Factors Affecting Long-Term Soil Organic Carbon Storage in Greek Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Methods
2.3. Statistical Analysis
3. Results
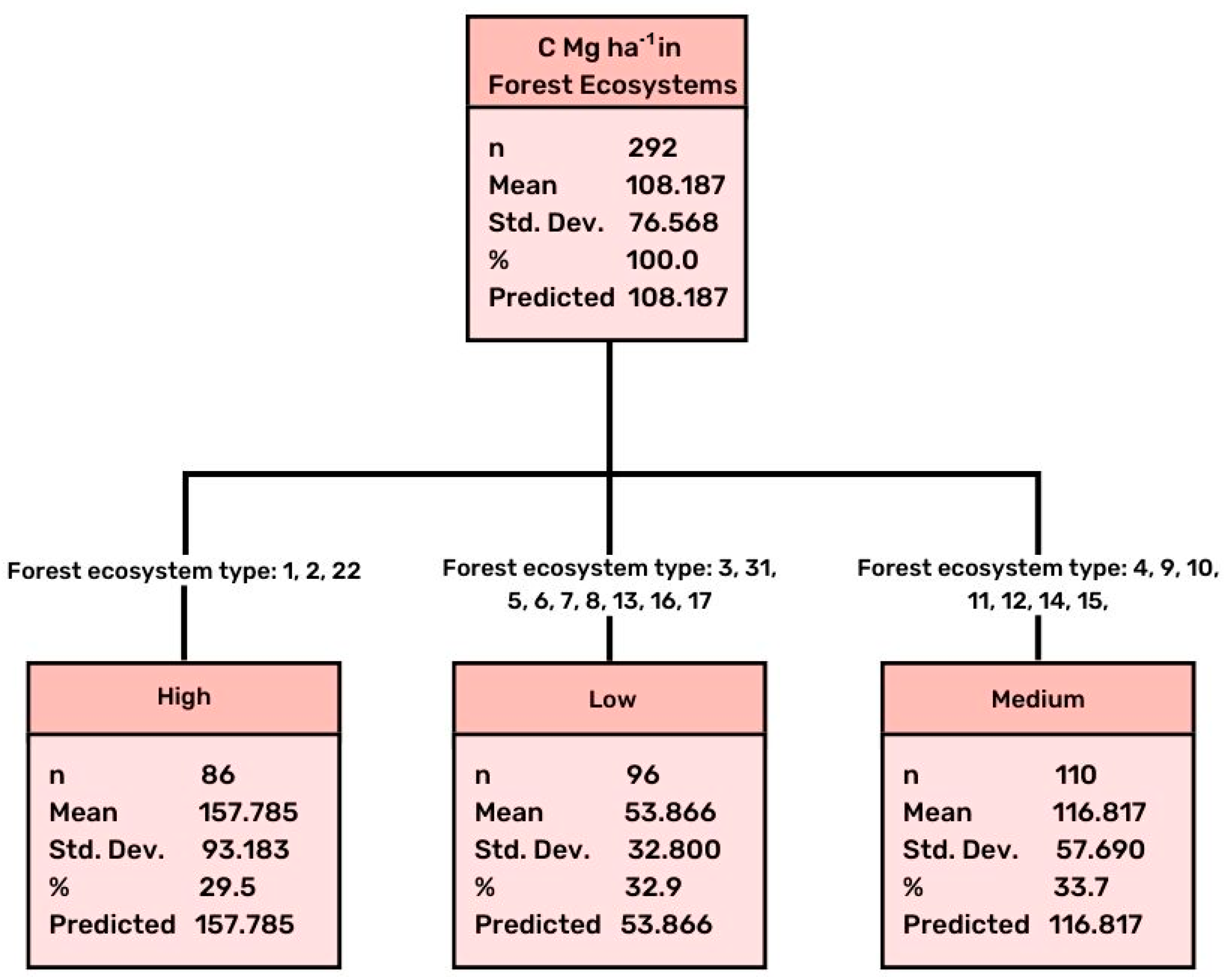
3.1. Differentiation of Soil C according to the Type (Dominant Tree Species) of Forest Ecosystem
3.2. Effect of Soil Depth on Stored C
3.3. Effect of Land Management Practices
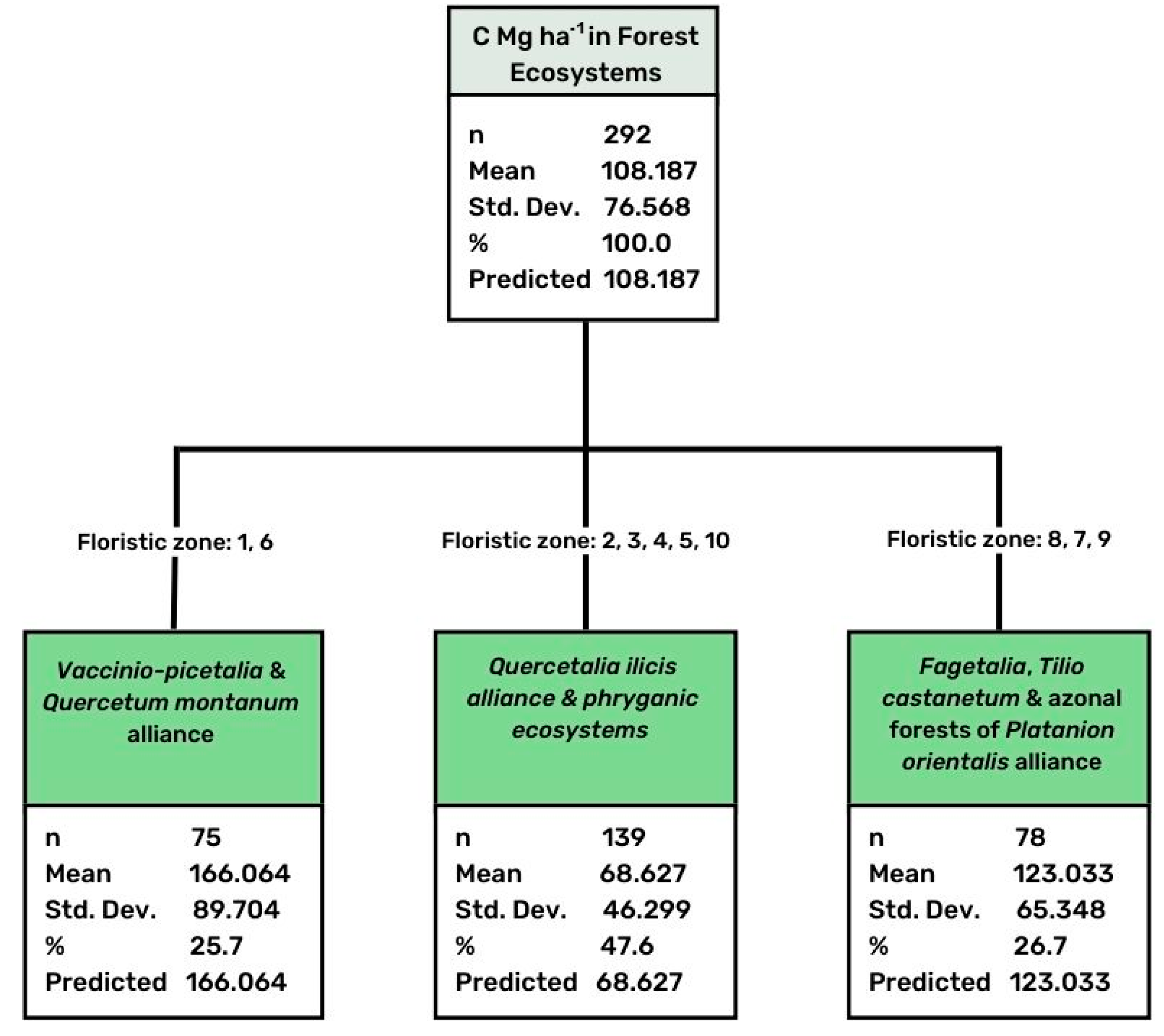
3.4. Differentiation of Soil Carbon in Relation to Floristic Zone
3.5. Differentiation According to the Climate Type
4. Discussion
4.1. Main Drivers Forming the Long-Term Ecosystem Storage Capacity
4.1.1. The Type of Forest Ecosystem (Dominant Tree Species)
4.1.2. Land Management Practices
4.1.3. Soil Depth
4.1.4. Floristic Zone
4.1.5. Climate Type
4.1.6. Analysis of the Combined Impacts
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Köhl, M.; Lasco, R.; Cifuentes, M.; Jonsson, Ö.; Korhonen, K.T.; Mundhenk, P.; de Jesus Navar, J.; Stinson, G. Changes in forest production, biomass and carbon: Results from the 2015 UN FAO Global Forest Resource Assessment. For. Ecol. Manag. 2015, 352, 21–34. [Google Scholar] [CrossRef]
- Bellassen, V.; Luyssaert, S. Carbon sequestration: Managing forests in uncertain times. Nature 2014, 506, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Ameray, A.; Bergeron, Y.; Valeria, O.; Montoro Girona, M.; Cavard, X. Forest Carbon Management: A Review of Silvicultural Practices and Management Strategies Across Boreal, Temperate and Tropical Forests. Curr. For. Rep. 2021, 7, 245–266. [Google Scholar] [CrossRef]
- Dixon, R.K.; Brown, S.; Houghton, R.A.; Solomon, A.M.; Trexler, M.C.; Wisniewski, J. Carbon pools and flux of global forest ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef]
- Sedjo, R.A. The carbon cycle and global forest ecosystem. Water Air Soil Pollut. 1993, 70, 295–307. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Chhabra, A.; Palria, S.S.; Dadhwal, V.K. Soil organic carbon pool in Indian forests. For. Ecol. Manag. 2003, 173, 187–199. [Google Scholar] [CrossRef]
- Lal, R. Soil management and restoration for C sequestration to mitigate the accelerated greenhouse effect. Prog. Environ. Sci. 1999, 1, 307–326. [Google Scholar]
- Joos, F.; Prentice, I.C.; Sitch, S.; Meyer, R.; Hooss, G.; Plattner, G.-K.; Gerber, S.; Hasselmann, K. Global warming feedbacks on terrestrial carbon uptake under the Intergovernmental Panel on Climate Change (IPCC) Emission Scenarios. Glob. Biogeochem. Cycles 2001, 15, 891–907. [Google Scholar] [CrossRef]
- Schimel, D.S.; House, J.I.; Hibbard, K.A.; Bousquet, P.; Ciais, P.; Peylin, P.; Braswell, B.H.; Apps, M.J.; Baker, D.; Bondeau, A.; et al. Recent patterns and mechanisms of carbon exchange by terrestrial ecosystems. Nature 2001, 414, 169–172. [Google Scholar] [CrossRef]
- Stolte, J.; Tesfai, M.; Øygarden, L.; Kværnø, S.; Keizer, S.J.; Verheijen, F.; Panagos, P.; Ballabio, C.; Hessel, R. Soil Threats in Europe; EUR 27607 EN; European Union: Luxembourg, 2016. [Google Scholar] [CrossRef]
- Grilli, E.; Carvalho, S.C.P.; Chiti, T.; Coppola, E.; D’Ascoli, R.; La Mantia, T.; Marzaioli; Mastrocicco, M.; Pulido, F.; Rutigliano, F.A.; et al. Critical range of soil organic carbon in southern Europe lands under desertification risk. J. Environ. Manag. 2021, 287, 112285. [Google Scholar] [CrossRef]
- Paul, K.I.; Polglase, P.J.; Nyakuengama, J.G.; Khanna, P.K. Change in soil carbon following afforestation. For. Ecol. Manag. 2002, 168, 241–257. [Google Scholar] [CrossRef]
- Lal, R. Forest soils and carbon sequestration. For. Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Chiti, T.; Díaz-Pinés, E.; Rubio, A. Soil organic carbon stocks of conifers, broadleaf and evergreen broadleaf forests of Spain. Biol. Fertil. Soils 2012, 48, 817–826. [Google Scholar] [CrossRef]
- Schlesinger, W.H. Biogeochemistry: An Analysis of Global Change, 2nd ed.; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Hyvönen, R.; Ågren, G.I.; Linder, S.; Persson, T.; Cotrufo, M.F.; Ekblad, A.; Freeman, M.; Grelle, A.; Janssens, I.A.; Jarvis, P.G.; et al. The likely impact of elevated [CO2], nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: A literature review. New Phytol. 2007, 173, 463–480. [Google Scholar] [CrossRef]
- Landsberg, J.; Sands, P. Physiological Ecology of Forest Production Principles, Processes and Models; Elsevier: Oxford, UK, 2011. [Google Scholar]
- Çömez, A.; Güner, Ş.T.; Tolunay, D. The effect of stand structure on litter decomposition in Pinus sylvestris L. stands in Turkey. Ann. For. Sci. 2021, 78, 19. [Google Scholar] [CrossRef]
- Whittaker, R.H.; Likens, G.E. Primary production: The biosphere and man. Hum. Ecol. 1973, 1, 357–369. [Google Scholar] [CrossRef]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How strongly can forest management influence soil carbon sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Schulp, C.J.E.; Nabuurs, G.-J.; Verburg, P.H.; de Waal, R.W. Effect of tree species on carbon stocks in forest floor and mineral soil and implications for soil carbon inventories. For. Ecol. Manag. 2008, 256, 482–490. [Google Scholar] [CrossRef]
- Camponi, L.; Cardelli, V.; Cocco, S.; Serrani, D.; Salvucci, A.; Cutini, A.; Agnelli, A.; Fabbio, G.; Bertini, G.; Roggero, P.P. Effect of coppice conversion into high forest on soil organic C and nutrients stock in a Turkey oak (Quercus cerris L.) forest in Italy. J. Environ. Manag. 2022, 312, 114935. [Google Scholar] [CrossRef]
- Gong, C.; Tan, O.; Liu, G.; Xu, M. Forest thinning increases soil carbon stocks in China. For. Ecol. Manag. 2021, 482, 118812. [Google Scholar] [CrossRef]
- Currie, W.S.; Yanai, R.D.; Piatek, K.B.; Prescott, C.E.; Goodale, C.L. Processes Affecting Carbon Storage in the Forest Floor and in Downed Woody Debris. In The Potential of U.S. Forest Soils to Sequester Carbon and Mitigate the Greenhouse Effect; CRC Press: Boca Raton, FL, USA, 2002; p. 23. ISBN 9780429137020. [Google Scholar]
- Borys, A.; Suckow, F.; Reyer, C.; Gutsch, M.; Lasch-Born, P. The impact of climate change under different thinning regimes on carbon sequestration in a German forest district. Mitig. Adapt Strateg. Glob. Chang. 2016, 21, 861–881. [Google Scholar] [CrossRef]
- Deng, L.; Liu, G.B.; Shangguan, Z.P. Land-use conversion and changing soil carbon stocks in China’s ‘Grain-for-Green’ Program: A synthesis. Glob. Chang. Biol. 2014, 20, 3544–3556. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Wei, X.; Shao, M.; Gale, W.; Li, L. Global pattern of soil carbon losses due to the conversion of forests to agricultural land. Sci. Rep. 2014, 4, 4062. [Google Scholar] [CrossRef]
- Le Quéré, C.; Raupach, M.R.; Canadell, J.G.; Marland, G.; Bopp, L.; Ciais, P.; Conway, T.J.; Doney, S.C.; Feely, R.A.; Foster, P.; et al. Trends in the sources and sinks of carbon dioxide. Nat. Geosci. 2009, 2, 831–836. [Google Scholar] [CrossRef]
- United Nations Framework Convention on Climate Change (UNFCCC). Decision 5/CMP.1. Modalities and Procedures for Afforestation and Reforestation Project Activities under the Clean Development Mechanism in the First Commitment Period of the Kyoto Protocol; FCCC/KP/CMP/2005/8/Add.1. United Nations: New York, NY, USA, 2005; p. 61. Available online: http://cdm.unfccc.int/Reference/COPMOP/08a01.pdf#page=61 (accessed on 30 April 2023).
- IPCC. Climate Change 2014: Mitigation of Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Heimann, M.; Reichstein, M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 2008, 451, 289–292. [Google Scholar] [CrossRef]
- Ganatsas, P.; Tsakaldimi, M.; Karydopoulos, T.; Petaloudi, L.-M.; Papaemmanouil, A.; Papadopoulos, S.; Gerochristou, S. Carbon Pools in a 77 Year-Old Oak Forest under Conversion from Coppice to High Forest. Sustainability 2022, 14, 13764. [Google Scholar] [CrossRef]
- Ganatsas, P.; Papaioannou, A. Estimation of accumulation of organic matter and nutrients in forest floor and forest soils of spruce and beech ecosystems in Elatia Drama, northern Greece. In Scientific Annals of the Department of Forestry& Natural Environment; AUTH: Thessaloniki, Greece, 1996; Volume 39/2. (In Greek) [Google Scholar]
- Tomasella, J.; Hodnett, M.G. Estimating soil water retention characteristics from limited data in Brazilian Amazonia. Soil Sci. 1998, 163, 190–202. [Google Scholar] [CrossRef]
- Martín, M.A.; Reyes, M.; Taguas, F.J. Estimating soil bulk density with information metrics of soil texture. Geoderma 2017, 287, 66–70. [Google Scholar] [CrossRef]
- Ganatsas, P. Stand Structure and Natural Regeneration of Spruce Forest in Elatia, Drama. Ph.D. Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1993. (In Greek). [Google Scholar] [CrossRef]
- Zagas, T. Conditions of Natural Reforestation of Scotch Pine in an Area of Rodopi. Ph.D. Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1990. (In Greek). [Google Scholar] [CrossRef]
- Papaioannou, A. Relations between Site Productivity and Humus Characteristics in Black Pine and Beach Forests in Northern Greece. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1993. (In Greek). [Google Scholar] [CrossRef]
- Mantzavelas, A. Typologie des Stations: Un Outil Traitement Statistique des Données Phytoécologiques et d’ Aide à la Decision en Aménagement Forestier. Application à la Foret Domaniale de Kerdylio (Grèce). Ph.D. Thesis, Universite Henri Poincaré, Nancy, France, 1994. [Google Scholar]
- Stamatopoulos, E. The Regeneration of Abies cephalonica in the Park of Parnitha. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1995. (In Greek). [Google Scholar] [CrossRef]
- Spanos, I. Analysis of Stand Structure and Natural Regeneration of Pinus brutia Forest in Thasos Island, Greece. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1993; p. 170. (In Greek). [Google Scholar]
- Pentarakis, K. Analysis of Stand Structure and Natural Regeneration of Pinus brutia Forest in Crete, Greece. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1993. (In Greek). [Google Scholar]
- Tsitsoni, T. Stand Structure and Conditions Determining Natural Regeneration after Fire in the Aleppo Pine Forest of Kassandra Peninsula (Chalkidiki, Greece). Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1991. (In Greek). [Google Scholar] [CrossRef]
- Theodoropoulos, K. Definition and Classification of Plantsociological Units in University Forest Taxiarchis, Chalkidiki. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1991. (In Greek). [Google Scholar] [CrossRef]
- Gouvas, M. The Plant Communities of the Hymettus Mountain. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2001. (In Greek). [Google Scholar] [CrossRef]
- Kostantinidou, E. Dynamic Evolution of the Form and Biomass of the Root System of Aleppo pine in the Area of Kassandra, Chakidiki. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1998. (In Greek). [Google Scholar] [CrossRef]
- Aslanidou, M. Evaluation of Ecological Factors that Affect the Appearance and Growth of the Forest Species in N. E. Chalkidiki. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2000. (In Greek). [Google Scholar] [CrossRef]
- Pipinis, E. Analysis of Stand Structure, Site Requirements and Potential Utilization of Platanus orientalis. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2003. (In Greek). [Google Scholar] [CrossRef]
- Kostantinidis, P. Investigation of the Relation between Physiographic Units in Forest of Aleppo Pine (Pinus halepensis Mil.) and the Plant Communities Which Have Occurred in Them. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1990. (In Greek). [Google Scholar] [CrossRef]
- Katsaros, L. The Importance of Ilex aquifolium from the Forest Policy, Ecological and Forest Botany Point of View. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1991. (In Greek) [Google Scholar] [CrossRef]
- Stampoulidis, A. Analysis of Beech (Fagus sylvatica L. s.l.) Natural Regeneration in the Late Successional Stands of the Species on the Grammos Mountain. Ph.D. Dissertation, Democritus University of Thrace, Orestiada, Greece, 2016. (In Greek). [Google Scholar] [CrossRef]
- Tantos, V. Nutrients Cycling in an Abies borisii regis Ecosystem. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1997. [Google Scholar] [CrossRef]
- Thanasis, G. Research on the Pinus nigra Reforestations in the Area of Mount Olympus. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2004. (In Greek). [Google Scholar] [CrossRef]
- Radoglou, K. Site Influence on the Success of Reforestation and on the Ecophysiological Condition of Trees of the Kedrinos Hill. Thessaloniki. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1987. (In Greek). [Google Scholar] [CrossRef]
- Goudelis, G. Comparative Research on Reforestations of Experimental University Forest of Taxiarchis-Chalkidiki (Behaviour of Artificial Stands). Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1992. (In Greek). [Google Scholar] [CrossRef]
- Mela, F.; Ganatsas, P. Effect of land preparation methods on restoration success of degraded oak forest ecosystems. Dendrobiology 2023, 89, 56–64. [Google Scholar] [CrossRef]
- Seilopoulos, D. Influence of Forest Fires on Soil Properties. Ph.D. Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 1991. (In Greek). [Google Scholar] [CrossRef]
- Jandl, R.; Ledermann, T.; Kindermann, G.; Weiss, P. Soil Organic Carbon Stocks in Mixed-Deciduous and Coniferous Forests in Austria. Front. For. Glob. Chang. 2021, 4, 688851. [Google Scholar] [CrossRef]
- Panchal, P.; Preece, C.; Peñuelas, J.; Giri, J. Soil carbon sequestration by root exudates. Trends Plant Sci. 2022, 27, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.; Prescott, C.E.; Abaker, W.E.A.; Augusto, L.; Cécillon, L.; Ferreira, G.W.D.; James, J.; Jandl, R.; Katzensteiner, K.; Laclau, J.-P.; et al. Tamm Review: Influence of forest management activities on soil organic carbon stocks: A knowledge synthesis. For. Ecol. Manag. 2020, 466, 118127. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M.; Jordán, A.; Zavala, L.M.; De la Rosa, D.; Abd-Elmabod, S.K.; Anaya-Romero, M. Impact of Land Use and Land Cover Changes on Organic Carbon Stocks in Mediterranean Soils (1956–2007). Land Degrad. Dev. 2015, 26, 168–179. [Google Scholar] [CrossRef]
- Balesdent, J.; Basile-Doelsch, I.; Chadoeuf, J.; Cornu, S.; Derrien, D.; Fekiacova, Z.; Hatté, C. Atmosphere–soil carbon transfer as a function of soil depth. Nature 2018, 559, 599–602. [Google Scholar] [CrossRef]
- Eimil-Fraga, C.; Rodríguez-Soalleiro, R.; Sánchez-Rodríguez, F.; Pérez-Cruzado, C.; Álvarez-Rodríguez, E. Significance of bedrock as a site factor determining nutritional status and growth of maritime pine. For. Ecol. Manag. 2014, 331, 19–24. [Google Scholar] [CrossRef]
- Cardelli, V.; Cocco, S.; Agnelli, A.; Nardi, S.; Pizzeghello, D.; Fernández-Sanjurjo, M.J.; Corti, G. Chemical and Biochemical Properties of Soils Developed from Different Lithologies in Northwestern Spain (Galicia). Forests 2017, 8, 135. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, M.; Zhu, Y.; Liu, Z. Impacts of land use and plant characteristics on dried soil layers in different climatic regions on the Loess Plateau of China. Agric. For. Meteorol. 2011, 151, 437–448. [Google Scholar] [CrossRef]
- Chauvier, Y.; Thuiller, W.; Brun, P.; Lavergne, S.; Descombes, P.; Karger, D.N.; Renaud, J.; Zimmermann, N.E. Influence of climate, soil, and land cover on plant species distribution in the European Alps. Ecol. Monogr. 2021, 91, e01433. [Google Scholar] [CrossRef]
- Dafis, S. Classification of forest vegetation of Greece. Sch. Agric. For. Sci. Yearb. 1973, 15, 75–90. (In Greek) [Google Scholar]
- Thirwood, J.V. Man and the Mediterranean Forest; Academic Press: Cambridge, MA, USA, 1981. [Google Scholar]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Grace, J.; José, J.S.; Meir, P.; Miranda, H.S.; Montes, R.A. Productivity and carbon fluxes of tropical savannas. J. Biogeogr. 2006, 33, 387–400. [Google Scholar] [CrossRef]
- Becknell, J.M.; Kucek, L.K.; Powers, J.S. Aboveground biomass in mature and secondary seasonally dry tropical forests: A literature review and global synthesis. For. Ecol. Manag. 2012, 276, 88–95. [Google Scholar] [CrossRef]
- Massaccesi, L.; De Feudis, M.; Leccese, A.; Agnelli, A. Altitude and Vegetation Affect Soil Organic Carbon, Basal Respiration and Microbial Biomass in Apennine Forest Soils. Forests 2020, 11, 710. [Google Scholar] [CrossRef]
- Adger, W.N. Adaptation to climate change in the developing world. Prog. Dev. Stud. 2003, 3, 179–195. [Google Scholar] [CrossRef]


| Type of the Forest Ecosystem | Location | Climate Type | Vegetation Zone | Altitude | Bedrock Type | Reference Based on |
|---|---|---|---|---|---|---|
| Natural forests | ||||||
| Picea abies (L.) H. Karst. | Rhodopi mountain | Dfb | Vaccinio-Piceion | 1000–1500 | Granite, granodiorites | [38] |
| Pinus sylvestris L. | Rhodopi mountain | Dfb | Vaccinio-Piceion | 1000–1500 | Granite, granodiorites | [39] |
| Fagus sylvatica L. | Rhodopi mountain | Dfb | Fagion sylvaticae | 1000–1500 | Granite, granodiorites | [40] |
| Fagus sylvatica | Pieria mountain | Dsb | Fagion sylvaticae | 1000–1500 | Gneiss, flysch, | [40] |
| Fagus sylvatica | Voras mountain | Dfb | Fagion sylvaticae | 1000–1500 | Crystalline rocks with appea-rances of schists and granites | [40] |
| Fagus sylvatica | Ossa mountain | Cfa | Fagion sylvaticae | 1000–1500 | Metabasic rocks, prasinites, glaucophanites, schists and marble inclusions | [40] |
| Pinus nigra J.F. Arnold | Pindos, Rhodopi mountain, Pieria mountain | Dsb | Fagion sylvaticae | 1000–1500 | Schists with marble inclusions | [40] |
| Quecus frainetto Ten. | Kerdylia mountain | Cfa | Quercion frainetto | 400–1000 | Gneiss, schists, hornblende | [41] |
| Abies cephalonica Loudon | Parnitha national park | Cfa | Abietion cephalonicae | 550–1413 | Limestone, Schists | [42] |
| Pinus brutia Ten. | Thassos island | Csa | Quercion ilicis | 30–700 | Limestone | [43] |
| Pinus brutia | Creta island | Csa | Quercion ilicis | 300–1000 | Limestone | [44] |
| Pinus halepensis Miller | Kassandra, Chalkidiki | Csa | Quercion ilicis | 30–400 | Marges, Conglomerates, Limestone | [45] |
| Quecus frainetto | Taxiarchis, Chalkidiki | Cfa | Quercion frainetto | 600–1000 | Limestone | [34] |
| Quercus dalechampii Ten. | Taxiarchis, Chalkidiki | Cfa | Quercetum montanum | 900–1000 | Granites | [46] |
| Quecus frainetto | Taxiarchis, Chalkidiki | Cfa | Quercion frainetto | 600–1000 | Limestone | [46] |
| Quercus ilex L. | Chalkidiki | Csa | Quercion ilicis | 30–500 | Limestone | [46] |
| Quercus coccifera L. | Chalkidiki | Csb | Ostryo carpinion | 400–700 | Limestone | [46] |
| Pinus halεpensis | Hymettus mountain | Csa | Quercion ilicis | 280–680 | Schists, marble | [47] |
| Quercus coccifera shrubland | Hymettus mountain | Csa | Cisto-Micromerietea julianae | 50–950 | Schists, marble | [47] |
| Pinus halepensis | Kassandra, Chalkidiki | Csa | Quercion ilicis | 50–400 | Marges, limestone, conglomerates | [48] |
| Quercus frainetto | Arnaia, Chalkidiki | Cfa | Quercion confertae | 350–800 | Gneiss, Phyllites | [49] |
| Quercus dalechampii | Arnaia, Chalkidiki | Cfa | Quercetum montanum | 350–800 | Gneiss, Phyllites | [49] |
| Castanea sativa Mill. | Arnaia, Chalkidiki | Cfa | Tilio castanetum | 350–800 | Gneiss, Phyllites | [49] |
| Fagus moesiaca (K. Malý) Czecz. | Arnaia, Chalkidiki | Cfa | Fagion moesiacae | 350–800 | Gneiss, Phyllites | [49] |
| Fagus moesiaca & Taxus baccata L. | Arnaia, Chalkidiki | Cfa | Fagion moesiacae | 350–800 | Gneiss, Phyllites | [49] |
| Quercus pubescens Willd. | Arnaia, Chalkidiki | Csb | Ostryo carpinion | 350–400 | Gneiss, Phyllites | [49] |
| Platanus orientalis L. | Rivers in the area Trikala and Karditsa | Cfa | Platanion orientalis | 760–840 | Alluvial | [50] |
| Pinus halepensis | Sithonia, Chalkidiki | Csa | Quercion ilicis | 300–550 | Phyllites, Marges, Schists, Psammites | [51] |
| Fagus sylvatica with Ilex aquifolium L. | Olympos mountain | Dsb | Fagion sylvaticae | 1200–1500 | Limestones, Dolomites, Gneiss, Amphibolites | [52] |
| Fagus sylvatica | Grammos mountain | Dfb | Fagion sylvaticae | 1600–2180 | Flysch, Marges, Psammites, Conglomerates | [53] |
| Abies borisii-regis Mattf. | Pertouli, Pindos | Dsb | Fagion moesiacae | 1100–1500 | Flysch | [54] |
| Planted forests | ||||||
| Pinus nigra | Olympos mountain | Dsb | Fagion sylvaticae | 800–1450 | Gneiss | [55] |
| Pinus pinea L. | Thessaloniki | Csb | Ostryo carpinion | 100–300 | Gneiss, Ophioliths, Phyllites, Gabbro | [56] |
| Pinus brutia | Thessaloniki | Csb | Ostryo carpinion | 100–300 | Gneiss, Ophioliths, Phyllites, Gabbro | [56] |
| Pinus nigra | Taxiarchis, Chalkidiki | Cfa | Quercion confertae | 750–1040 | Granites-Phyllites | [57] |
| Pinus brutia | Chalkidiki | Csa | Quercion ilicis | 300–600 | Phyllites | [57] |
| Pinus pinea | Chalkidiki | Csa | Quercion ilicis | 300–600 | Phyllites | [57] |
| Pinus pinaster Aiton | Chalkidiki | Csa | Quercion ilicis | 300–600 | Phyllites | [57] |
| Pinus radiata D. Don | Chalkidiki | Csa | Quercion ilicis | 300–600 | Phyllites | [57] |
| Quercus pubescens | Lagadas | Cfa | Quercion confertae | 500–800 | Crystalline and igneous rocks | [58] |
| Burned forest | ||||||
| Pinus halepensis | Kassandra, Chalkidiki | Csa | Quercion ilicis | 30–400 | Marges, conglomerate, limestone | [45] |
| Pinus halepensis | Chalkidiki | Csa | Quercion ilicis | 0–400 | Gneiss-Marges | [59] |
| Type of Forest Ecosystem (Code) | Mean in Mg ha−1 | N | Std. Error of Mean |
|---|---|---|---|
| 1 | 255.59800 | 5 | 66.617305 |
| 2 | 150.69340 | 65 | 10.041908 |
| 3 | 47.74474 | 19 | 7.832819 |
| 4 | 108.57815 | 27 | 7.484601 |
| 5 | 49.44000 | 5 | 4.578876 |
| 6 | 68.70250 | 16 | 12.133660 |
| 7 | 62.29000 | 6 | 17.849855 |
| 8 | 41.05000 | 8 | 11.187599 |
| 9 | 99.46200 | 5 | 19.230321 |
| 10 | 124.03488 | 43 | 9.482236 |
| 11 | 132.65200 | 5 | 27.342324 |
| 12 | 134.52000 | 5 | 18.086965 |
| 13 | 29.23000 | 5 | 3.114782 |
| 14 | 93.71333 | 6 | 6.025550 |
| 15 | 117.06524 | 21 | 17.229275 |
| 16 | 53.17434 | 27 | 2.870207 |
| 17 | 46.31795 | 14 | 8.766629 |
| 22 | 167.76600 | 5 | 33.432808 |
| 31 | 74.94250 | 5 | 5.482308 |
| Total | 108.18677 | 292 | 4.480820 |
| Management Type | Code | Mean (Mg ha−1) | N | Std. Error of Mean |
|---|---|---|---|---|
| Managed high forest | 1 | 138.47876 | 113 | 6.947476 |
| Reforestation of Pinus nigra | 2 | 107.04727 | 70 | 10.527522 |
| Βurned forests | 3 | 123.23750 | 8 | 16.356792 |
| Secondary managed forests | 4 | 64.12633 | 30 | 6.799467 |
| Riparian (non-managed) forests | 5 | 117.06524 | 21 | 17.229275 |
| Protected natural forests | 6 | 53.17434 | 27 | 2.870207 |
| Garrigue/phrygana-degraded highly | 7 | 47.44780 | 13 | 9.390071 |
| Old growth forests | 8 | 167.76600 | 5 | 33.432808 |
| Shrublands of low altitude | 9 | 37.94200 | 5 | 5.088561 |
| Total | 108.18677 | 292 | 4.480820 |
| Floristic Zone | Code | Mean (in Mg ha−1) | N | Std. Error of Mean |
|---|---|---|---|---|
| Vaccinio-picetalia | 1 | 194.12500 | 10 | 39.651069 |
| Pinion nigrae-Abietum cephalonicae | 2 | 66.79501 | 43 | 7.528515 |
| Quercetalia ilicis | 3 | 80.33729 | 59 | 5.964230 |
| Ostryo-carpinion | 4 | 41.05000 | 8 | 11.187599 |
| Quercetalia pubescentis | 5 | 61.36938 | 16 | 11.174760 |
| Quercetum montanum | 6 | 161.74662 | 63 | 10.341316 |
| Tilio castanetum | 7 | 134.52000 | 5 | 18.086965 |
| Fagetalia | 8 | 124.71500 | 54 | 8.377874 |
| Platanion orientalis | 9 | 117.06524 | 21 | 17.229275 |
| Garrigue/phrygana | 10 | 47.44780 | 13 | 9.390071 |
| Total | 108.18677 | 292 | 4.480820 |
| Climate Type | N | Mean (Mg ha−1) | Std. Error |
|---|---|---|---|
| Csa | 72 | 74.39 b | 5.362 |
| Csb | 57 | 74.59 b | 7.891 |
| Dsb | 40 | 153.86 a | 13.947 |
| Dfb | 74 | 156.30 a | 9.503 |
| Cfa | 49 | 86.96 b | 7.864 |
| Total | 292 | 108.18 | 4.481 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganatsas, P.; Tsakaldimi, M.; Petaloudi, L.-M. Factors Affecting Long-Term Soil Organic Carbon Storage in Greek Forests. Forests 2023, 14, 1518. https://doi.org/10.3390/f14081518
Ganatsas P, Tsakaldimi M, Petaloudi L-M. Factors Affecting Long-Term Soil Organic Carbon Storage in Greek Forests. Forests. 2023; 14(8):1518. https://doi.org/10.3390/f14081518
Chicago/Turabian StyleGanatsas, Petros, Marianthi Tsakaldimi, and Lydia-Maria Petaloudi. 2023. "Factors Affecting Long-Term Soil Organic Carbon Storage in Greek Forests" Forests 14, no. 8: 1518. https://doi.org/10.3390/f14081518
APA StyleGanatsas, P., Tsakaldimi, M., & Petaloudi, L.-M. (2023). Factors Affecting Long-Term Soil Organic Carbon Storage in Greek Forests. Forests, 14(8), 1518. https://doi.org/10.3390/f14081518









