karst Forests of Southwest China. Forests. 2023; 14(7):1467. https://doi.org/10.3390/f14071467
Chicago/Turabian StyleTang, Tiangang, Peilei Hu, Wei Zhang, Dan Xiao, Li Tang, Jun Xiao, Jie Zhao, and Kelin Wang. 2023. "The Role of Bedrock Geochemistry and Climate in Soil Organic Matter Stability in Subtropical Karst Forests of Southwest China" Forests 14, no. 7: 1467. https://doi.org/10.3390/f14071467
APA StyleTang, T., Hu, P., Zhang, W., Xiao, D., Tang, L., Xiao, J., Zhao, J., & Wang, K. (2023). The Role of Bedrock Geochemistry and Climate in Soil Organic Matter Stability in Subtropical Karst Forests of Southwest China. Forests, 14(7), 1467. https://doi.org/10.3390/f14071467









