Molecular Characterization and Phylogenetic Analysis of the Pine Tortoise Scale Insect Toumeyella parvicornis (Cockerell) (Hemiptera: Coccidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
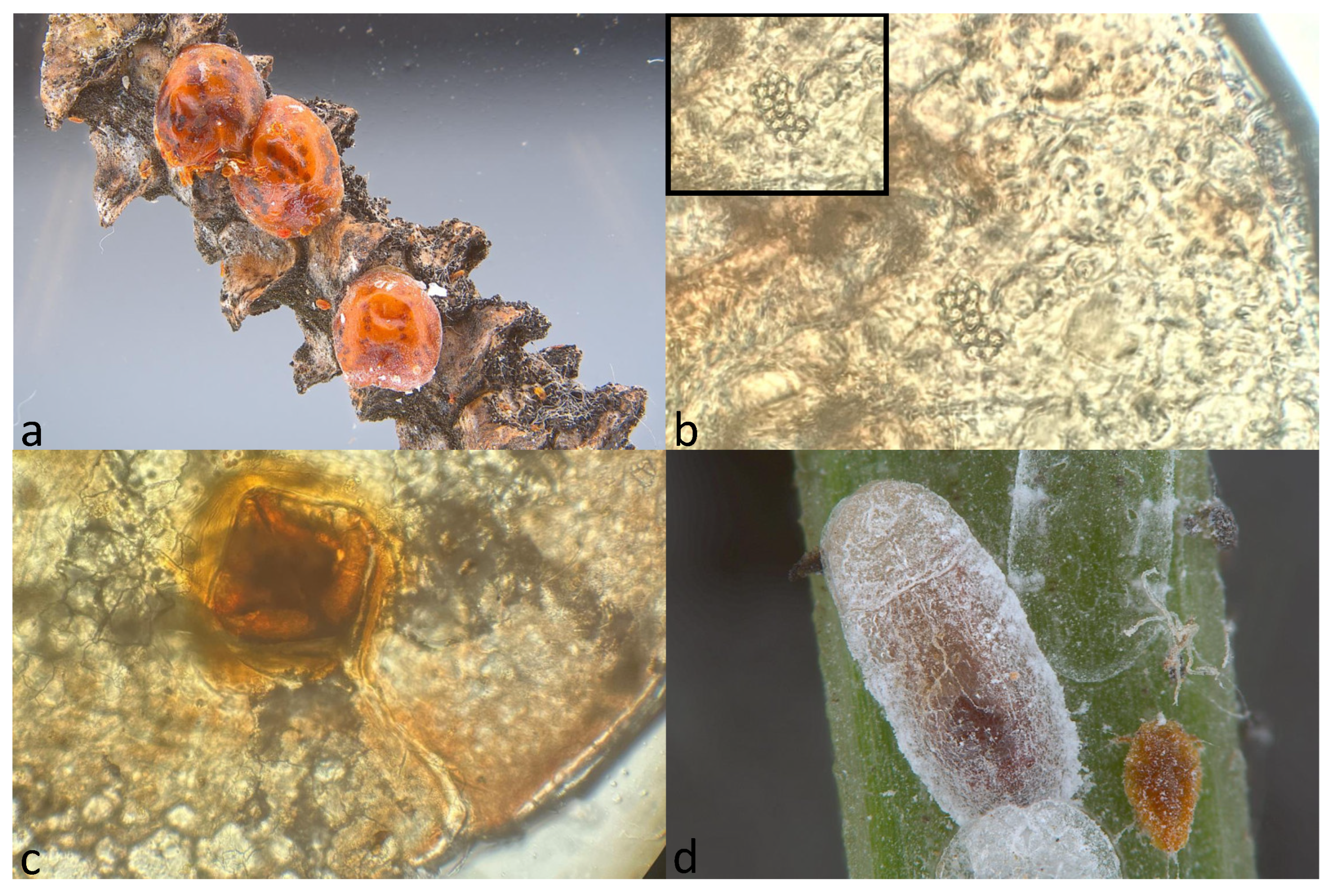
2.1. Collection of Toumeyella parvicornis Specimens and Morphological Identification
2.2. DNA Extraction, PCR, and Sequencing
2.3. Phylogenetic Analysis
3. Results
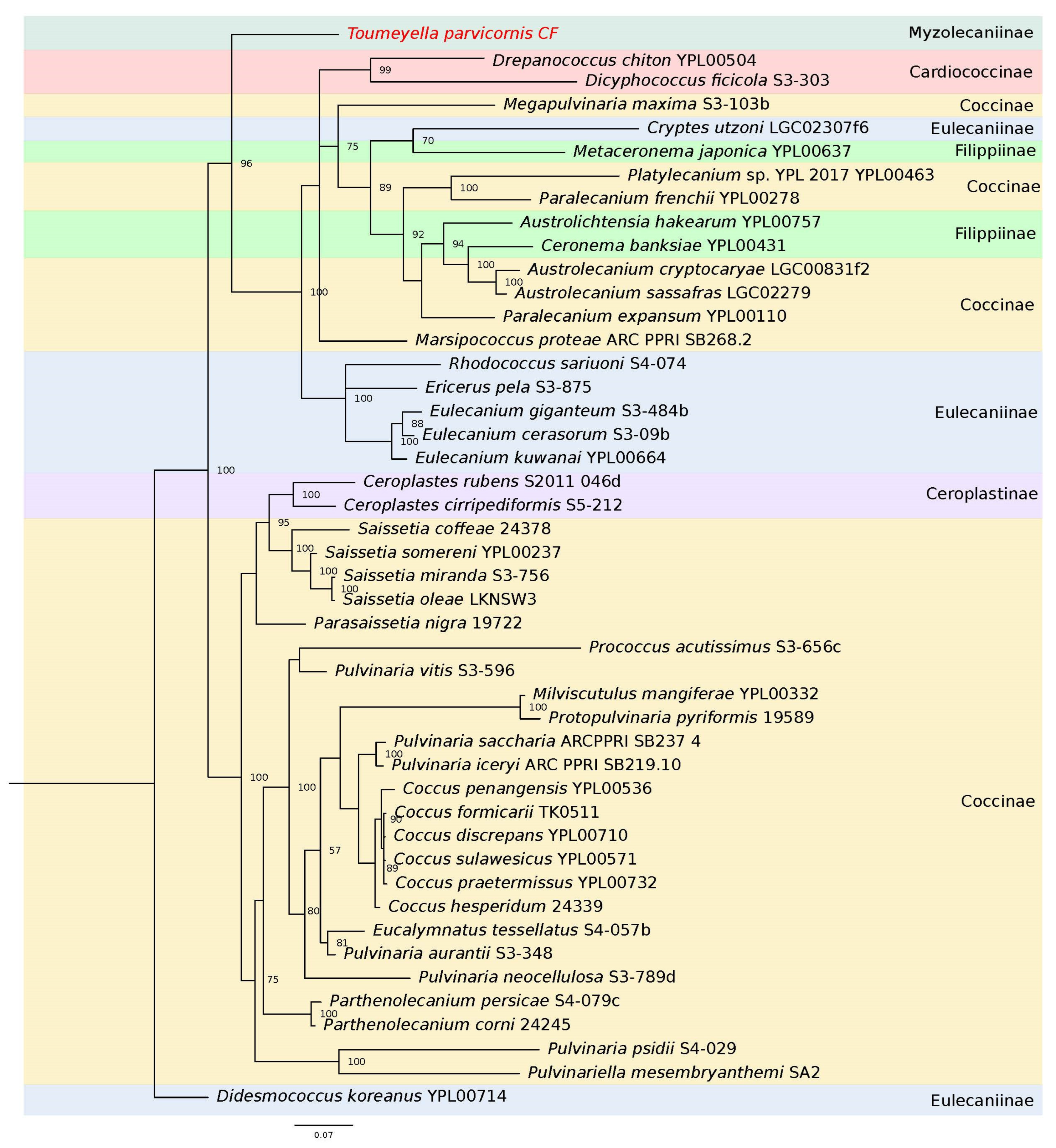
3.1. Phylogenetic Relationships of the COI
3.2. Phylogenetic Relationships of the 28S Ribosomal RNA Gene
3.3. Phylogenetic Relationships of the Wingless Gene
3.4. Molecular Characterization of the Elongation Factor 1-Alpha
3.5. Genetic Characterization of the Histone H3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodgson, C.J. Chapter 1.1.3.4. Classification of the Coccidae and related Coccoid families. In Soft Scale Insects—Their Biology, Natural Enemies and Control; Ben-Dov, Y., Hodgson, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; pp. 157–201. [Google Scholar]
- Gill, R.; Kosztarab, M. Chapter 3.1. Economic Importance. In Soft Scale Insects—Their Biology, Natural Enemies and Control; Ben-Dov, Y., Hodgson, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; pp. 161–163. [Google Scholar]
- Pellizzari, G.; Germain, J.F. Chapter 9.3. Scales (Hemiptera, Superfamily Coccoidea). BioRisk 4 Alien Terr. Arthropods Eur. 2010, 4, 475–510. [Google Scholar]
- Dos Santos, M.M.; Carvalho Reis, L.A.; Ferreira, E.A.; Rocha De Souza, M.W.; Gomes, J.B.; Da Silva, I.M.; Serrão, J.E.; Soares, M.A.; Zanuncio, J.C. Physiological aspects of Olea europaea (Oleaceae) attacked by Saissetia oleae (Hemiptera: Coccidae). Fla. Entomol. 2022, 105, 206–210. [Google Scholar] [CrossRef]
- Assouguem, A.; Kara, M.; Mechchate, H.; Alzain, M.N.; Noman, O.M.; Imtara, H.; Hano, C.; Ibrahim, M.N.; Benmessaoud, S.; Farah, A.; et al. Evaluation of the effect of different concentrations of Spirotetramat on the Diaspine scale Parlatoria ziziphi in citrus orchards. Agronomy 2021, 11, 1840. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kour, R.; Gani, M.; Guroo, M.A.; Bali, K. Potential of wax degrading Bacteria for management of the citrus mealybug, Planococcus citri. BioControl 2022, 67, 49–61. [Google Scholar] [CrossRef]
- Hamon, A.; Williams, M. The Soft Scale Insects of Florida (Homoptera: Coccoidea: Coccidae); Florida Department of Agriculture and Consumer Services, Division of Plant Industry: Gainesville, FL, USA, 1984; Volume 11. [Google Scholar]
- Bi, S.; Qian, G.; Song, X.; Zhang, S.; Zhou, X.; Zou, Y. Studies on dominant natural enemies of Ceroplastes rubens Maskell in tea gardens at different altitudes. Int. J. Trop. Insect Sci. 2022, 42, 2845–2852. [Google Scholar] [CrossRef]
- Villanueva, R.T.; Gauthier, N.; Ahmed, M.Z. First record of Coccus hesperidum L. (Hemiptera: Coccidae) in industrial hemp in Kentucky. Fla. Entomol. 2020, 103, 514–515. [Google Scholar]
- Gallego, D.; Riba, J.M.; Molina, N.; González, E.; Di Sora, N.; Núñez, L.; Closa, A.M.; Comparini, C.; Leza, M. Las invasiones silenciosas de escolítidos: El caso del género Xylosandrus (Coleoptera, Curculionidae, Scolytinae). Foresta 2020, 78, 78–83. [Google Scholar]
- Sax, D.F.; Brown, J.H. The paradox of invasion. Glob. Ecol. Biogeogr. 2000, 9, 363–371. [Google Scholar] [CrossRef]
- Williams, M.; Kondo, T. Status and current composition of the scale insect genus Toumeyella (Hemiptera: Coccidae). In Proceedings of the XI International Symposium of Scale Insect Studies, Oeiras, Portugal, 24–27 September 2007; pp. 24–27. [Google Scholar]
- Bragard, C.; Baptista, P.; Chatzivassiliou, E.; Di Serio, F.; Gonthier, P.; Jaques Miret, J.A.; Fejer Justesen, A.; Magnusson, C.S.; Milonas, P.; Navas-Cortes, J.A.; et al. Pest categorisation of Toumeyella parvicornis. EFSA J. 2022, 20, e07146. [Google Scholar]
- EPPO. Available online: https://gd.eppo.int/taxon/TOUMPA/hosts (accessed on 8 June 2023).
- Garonna, A.; Foscari, A.; Russo, E.; Jesu, G.; Somma, S.; Cascone, P.; Guerrieri, E. The spread of the non-native pine tortoise scale Toumeyella parvicornis (Hemiptera: Coccidae) in Europe: A major threat to Pinus pinea in Southern Italy. iForest—Biogeosciences For. 2018, 11, 628–634. [Google Scholar] [CrossRef] [Green Version]
- Di Sora, N.; Rossini, L.; Contarini, M.; Chiarot, E.; Speranza, S. Endotherapic treatment to control Toumeyella parvicornis Cockerell infestations on Pinus pinea L. Pest Manag. Sci. 2022, 78, 2443–2448. [Google Scholar] [CrossRef] [PubMed]
- Di Sora, N.; Rossini, L.; Contarini, M.; Mastrandrea, G.; Speranza, S. Toumeyella parvicornis versus endotherapic abamectin: Three techniques, 1 year after. Pest Manag. Sci. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L.; Williams, M.L. Tests of male soft scale insects (Homoptera: Coccidae) from America North of Mexico, Including a Key to the Species. Syst. Entomol. 1990, 15, 339–358. [Google Scholar] [CrossRef]
- Garonna, A.P.; Scarpato, S.; Vicinanza, F.; Espinosa, B. First report of Toumeyella parvicornis (Cockerell) in Europe (Hemiptera: Coccidae). Zootaxa 2015, 3949, 142–146. [Google Scholar] [CrossRef]
- Williams, M.; Hodges, G. Chapter 1.1.3.3 Taxonomic characters—Nymphs. In Soft Scale Insects—Their Biology, Natural Enemies and Control; Ben-Dov, Y., Hodgson, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; pp. 143–156. [Google Scholar]
- Bold System. Available online: https://www.boldsystems.org/index.php/Public_SearchTerms (accessed on 8 June 2023).
- Ratnasingham, S.; Hebert, P.D.N. Bold: The barcode of life data system (Http://www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Amouroux, P.; Crochard, D.; Germain, J.F.; Correa, M.; Ampuero, J.; Groussier, G.; Kreiter, P.; Malausa, T.; Zaviezo, T. Genetic diversity of armored scales (Hemiptera: Diaspididae) and soft scales (Hemiptera: Coccidae) in Chile. Sci. Rep. 2017, 7, 2014. [Google Scholar] [CrossRef]
- Cho, S.; Mitchell, A.; Regier, J.C.; Miller, C.; Poole, R.W.; Friedlander, T.P.; Zhao, S. A highly conserved nuclear gene for low-level phylogenetics: Elongation factor-1 alpha recovers morphology-based tree for heliothine moths. Mol. Biol. Evol. 1995, 12, 650–656. [Google Scholar]
- Hardy, N.B.; Gullan, P.J.; Hodgson, C.J. A subfamily-level classification of mealybugs (Hemiptera: Pseudococcidae) based on integrated molecular and morphological data. Syst. Entomol. 2008, 33, 51–71. [Google Scholar] [CrossRef]
- Brower, A.V.Z.; DeSalle, R. Patterns of mitochondrial versus nuclear DNA sequence divergence among Nymphalid butterflies: The utility of wingless as a source of characters for phylogenetic inference. Insect Mol. Biol. 1998, 7, 73–82. [Google Scholar] [CrossRef]
- Zahniser, J.N.; Dietrich, C.H. Phylogeny, evolution, and historical biogeography of the grassland leafhopper tribe Chiasmini (Hemiptera: Cicadellidae: Deltocephalinae). Zool. J. Linn. Soc. 2015, 175, 473–495. [Google Scholar] [CrossRef] [Green Version]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; Varlamov, A.; Vaskin, Y.; Efremov, I.; German Grehov, O.G.; Kandrov, D.; Rasputin, K.; Syabro, M.; et al. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hebert, P.D.N.; Ratnasingham, S.; De Waard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. Lond. Ser. B—Biol. Sci. 2003, 270, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.F.; Kelso, S.; Jackson, M.D.; Kits, J.H.; Miranda, G.F.G.; Skevington, J.H. Diptera-specific Polymerase Chain Reaction amplification primers of use in molecular phylogenetic research. Ann. Entomol. Soc. Am. 2011, 104, 976–997. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, W.C. The systematics of insect ribosomal DNA. In The Hierarchy of Life: Molecules and Morphology in Phylogenetic Analysis; Fernholm, B., Bremer, K., Jornvall, H., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 307–321. [Google Scholar]
- Chesters, D. The phylogeny of insects in the data-driven era. Syst. Entomol. 2020, 45, 540–551. [Google Scholar] [CrossRef]
- Normark, B.B.; Okusu, A.; Morse, G.E.; Peterson, D.A.; Itioka, T.; Schneider, S.A. Phylogeny and classification of armored scale insects (Hemiptera: Coccomorpha: Diaspididae). Zootaxa 2019, 4616, 1–98. [Google Scholar] [CrossRef] [Green Version]
- Kondo, T.; Williams, M. Redescription of the Mexican soft scale insect Toumeyella sallei (Signoret, 1874), Comb. n.(Insecta: Hemiptera: Coccidae). Ann. Naturhistorischen Mus. Wien. Ser. B für Bot. Zool. 2003, 105, 211–215. [Google Scholar]
- Kondo, T.; Williams, M.L. Redescriptions of Neolecanium leucaenae Ckll., Toumeyella cerifera Ferris and T. sonorensis Ckll. and Parrott and their transfer to Neotoumeyella gen. nov. (Hemiptera: Coccidae), with descriptions of two new species from the Southeastern U.S.A. and Colombia, South America. Int. J. Insect Sci. 2020, 1, S2827. [Google Scholar]
- Miller, D.R.; Hodgson, C.J. Chapter 1.1.3.7 Phylogeny. In Soft Scale Insects—Their Biology, Natural Enemies and Control; Ben-Dov, Y., Hodgson, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; pp. 229–250. [Google Scholar]
- Choi, J.; Lee, S. Molecular phylogeny of the Family Coccidae (Hemiptera, Coccomorpha), with a discussion of their waxy ovisacs. Syst. Entomol. 2020, 45, 396–414. [Google Scholar] [CrossRef]
- Lin, Y.P.; Kondo, T.; Gullan, P.; Cook, L.G. Delimiting genera of scale insects: Molecular and morphological evidence for synonymising Taiwansaissetia Tao, Wong and Chang with Coccus Linnaeus (Hemiptera: Coccoidea: Coccidae). Syst. Entomol. 2013, 38, 249–264. [Google Scholar] [CrossRef]
- Oh, M.; Kim, S.; Lee, S. Revisiting the phylogeny of the family Miridae (Heteroptera: Cimicomorpha), with updated insights into its origin and life history evolution. Mol. Phylogenetics Evol. 2023, 184, 107796. [Google Scholar] [CrossRef]
- Caudullo, G.; Welk, E.; Jesús, S.-M.-A. Chorological maps for the main European woody species. Data Br. 2017, 12, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Di Sora, N.; Mannu, R.; Rossini, L.; Contarini, M.; Gallego, D.; Speranza, S. Using species distribution models (SDMs) to estimate the suitability of European Mediterranean non-native area for the establishment of Toumeyella parvicornis (Hemiptera: Coccidae). Insects 2023, 14, 46. [Google Scholar] [CrossRef]
- Hamelin, R.C.; Roe, A.D. Genomic biosurveillance of forest invasive alien enemies: A story written in code. Evol. Appl. 2020, 13, 95–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, P.V.; dos Santos, A.R.; Olive, E.L.; Britto, K.B.; de Almeida, F.A.N.; Pacheco da Silva, V.C.; Machado, C.B.; Fornazier, M.J.; Ventura, J.A.; Culik, M.P.; et al. Molecular Species Delimitation Using COI Barcodes of Mealybugs (Hemiptera: Pseudococcidae) from Coffee Plants in Espírito Santo, Brazil. Diversity 2023, 15, 305. [Google Scholar] [CrossRef]
- Wang, X.B.; Deng, J.; Zhang, J.T.; Zhou, Q.S.; Zhang, Y.Z.; Wu, S.A. DNA barcoding of common soft scales (Hemiptera: Coccoidea: Coccidae) in China. Bull. Entomol. Res. 2015, 105, 545–554. [Google Scholar] [CrossRef]
- Chua, P.Y.S.; Bourlat, S.J.; Ferguson, C.; Korlevic, P.; Zhao, L.; Ekrem, T.; Meier, R.; Lawniczak, M.K.N. Future of DNA-based insect monitoring. Trends Genet. 2023, 39, 531–544. [Google Scholar] [CrossRef]
- Latina, R.A.; Lantican, D.V.; Guerrero, M.S.; Rubico, E.C.; Laquinta, J.F.; Caoili, B.L. Species-specific PCR-based marker for rapid detection of Aspidiotus rigidus Reyne (Hemiptera: Diaspididae). J. Asia-Pac. Entomol. 2022, 25, 101848. [Google Scholar] [CrossRef]
- Serrana, J.M.; Ishitani, N.; Carvajal, T.M.; Almarinez, B.J.M.; Barrion, A.T.; Amalin, D.M.; Watanabe, K. Unravelling the genetic structure of the coconut scale insect pest (Aspidiotus rigidus Reyne) outbreak populations in the Philippines. Insects 2019, 10, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulme, P. Invasion pathways at a crossroad: Policy and research challenges for managing alien species introductions. J. Appl. Ecol. 2015, 52, 1418–1424. [Google Scholar] [CrossRef]
- Cesari, M.; Maistrello, L.; Ganzerli, F.; Dioli, P.; Rebecchi, L.; Guidetti, R. A pest alien invasion in progress: Potential pathways of origin of the brown marmorated stink bug Halyomorpha halys populations in Italy. J. Pest Sci. 2015, 88, 1–7. [Google Scholar]




| Primers | Sequence 5′-3′ | Reference | |
|---|---|---|---|
| Forward | Reverse | ||
| PCO-F1—LepR | CCTTCAACTAATCATAAAAATATYAG | TAAACTTCTGGATGTCCAAAAAATCA | Amouroux et al. [23] |
| C-28SLong-F-C-28SLong-R | GAGAGTTMAASAGTACGTGAAAC | TCGGARGGAACCAGCTACTA | Amouroux et al. [23] |
| M3-rcM44.9 | CACATYAACATTGTCGTSATYGG | CTTGATGAAATCYCTGTGTCC | Cho et al. [24] |
| M44-t-rcM53.2 | CGAACGTGAACGTGGTATCAC * | GCAATGTGRGC[I]GTGTGGCA | Cho et al. [24] |
| scale_wg_F-LEPWG2 | CTGGTTCGTGCACGACGMGRACSTGYTGGATG | ACT[I]CGCARCACCARTGGAATGTRCA | Hardy et al. [25] Brower & DeSalle [26] |
| H3 HexA-f-HexA-r | ATGGCTCGTACCAAGCAGACGGC | ATATCCTTGGGCATGATGGTGAC | Zahniser et al. [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Sora, N.; Turco, S.; Brugneti, F.; Rossini, L.; Mazzaglia, A.; Contarini, M.; Speranza, S. Molecular Characterization and Phylogenetic Analysis of the Pine Tortoise Scale Insect Toumeyella parvicornis (Cockerell) (Hemiptera: Coccidae). Forests 2023, 14, 1585. https://doi.org/10.3390/f14081585
Di Sora N, Turco S, Brugneti F, Rossini L, Mazzaglia A, Contarini M, Speranza S. Molecular Characterization and Phylogenetic Analysis of the Pine Tortoise Scale Insect Toumeyella parvicornis (Cockerell) (Hemiptera: Coccidae). Forests. 2023; 14(8):1585. https://doi.org/10.3390/f14081585
Chicago/Turabian StyleDi Sora, Nicolò, Silvia Turco, Federico Brugneti, Luca Rossini, Angelo Mazzaglia, Mario Contarini, and Stefano Speranza. 2023. "Molecular Characterization and Phylogenetic Analysis of the Pine Tortoise Scale Insect Toumeyella parvicornis (Cockerell) (Hemiptera: Coccidae)" Forests 14, no. 8: 1585. https://doi.org/10.3390/f14081585
APA StyleDi Sora, N., Turco, S., Brugneti, F., Rossini, L., Mazzaglia, A., Contarini, M., & Speranza, S. (2023). Molecular Characterization and Phylogenetic Analysis of the Pine Tortoise Scale Insect Toumeyella parvicornis (Cockerell) (Hemiptera: Coccidae). Forests, 14(8), 1585. https://doi.org/10.3390/f14081585










