The SSR Genetic Diversity of Wild Red Fruit Lycium (Lycium barbarum) in Northwest China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Extraction of Genomic DNA
2.3. SSR Primers
2.4. PCR Amplification and Capillary Electrophoresis
2.5. Data Reading and Processing
3. Results
3.1. Primer Screening and Polymorphism Analysis
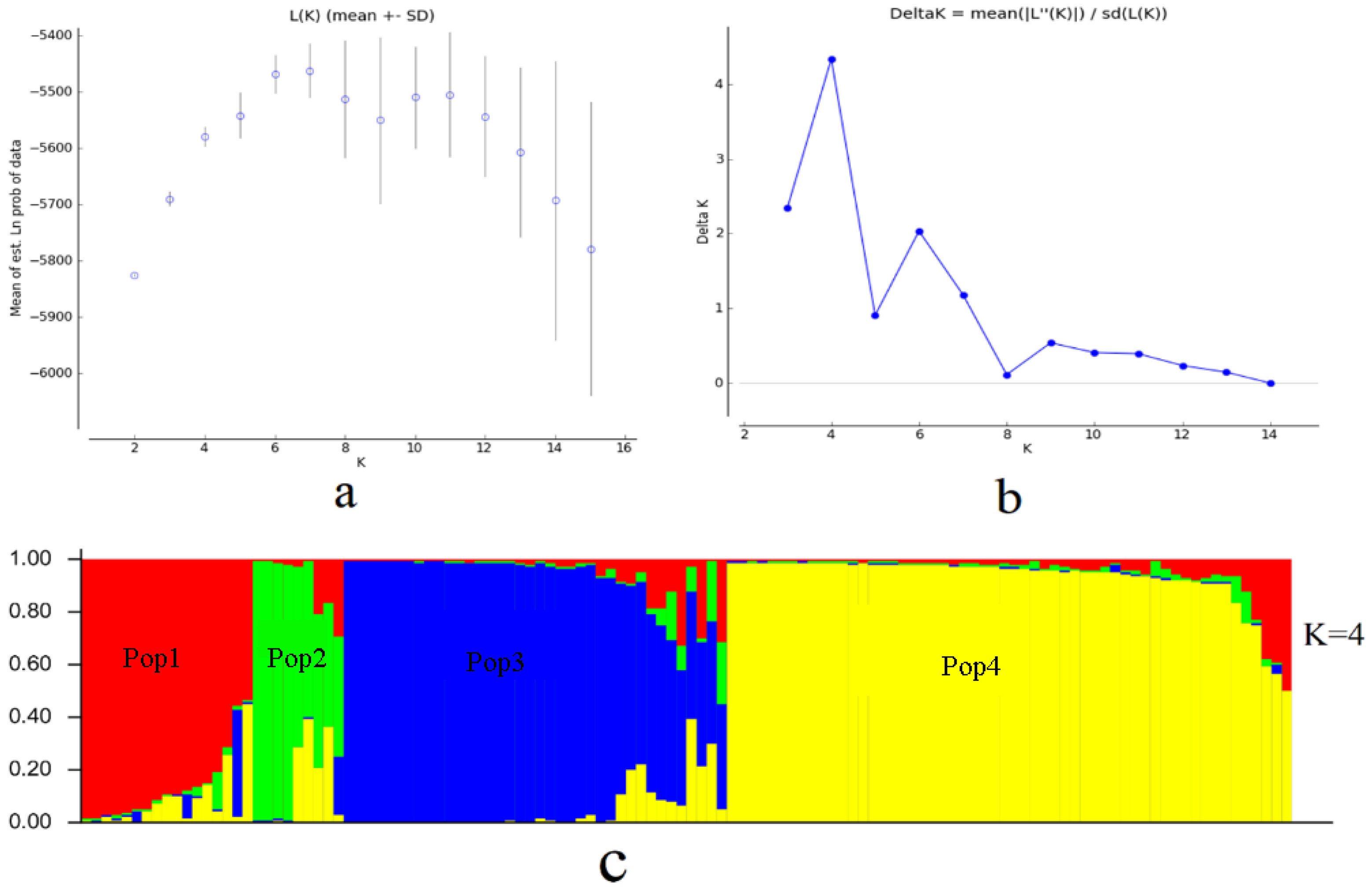
3.2. Structure Clustering Analysis
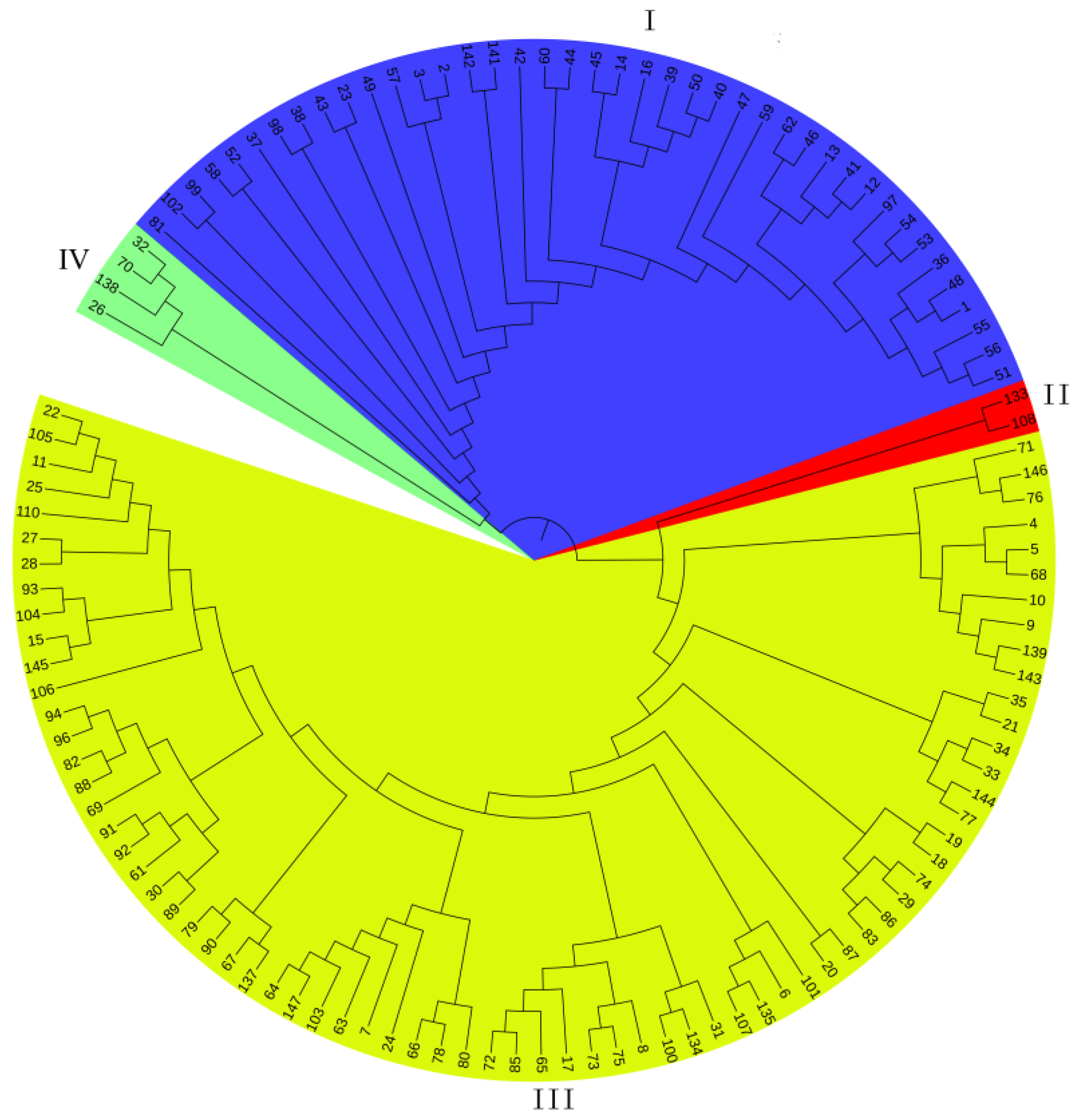
3.3. UPGMA Cluster Analysis
3.4. Molecular Analysis of Variance
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, R.C.; So, K.F. Use of anti-aging herbal medicine, Lycium barbarum, against aging-associated diseases. What do we know so far? Cell Mol. Neurobiol. 2008, 28, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Wei, A.; Jianhua, Z.; Yanlong, L.; Yunfang, F.; Jinhuan, C.; Youlong, C.; Xiangqiang, Z. Constructing the wolfberry (Lycium spp.) genetic linkage map using AFLP and SSR markers. J. Integr. Agric. 2022, 21, 131–138. [Google Scholar] [CrossRef]
- Choudhury, D.R.; Kumar, R.; Maurya, A.; Semwal, D.P.; Rathi, R.S.; Gautam, R.K.; Trivedi, A.K.; Bishnoi, S.K.; Ahlawat, S.P.; Singh, K.; et al. SSR and SNP Marker-Based Investigation of Indian Rice Landraces in Relation to Their Genetic Diversity, Population Structure, and Geographical Isolation. Agriculture 2023, 13, 823. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, G.; Chen, W.; Zhang, Z.; Zou, X. Identification and development of polymorphic EST-SSR markers by sequence alignment in pepper, Capsicum annuum (Solanaceae). Am. J. Bot. 2012, 99, e59–e61. [Google Scholar] [CrossRef] [Green Version]
- Ismail, N.A.; Rafii, M.Y.; Mahmud, T.M.M.; Hanafi, M.M.; Miah, G. Genetic Diversity of Torch Ginger (Etlingera elatior) Germplasm Revealed by ISSR and SSR Markers. Biomed. Res. Int. 2019, 2019, 5904804. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Rashid, M.A.R.; Li, X.; Yao, C.; Lu, L.; Bai, J.; Li, Y.; Xu, N.; Yang, Q.; Zhang, L.; et al. Collection and Evaluation of Genetic Diversity and Population Structure of Potato Landraces and Varieties in China. Front. Plant Sci. 2019, 10, 139. [Google Scholar] [CrossRef] [Green Version]
- Banks, S.C.; Cary, G.J.; Smith, A.L.; Davies, I.D.; Peakall, R. How does ecological disturbance influence genetic diversity? Trends Ecol. Evol. 2013, 28, 670–679. [Google Scholar] [CrossRef]
- Rao, V.R.; Hodgkin, T. Genetic diversity and conservation and utilization of plant genetic resources. Plant Cell Tissue Organ. Cult. 2002, 68, 1–19. [Google Scholar]
- Zhao, W.G.; Chung, J.W.; Cho, Y.I.; Rha, W.H.; Lee, G.A.; Ma, K.H.; Han, S.H.; Bang, K.H.; Park, C.B.; Kim, S.M.; et al. Molecular genetic diversity and population structure in Lycium accessions using SSR markers. Comptes Rendus Biol. 2010, 333, 793–800. [Google Scholar] [CrossRef]
- Chen, C.; Xu, M.; Wang, C.; Qiao, G.; Wang, W.; Tan, Z.; Wu, T.; Zhang, Z. Characterization of the Lycium barbarum fruit transcriptome and development of EST-SSR markers. PLoS ONE 2017, 12, e0187738. [Google Scholar] [CrossRef] [Green Version]
- Info, M.N. Analysis of Gene Diversity in Subdivided Populations. Proc. Natl. Acad. Sci. USA 1973, 70, 3321–3323. [Google Scholar] [CrossRef]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: Dominant markers and null alleles. Mol. Ecol. Notes 2007, 7, 574–578. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [Green Version]
- Peakall, R.O.D.; Smouse, P.E. Genalex 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Das, A.K.; Kumar, S.; Rahim, A. Estimating microsatellite based genetic diversity in Rhode Island Red chicken. Iran. J. Vet. Res. 2015, 16, 274–277. [Google Scholar] [PubMed]
- Keller, L.F.; Waller, D.M. Inbreeding effects in wild populations. Trends Ecol. Evol. 2002, 17, 230–241. [Google Scholar] [CrossRef]
- Wright, S. Variability within and among Natural Populations; University of Chicago Press: Chicago, IL, USA, 1978. [Google Scholar]
- Li, L.; Fang, Z.; Zhou, J.; Chen, H.; Hu, Z.; Gao, L.; Chen, L.; Ren, S.; Ma, H.; Lu, L. An accurate and efficient method for large-scale SSR genotyping and applications. Nucleic Acids Res. 2017, 45, e88. [Google Scholar] [CrossRef] [Green Version]
- Fanjuan, M.; Xiangyang, X.; Fenglan, H.; Jingfu, L. Analysis of Genetic Diversity in Cultivated and Wild Tomato Varieties in Chinese Market by RAPD and SSR. Agric. Sci. China 2010, 9, 1430–1437. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Zhou, X.; Bao, S.; Zhuang, Y. Genetic diversity and population structure of worldwide eggplant (Solanum melongena L.) germplasm using SSR markers. Genet. Resour. Crop Evol. 2018, 65, 1663–1670. [Google Scholar] [CrossRef]
- Yin, Y.; An, W.; Zhao, J.; Wang, Y.; Fan, Y.; Cao, Y. SSR information in transcriptome and development of molecular markers in Lycium ruthenicum. J. Zhejiang AF Univ. 2019, 36, 422–428. [Google Scholar]
- Yisilam, G.; Wang, C.X.; Xia, M.Q.; Comes, H.P.; Li, P.; Li, J.; Tian, X.M. Phylogeography and Population Genetics Analyses Reveal Evolutionary History of the Desert Resource Plant Lycium ruthenicum (Solanaceae). Front. Plant Sci. 2022, 13, 915526. [Google Scholar] [CrossRef]
- Meng, H.H.; Gao, X.Y.; Huang, J.F.; Zhang, M.L. Plant phylogeography in arid Northwest China: Retrospectives and perspectives. J. Syst. Evol. 2015, 53, 33–46. [Google Scholar] [CrossRef]
- Shi, X.J.; Zhang, M.L. Phylogeographical structure inferred from cpDNA sequence variation of Zygophyllum xanthoxylon across north-west China. J. Plant Res. 2015, 128, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.L.; Li, Y.L.; Fan, Y.F.; Li, Z.; Liu, Z.J. Wolfberry genomes and the evolution of Lycium (Solanaceae). Commun. Biol. 2021, 4, 671. [Google Scholar] [CrossRef] [PubMed]
| No. | Longitude, E | Dimensionality, N | No. | Longitude, E | Dimensionality, N | No. | Longitude, E | Dimensionality, N | No. | Longitude, E | Dimensionality, N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 101.70 | 36.23 | 31 | 87.42 | 40.71 | 61 | 101.76 | 36.73 | 92 | 87.50 | 47.12 |
| 2 | 101.70 | 36.23 | 32 | 86.20 | 41.63 | 62 | 101.49 | 36.66 | 93 | 87.93 | 44.14 |
| 3 | 101.70 | 36.18 | 33 | 95.87 | 36.21 | 63 | 100.13 | 39.23 | 94 | 89.06 | 43.84 |
| 4 | 102.53 | 36.18 | 34 | 95.87 | 36.21 | 64 | 99.64 | 39.55 | 96 | 89.06 | 43.84 |
| 5 | 103.28 | 36.17 | 35 | 95.87 | 36.21 | 65 | 98.60 | 39.84 | 97 | 89.06 | 43.84 |
| 6 | 103.28 | 36.17 | 36 | 104.10 | 35.53 | 66 | 98.58 | 39.84 | 98 | 89.06 | 43.84 |
| 7 | 103.28 | 36.17 | 37 | 104.18 | 35.46 | 67 | 98.5 | 39.79 | 99 | 89.61 | 44.04 |
| 8 | 103.28 | 36.17 | 38 | 104.18 | 35.46 | 68 | 98.06 | 39.81 | 100 | 89.61 | 44.04 |
| 9 | 104.01 | 36.49 | 39 | 104.40 | 35.29 | 69 | 97.07 | 40.27 | 101 | 90.11 | 43.82 |
| 10 | 104.01 | 36.49 | 40 | 104.41 | 35.01 | 70 | 97.03 | 40.30 | 102 | 93.02 | 43.60 |
| 11 | 104.51 | 36.67 | 41 | 104.45 | 34.56 | 71 | 97.04 | 40.33 | 103 | 93.34 | 44.24 |
| 12 | 105.08 | 36.64 | 42 | 105.09 | 34.45 | 72 | 95.76 | 40.52 | 104 | 94.69 | 43.25 |
| 13 | 105.69 | 36.55 | 43 | 105.57 | 34.36 | 73 | 95.75 | 40.52 | 105 | 95.27 | 43.22 |
| 14 | 106.93 | 36.05 | 44 | 105.57 | 34.39 | 74 | 95.74 | 40.52 | 106 | 96.55 | 40.53 |
| 15 | 112.32 | 37.65 | 45 | 105.41 | 34.27 | 75 | 95.76 | 40.52 | 107 | 88.91 | 39.26 |
| 16 | 111.84 | 38.08 | 46 | 105.45 | 34.23 | 76 | 95.69 | 40.51 | 108 | 87.36 | 38.69 |
| 17 | 109.93 | 38.29 | 47 | 105.44 | 34.24 | 77 | 87.22 | 43.74 | 110 | 117.90 | 34.40 |
| 18 | 106.79 | 39.26 | 48 | 105.42 | 34.28 | 78 | 85.76 | 44.28 | 133 | 102.76 | 25.06 |
| 19 | 106.13 | 39.03 | 49 | 105.42 | 34.46 | 79 | 85.76 | 44.28 | 134 | 120.16 | 33.35 |
| 20 | 103.09 | 39.63 | 50 | 105.39 | 34.46 | 80 | 84.58 | 44.44 | 135 | 83.04 | 46.73 |
| 21 | 101.97 | 38.23 | 51 | 104.30 | 35.32 | 81 | 80.92 | 43.98 | 137 | 106.13 | 39.03 |
| 22 | 101.14 | 38.53 | 52 | 104.14 | 35.28 | 82 | 81.85 | 43.21 | 138 | 86.39 | 44.51 |
| 23 | 100.23 | 38.19 | 53 | 103.48 | 35.24 | 83 | 81.85 | 43.21 | 139 | 86.39 | 44.51 |
| 24 | 100.25 | 37.26 | 54 | 103.34 | 35.27 | 85 | 84.25 | 41.78 | 141 | 102.03 | 35.43 |
| 25 | 98.56 | 37.01 | 55 | 102.57 | 35.25 | 86 | 86.15 | 42.32 | 142 | 103.8 | 36.06 |
| 26 | 97.51 | 36.03 | 56 | 102.06 | 35.36 | 87 | 87.45 | 42.86 | 143 | 82.65 | 44.55 |
| 27 | 93.62 | 36.75 | 57 | 102.06 | 35.73 | 88 | 88.49 | 43.39 | 144 | 89.06 | 43.84 |
| 28 | 88.17 | 39.07 | 58 | 102.06 | 35.73 | 89 | 87.60 | 43.39 | 145 | 87.36 | 38.69 |
| 29 | 87.42 | 40.71 | 59 | 101.78 | 36.84 | 90 | 87.52 | 44.03 | 146 | 105.95 | 34.65 |
| 30 | 87.42 | 40.71 | 60 | 101.76 | 36.72 | 91 | 101.76 | 36.73 | 147 | 80.51 | 40.68 |
| Primer Name | Forward Primers | Reverse Primers | Target Fragment Length |
|---|---|---|---|
| B6 | CAAGAAGCAAATCTGCCACA | TCACCCGCTTTCCTATCAAC | 140 |
| B18 | CCTCCGATTTCGTGTGCTAT | AGCCCAGTTCTACCCCTTGT | 150 |
| E8 | AAGATTTGGGGAAGACGACG | CATGGCAGCATATTAGCGAAG | 150 |
| E14 | GGATGAATGAATGCTCGTGC | TCCTCTTCCCTTCAAAATACCC | 100 |
| E16 | CCAGTTGTACAGGTAGCTATGCG | GACTTTCCTTGGGCTCTCATG | 140 |
| E19 | CCCATACATACTCATCACCTCTGC | GTTGTCCAAACAACACACAGTGC | 140 |
| E13 | TTCCATTACACTTCGCTGCTC | CAGATGAAGAGGGACGAGACG | 150 |
| E18 | TGCTGCAGAGAACAACATTAGG | TCTCCCATTCAATCCAGTATGC | 150 |
| F1 | CAAGTCATCACGTGGACGC | CTCTGAAACGAGAAGGACGG | 120 |
| G6 | TTGGAACCAATGCTAATGGAAG | GGGACATCAGTTGGAAATTAG | 140 |
| H1 | ACAAACCGAAAGCGAGAAGA | GAACAACTCGTGGTGACCCT | 189 |
| H2 | TGCCACCTTGTATTGGTTCA | GGGTCTGTTTGGTTGTTTGA | 115 |
| H3 | CTCGTCTCCCTTCCAAACAA | ATGCACATCCTACCACCACA | 155 |
| H4 | TGGCAAGCTTACAGGTTGTG | CCCAAATTGATCTGTGTAGAGGA | 113 |
| H8 | AATGGATTGCTT | ATTCCAGCACCAATTTCAGC | 147 |
| H12 | CACCACAAACTCCTCAGCAA | TCTCCCTCGAGCAACTGAAT | 182 |
| H13 | AGGTGCATGAAAAGCCAAAA | AAAGAGGGTTTAGGCCCGTA | 226 |
| Primers | Na | Ne | Ho | He | I | PIC |
|---|---|---|---|---|---|---|
| B6 | 10 | 1.99 | 0.26 | 0.42 | 0.71 | 0.62 |
| B18 | 12 | 3.05 | 0.67 | 0.62 | 1.15 | 0.82 |
| E8 | 22 | 4.43 | 0.66 | 0.69 | 1.41 | 0.91 |
| E13 | 21 | 4.19 | 0.86 | 0.72 | 1.44 | 0.89 |
| E14 | 22 | 3.21 | 0.51 | 0.60 | 1.13 | 0.84 |
| E18 | 15 | 2.34 | 0.59 | 0.52 | 0.91 | 0.75 |
| E19 | 15 | 4.02 | 0.73 | 0.72 | 1.42 | 0.84 |
| F1 | 4 | 2.00 | 0.56 | 0.46 | 0.71 | 0.61 |
| G6 | 5 | 1.80 | 0.34 | 0.37 | 0.59 | 0.44 |
| H1 | 8 | 3.01 | 0.71 | 0.62 | 1.12 | 0.80 |
| H2 | 4 | 1.92 | 0.34 | 0.44 | 0.68 | 0.55 |
| H3 | 8 | 2.20 | 0.51 | 0.48 | 0.85 | 0.60 |
| H4 | 10 | 3.18 | 0.67 | 0.64 | 1.18 | 0.83 |
| H8 | 8 | 2.61 | 0.55 | 0.56 | 0.97 | 0.70 |
| H12 | 12 | 3.41 | 0.68 | 0.68 | 1.29 | 0.79 |
| H13 | 15 | 2.97 | 0.69 | 0.62 | 1.10 | 0.79 |
| average | 11.94 | 2.90 | 0.58 | 0.57 | 1.04 | 0.73 |
| Source of Variation | df | SS | MS | var | Percent (%) | Nm | Fst |
|---|---|---|---|---|---|---|---|
| Inter populations | 3 | 338.06 | 112.69 | 4.99 | 27.01 | 0.68 | 0.27 |
| Within populations | 116 | 1563.64 | 13.48 | 13.48 | 72.99 | / | / |
| Total | 119 | 1901.70 | 124.17 | 18.47 | 1.00 | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Li, J.; Song, J.; Guo, Q. The SSR Genetic Diversity of Wild Red Fruit Lycium (Lycium barbarum) in Northwest China. Forests 2023, 14, 1598. https://doi.org/10.3390/f14081598
Gao X, Li J, Song J, Guo Q. The SSR Genetic Diversity of Wild Red Fruit Lycium (Lycium barbarum) in Northwest China. Forests. 2023; 14(8):1598. https://doi.org/10.3390/f14081598
Chicago/Turabian StyleGao, Xiaoge, Jiajia Li, Jie Song, and Qirong Guo. 2023. "The SSR Genetic Diversity of Wild Red Fruit Lycium (Lycium barbarum) in Northwest China" Forests 14, no. 8: 1598. https://doi.org/10.3390/f14081598





