Melamine–Urea–Formaldehyde Resin Adhesive Modified with Recycling Lignin: Preparation, Structures and Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Melamine-Urea–Formaldehyde Resins
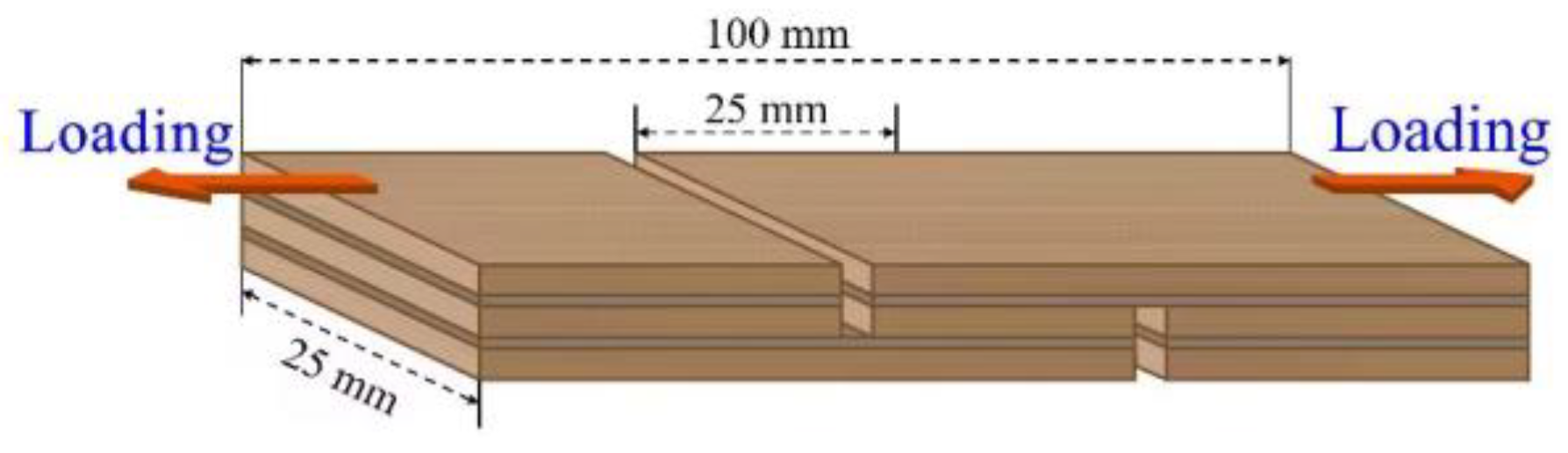
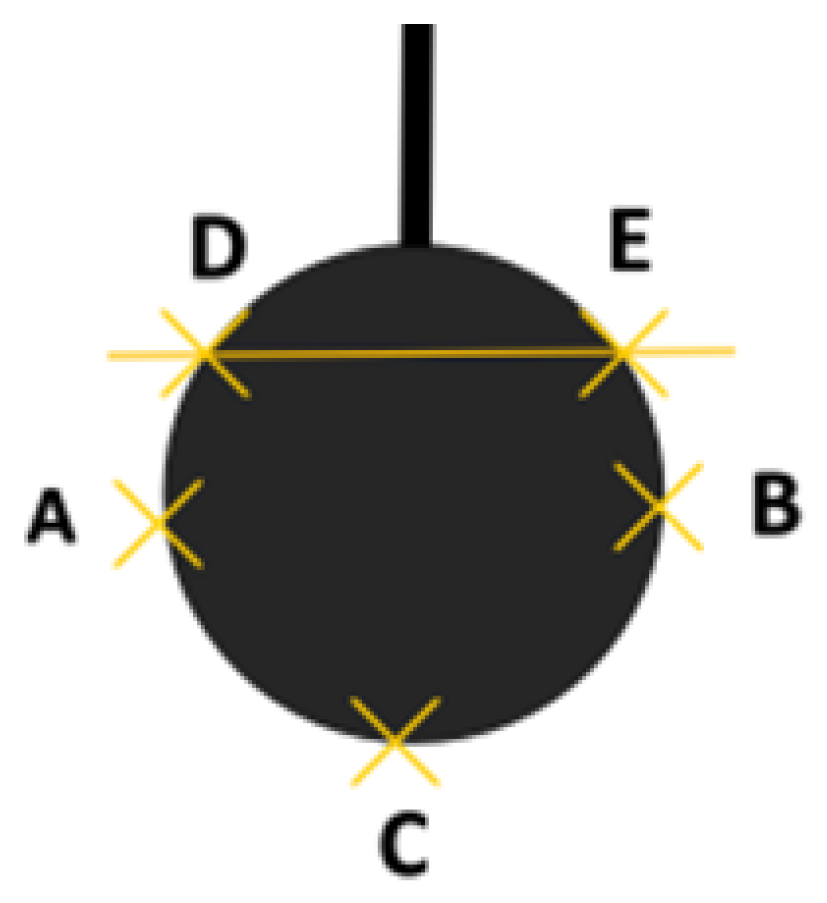
2.3. Preparation of Plywood and Test of Bonding Strength
2.4. Test of Wettability
2.5. Test of Fourier–Transform Infrared Spectroscopy (FTIR)
2.6. Test of Differential Scanning Calorimetry (DSC)
2.7. Test of Thermogravimetry (TG)
2.8. Test of X-ray Diffraction (XRD)
2.9. Test of Scanning Electron Microscopy (SEM)
2.10. Test of 13C Nuclear Magnetic Resonance (13C–NMR)
3. Results and Discussion
3.1. Basic Performance Analysis
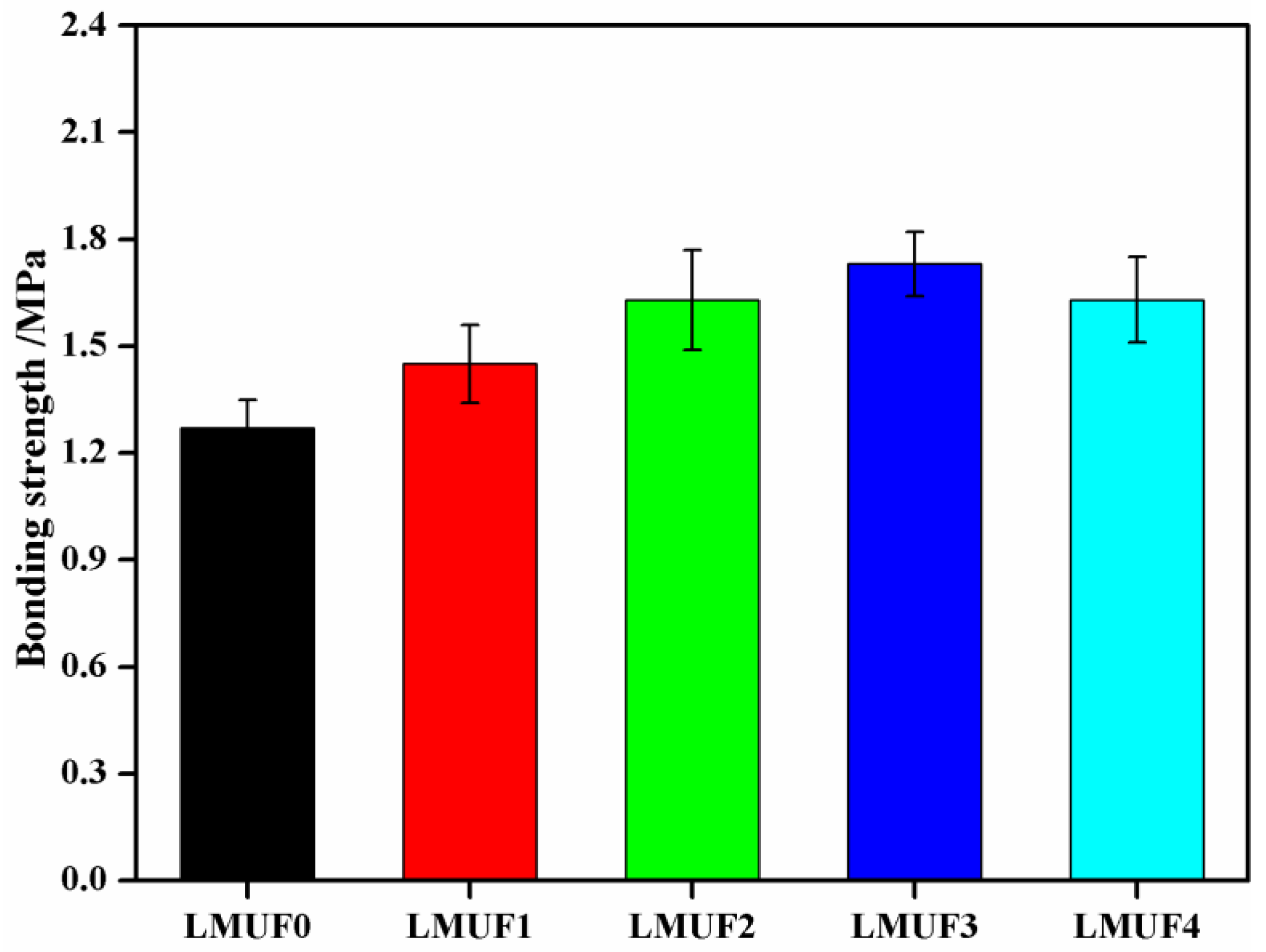
3.2. Bonding Strength Analysis
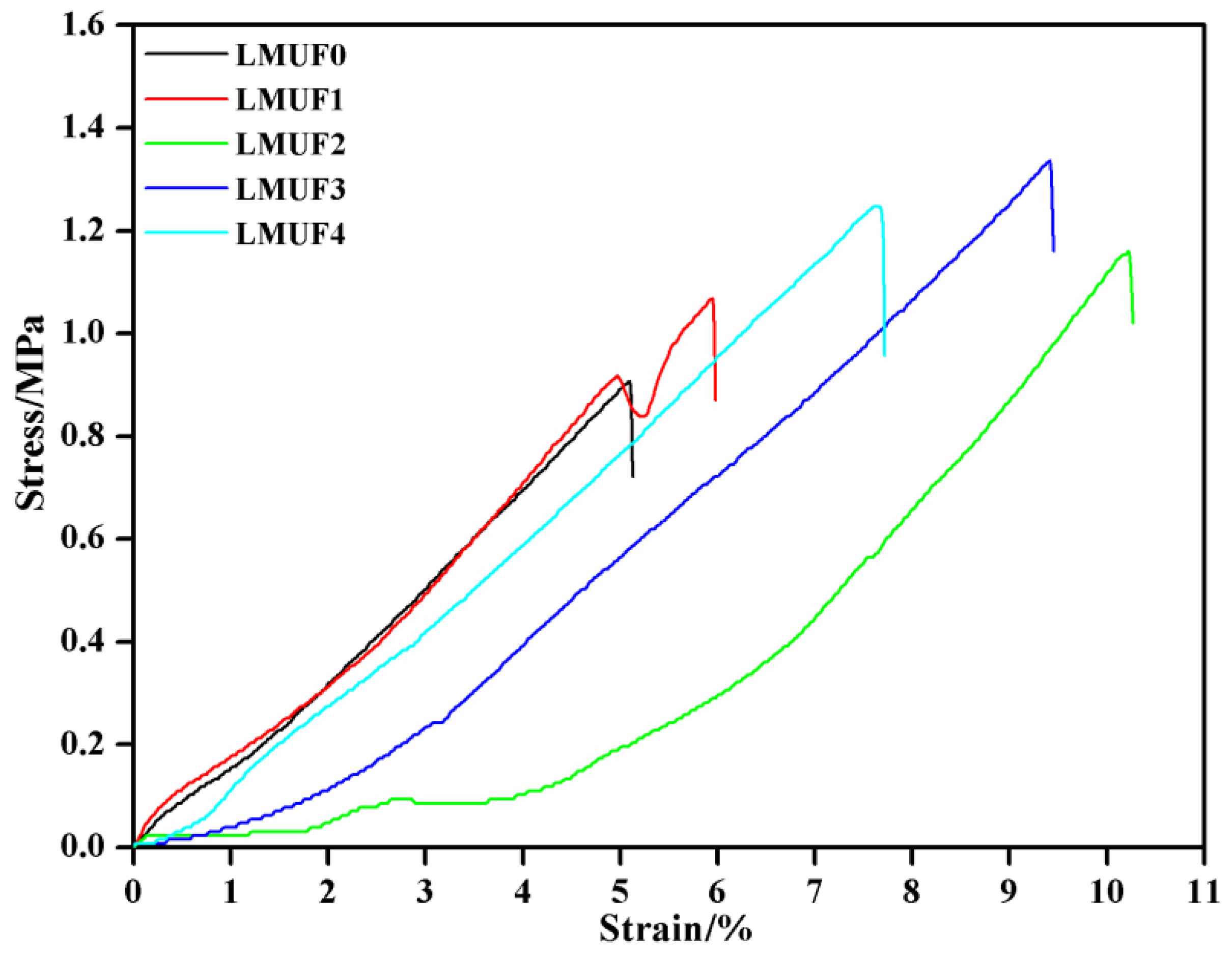
3.3. Flexibility Analysis
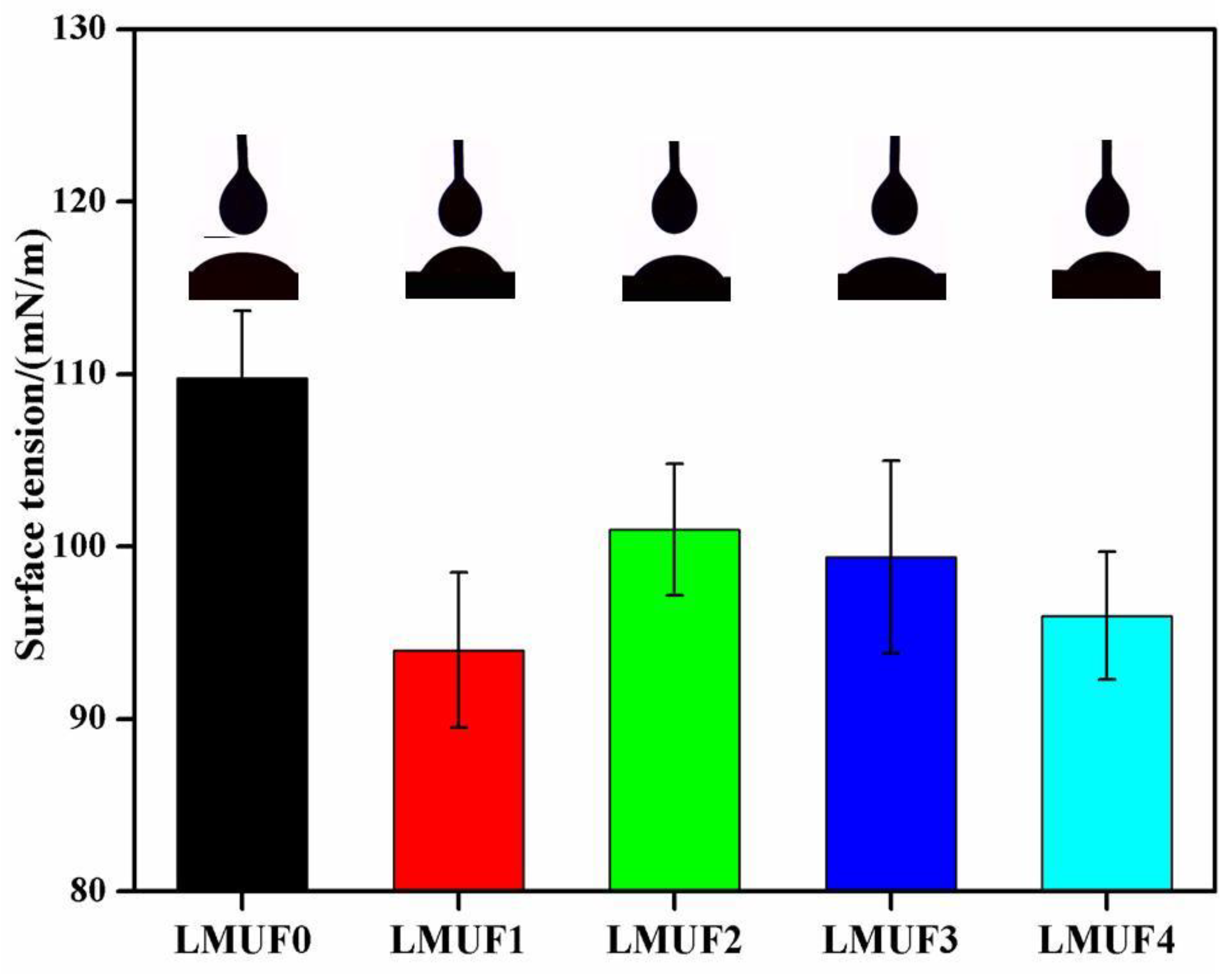
3.4. Wettability Analysis
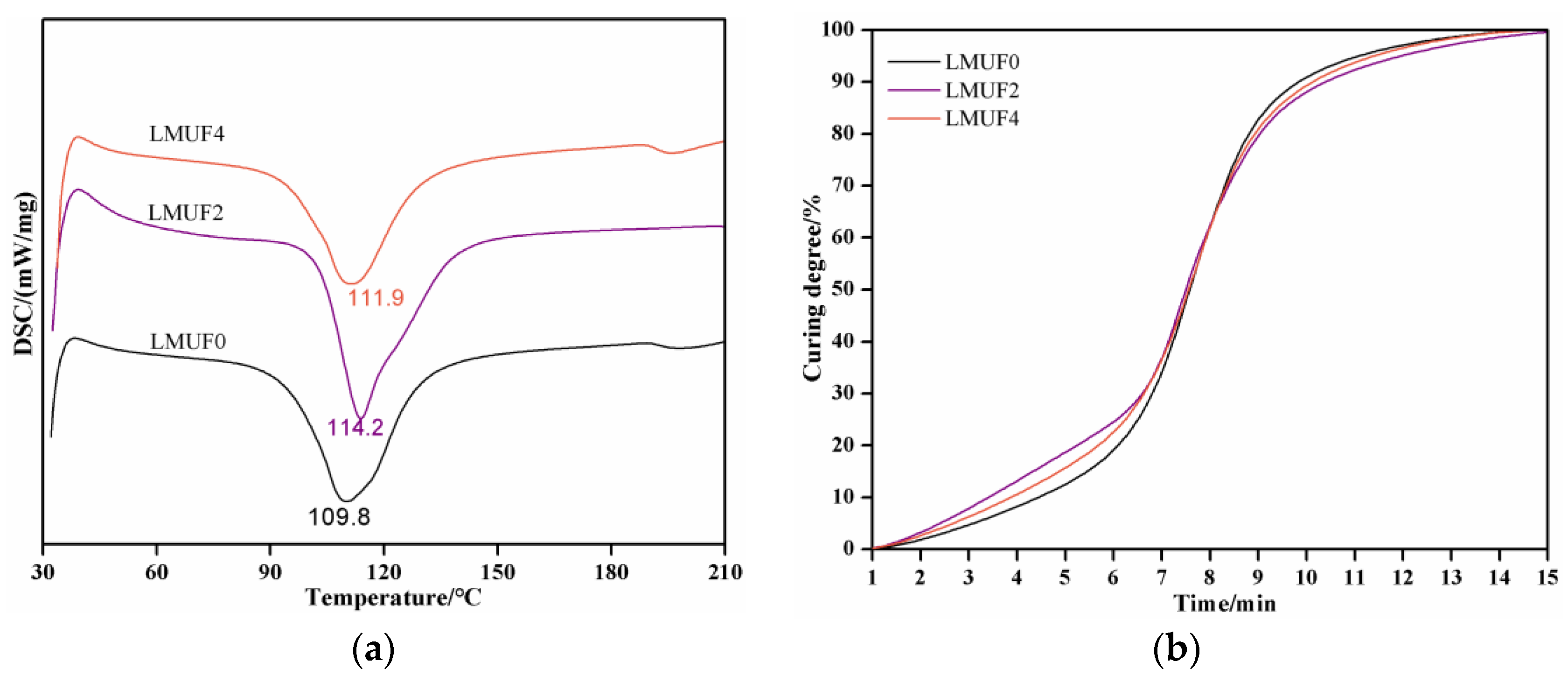
3.5. Curing PerformanceAnalysis
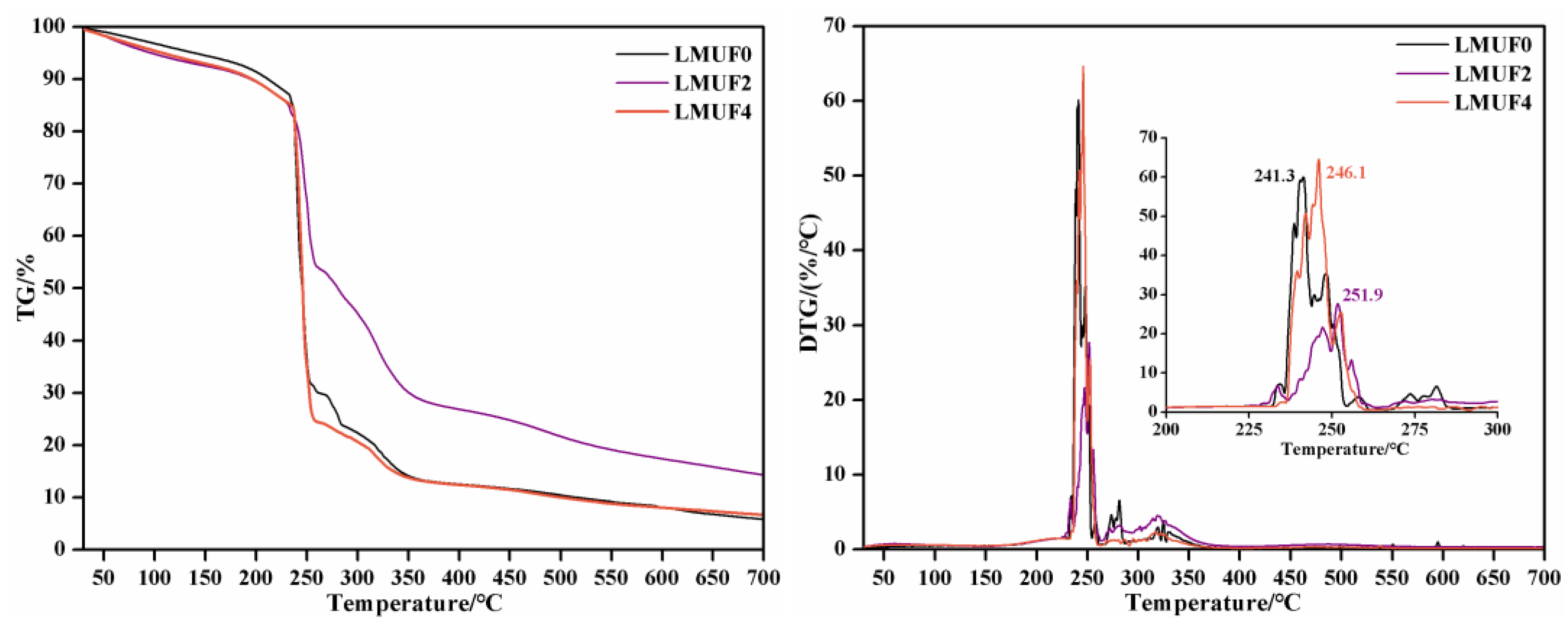
3.6. Thermal Properties Analysis
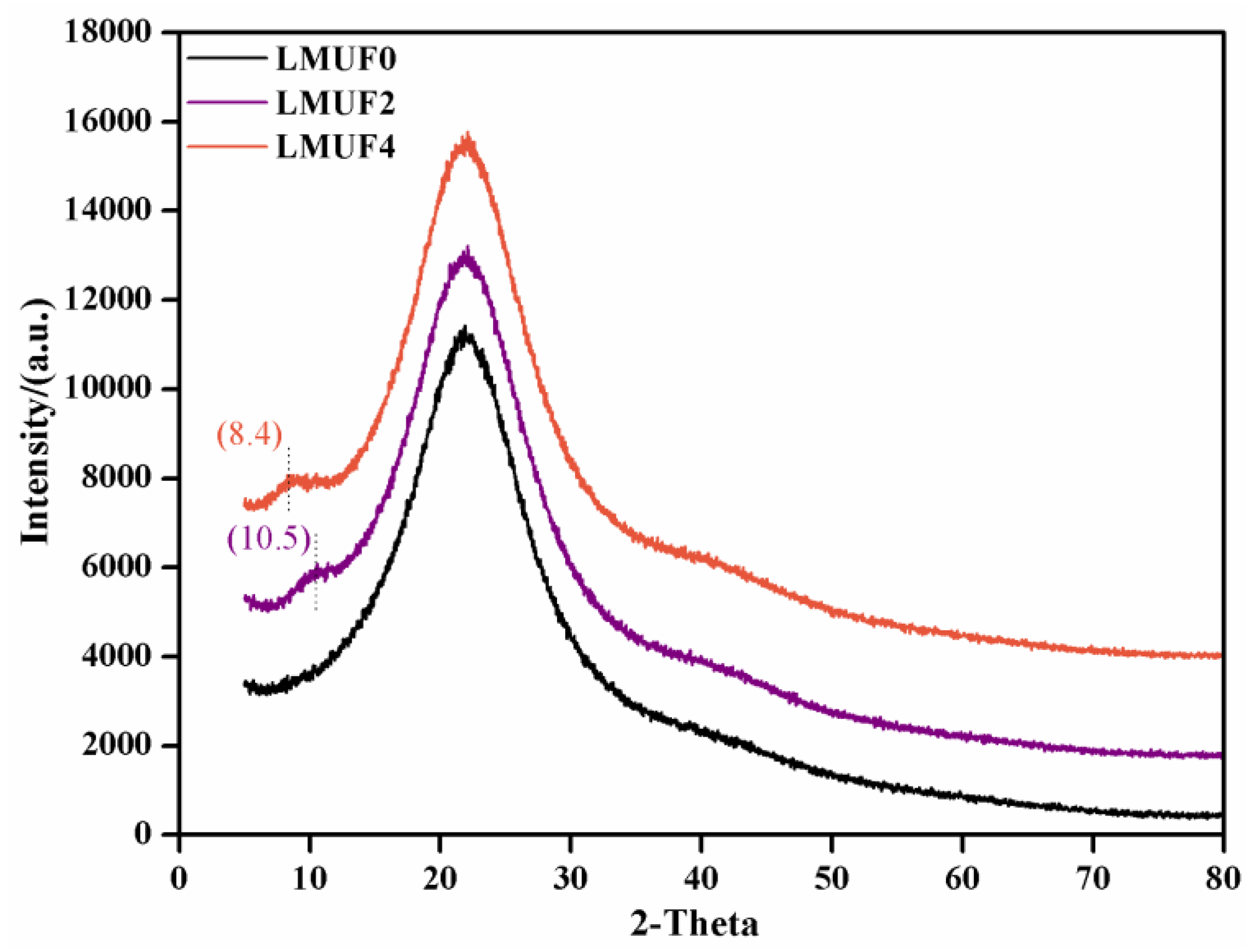
3.7. XRD and SEM Analysis
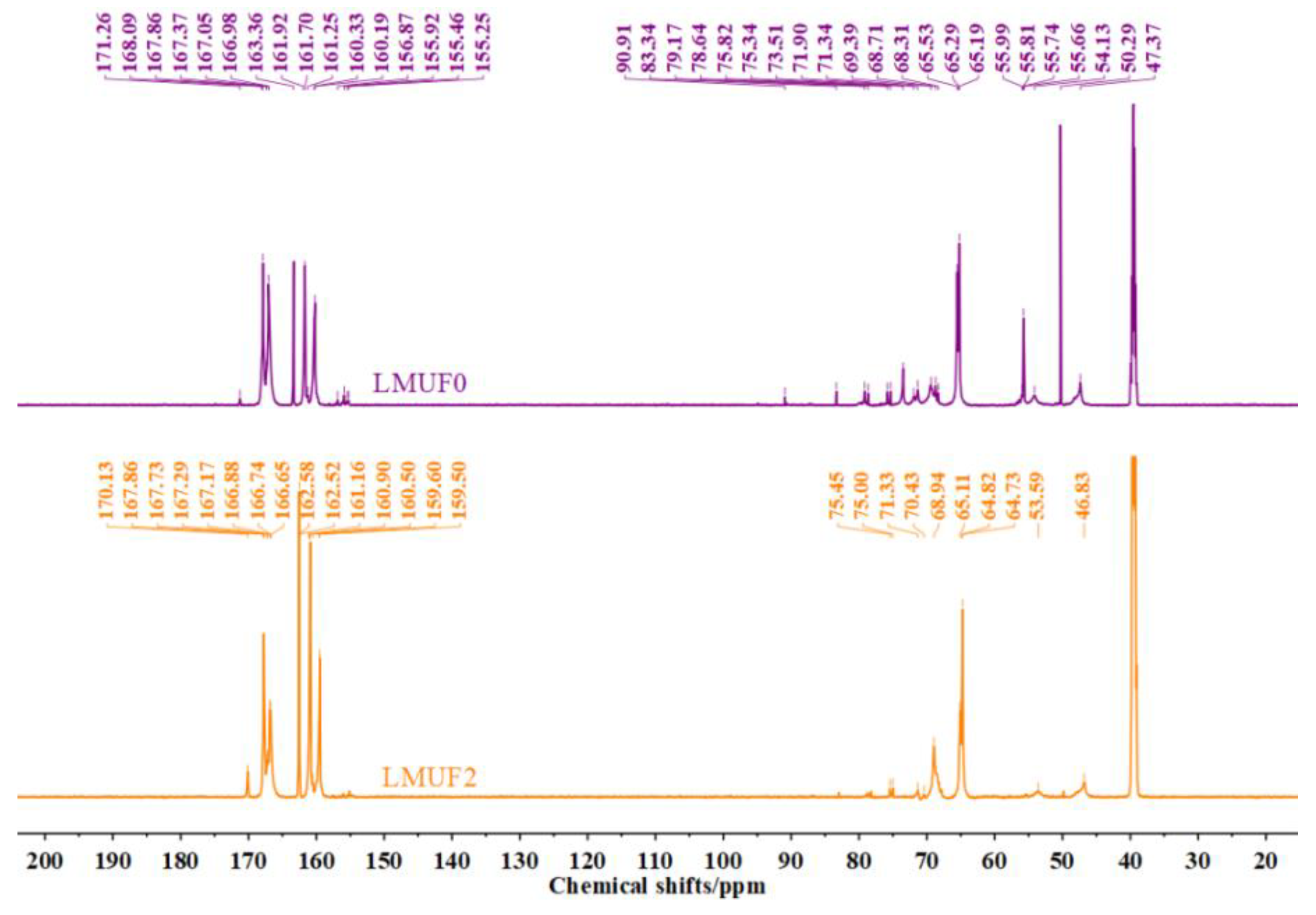
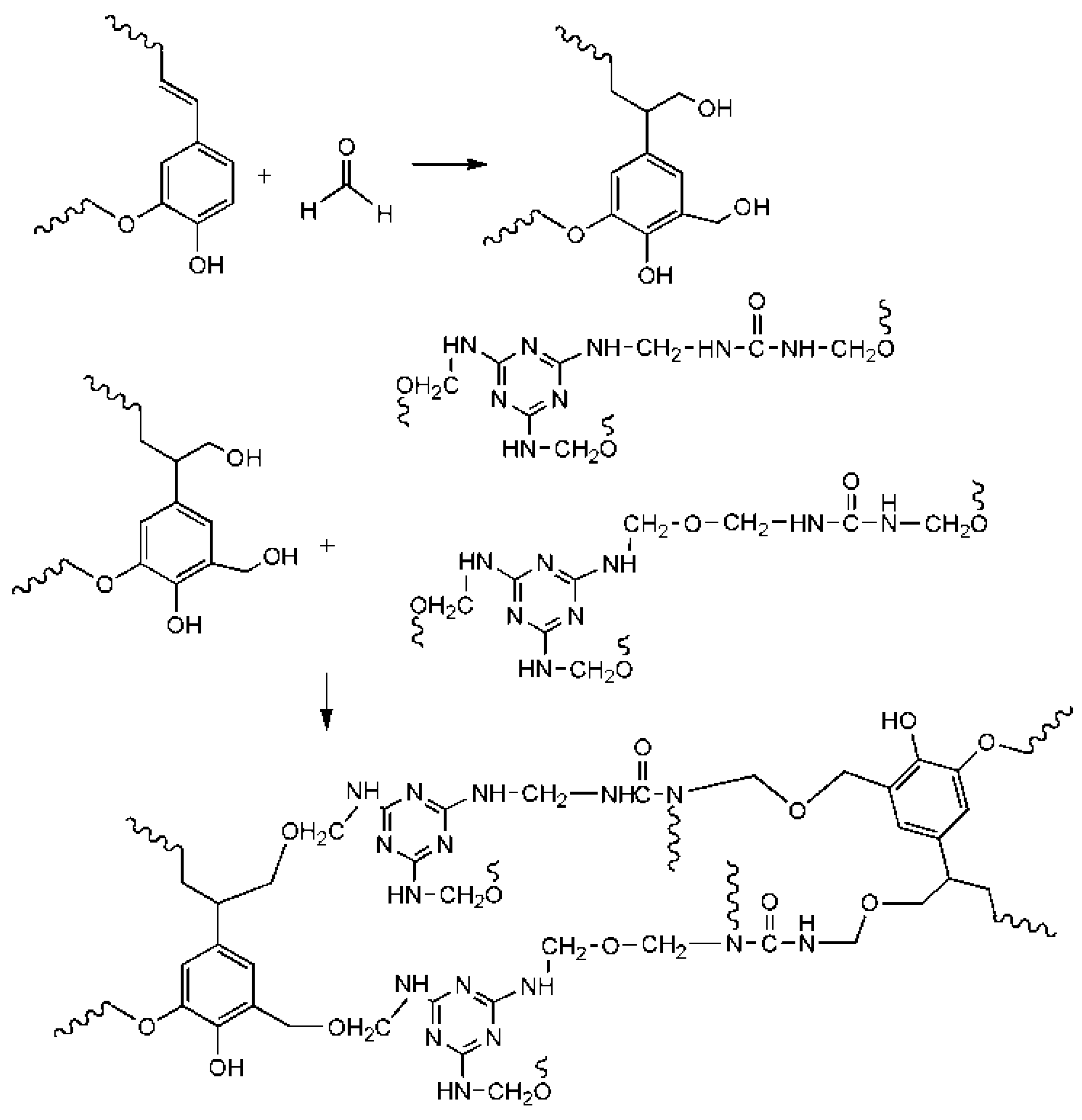
3.8. Modification Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, G.; Yang, T.; Zhang, M.; Li, H.; Wang, C.; Deng, Z.; Wang, Z. Research on the functional modification of plantation wood by inspiring of biomimetic mineralization. J. For. Eng. 2023, 8, 21–29. [Google Scholar]
- Tu, Y.; Liang, J.; Yu, L.; Wu, Z.; Xi, X.; Zhang, B.; Tian, M.; Li, D.; Xiao, G. Effects of plasma treatment on the surface characteristics and bonding performance of Pinus massoniana wood. Forests 2023, 14, 1346. [Google Scholar] [CrossRef]
- Wu, Z.; Deng, X.; Li, L.; Xi, X.; Tian, M.; Yu, L.; Zhang, B. Effects of heat treatment on interfacial properties of Pinus Massoniana wood. Coatings 2021, 11, 543. [Google Scholar] [CrossRef]
- Autengruber, M.; Lukacevic, M.; Gröstlinger, C.; Füssl, J. Finite-element-based prediction of moisture-induced crack patterns for cross sections of solid wood and glued laminated timber exposed to a realistic climate condition. Constr. Build. Mater. 2021, 271, 121775. [Google Scholar]
- Chu, D.; Mu, J.; Zhang, L. Promotion effect of NP fire retardant pre-treatment on heat-treated poplar wood. Part2: Hygroscopicity, leaching resistance, and thermal stability. Holzforschung 2017, 71, 217–223. [Google Scholar]
- Zhang, Y.; Chen, H.; Yang, Z.; Qin, L. Effect of pretreatment on surface roughness and wettability of preservative treated Masson pine. J. For. Eng. 2023, 8, 53–58. [Google Scholar]
- Li, H.; Liang, Y.; Li, P.; He, C. Conversion of Biomass Lignin to High-value Polyurethane: A Review. J. Bioresour. Bioprod. 2020, 5, 163–179. [Google Scholar]
- Xu, C.; Xu, Y.; Chen, M.; Zhang, Y.; Li, J.; Gao, Q.; Shi, S.Q. Soy protein adhesive with bio-based epoxidized daidzein for high strength and mildew resistance. Chem. Eng. J. 2020, 390, 124622. [Google Scholar] [CrossRef]
- Li, C.; Lei, H.; Wu, Z.; Xi, X.; Du, G.; Pizzi, A. Fully biobased adhesive from glucose and citric acid for plywood with high performance. ACS Appl. Mater. Interfaces 2022, 14, 23859–23867. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, Z.; Yang, F.; Zhang, J.; Pizzi, A.; Essawy, H.; Du, G.; Zhou, X. Development of easy-handled, formaldehyde-free, high-bonding performance bio-sourced wood adhesives by co-reaction of furfuryl alcohol and wheat gluten protein. Chem. Eng. J. 2023, 462, 142161. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Q.; Xi, X.; Lei, H.; Cao, M.; Du, G.; Wu, Z. Tannin-based wood adhesive with good water resistance crosslinked by hexanediamine. Int. J. Biol. Macromol. 2023, 234, 123644. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Pizzi, A.; Frihart, C.R.; Lorenz, L.; Gerardin, C. Tannin plywood bioadhesives with non-volatile aldehydes generation by specific oxidation of mono- and disaccharides. Int. J. Adhes. Adhes. 2020, 98, 102499. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, X.; Pizzi, A.; Petrissans, M.; Dumarcay, S.; Petrissans, A.; Zhou, X.; Du, G.; Colin, B.; Xi, X. Highly BranchedTannin-Tris (2-aminoethyl) amine–Urea Wood Adhesives. Polymers 2023, 15, 890. [Google Scholar] [CrossRef] [PubMed]
- Younesi-Kordkheili, H.; Pizzi, A. A comparison among lignin modification methods on the properties of lignin–phenol–formaldehyde resin as wood adhesive. Polymers 2021, 13, 3502. [Google Scholar] [CrossRef]
- Gao, W. New trends in wood adhesives and coatings market. China Wood Ind. 2022, 29, 43–44. [Google Scholar]
- Grinins, J.; Biziks, V.; Irbe, I.; Rizhikovs, J. Water related properties of birch wood modified with phenol formaldehyde (PF) resins. Key Eng. Mater. 2019, 800, 246–250. [Google Scholar] [CrossRef]
- Mu, Q.; Wei, C.; Feng, S. Studies on mechanical properties of sisal fiber/phenol formaldehyde resin in-situ composites. Polym. Compos. 2009, 30, 131–137. [Google Scholar] [CrossRef]
- Younesi-Kordkheili, H. Maleated lignin coreaction with phenol-formaldehyde resins for improved wood adhesives performance. Int. J. Adhes. Adhes. 2022, 113, 103080. [Google Scholar] [CrossRef]
- Savov, V.; Valchev, I.; Antov, P.; Yordanov, I.; Popski, Z. Effect of the adhesive system on the properties of fiberboard panels bonded with hydrolysis lignin and phenol-formaldehyde resin. Polymers 2022, 14, 1768. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, S.; Liang, J.; Li, L.; Xi, X.; Deng, X.; Zhang, B.; Lei, H. Plasma treatment induced chemical changes of alkali lignin to enhance the performances of lignin-phenol-formaldehyde resin adhesive. J. Renew. Mater. 2021, 9, 1959–1972. [Google Scholar]
- Merline, D.; Vukusic, S.; Abdala, A. Melamine formaldehyde: Curing studies and reaction mechanism. Polym. J. 2013, 45, 413–419. [Google Scholar]
- Pizzi, A. Handbook of Adhesive Technology; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Drage, T.C.; Arenillas, A.; Smith, K.M.; Pevida, C.; Piippo, S.; Snape, C.E. Preparation of carbon dioxide adsorbents from the chemical activation of urea–formaldehyde and melamine–formaldehyde resins. Fuel 2007, 86, 22–31. [Google Scholar]
- Tian, F.; Mao, W.; Zhu, C.; Xu, D.; Jia, C.; Xu, X. Effect of nano-montmorillonite modified phenol formaldehyde resin on properties of large particleboards. J. For. Eng. 2023, 8, 35–42. [Google Scholar]
- Park, B.D.; Lee, S.M.; Roh, J.K. Effects of formaldehyde/urea mole ratio and melamine content on the hydrolytic stability of cured urea-melamine-formaldehyde resin. Eur. J. Wood Prod. 2009, 67, 121–123. [Google Scholar]
- Wu, Z.; Lei, H.; Du, G.; Cao, M.; Xi, X.; Liang, J. Urea-formaldehyde resin prepared with concentrated formaldehyde. J. Adhes. Sci. Technol. 2016, 30, 2655–2666. [Google Scholar] [CrossRef]
- Xu, G.; Liang, J.; Zhang, B.; Wu, Z.; Lei, H.; Du, G. Performance and structures of urea-formaldehyde resins prepared with different formaldehyde solutions. Wood Sci. Technol. 2021, 55, 1419–1437. [Google Scholar]
- Dunky, M. Urea–formaldehyde (UF) adhesive resins for wood. Int. J. Adhes. Adhes. 1998, 18, 95–107. [Google Scholar] [CrossRef]
- Dorieh, A.; Pouresmaeel Selakjani, P.; Hassan Shahavi, M.; Pizzi, A.; Ghafari Movahed, S.; Farajollah Pour, M.; Aghaei, R. Recent developments in the performance of micro/nanoparticle-modified urea-formaldehyde resins used as wood-based composite binders: A review. Int. J. Adhes. Adhes. 2022, 114, 103106. [Google Scholar]
- Park, S.; Jeong, B.; Park, B.D. A comparison of adhesion behavior of urea-formaldehyde resins with melamine-urea-formaldehyde resins in bonding wood. Forests 2021, 12, 1037. [Google Scholar] [CrossRef]
- Li, T.; Guo, X.; Liang, J.; Wang, H.; Xie, X.; Du, G. Competitive formation of the methylene and methylene ether bridges in the urea-formaldehyde reaction in alkaline solution: A combined experimental and theoretical study. Wood Sci. Technol. 2015, 49, 475–493. [Google Scholar]
- Dorieh, A.; Mahmoodi, N.; Mamaghani, M.; Pizzi, A.; Mohammadi, Z.M. Comparison of the properties of urea-formaldehyde resins by the use of formalin or urea formaldehyde condensates. J. Adhes. Sci. Technol. 2018, 32, 2537–2551. [Google Scholar] [CrossRef]
- Song, F.; Liu, T.; Fan, Q.; Li, D.; Ou, R.; Liu, Z. Sustainable, high-performance, flame-retardant waterborne wood coatings via phytic acid based green curing agent for melamine-urea-formaldehyde resin. Prog. Org. Coat. 2022, 162, 106597. [Google Scholar]
- Ding, Z.; Ding, Z.; Zhang, D.; Chao, C. The hydrolytic stability and structure of the cured melamine-urea-formaldehyde resin. J. Adhes. 2022, 9, 1614–1634. [Google Scholar] [CrossRef]
- Nazerian, M.; Naderi, F.; Papadopoulos, A.N. Performance Evaluation of an Improved ANFIS Approach Using Different Algorithms to Predict the Bonding Strength of Glulam Adhered by Modified Soy Protein–MUF Resin Adhesive. J. Compos. Sci. 2023, 73, 93. [Google Scholar]
- Silva, D.A.L.; Lahr, F.A.R.; Varanda, L.D.; Christoforo, A.L.; Ometto, A.R. Environmental performance assessment of the melamine-urea-formaldehyde (MUF) resin manufacture: A case study in Brazil. J. Clean. Prod. 2015, 96, 299–307. [Google Scholar]
- Slabohm, M.; Stolze, H.; Militz, H. Evaluation of wet tensile shear strength and surface properties of finger-jointed acetylated beech (Fagus sylvatica L.) laminated veneer lumber. Eur. J. Wood Prod. 2023, 1–9. [Google Scholar] [CrossRef]
- Ozyhar, T.; Tschannen, C.; Thoemen, H.; Zoppe, J.O. Evaluating the use of calcium hydrogen phosphate dehydrate as a mineral-based fire retardant for application in melamine-urea-formaldehyde (MUF)-bonded wood-based composite materials. Fire Mater. 2022, 46, 595–604. [Google Scholar]
- Ozyhar, T.; Tschannen, C.; Hilty, F.; Thoemen, H.; Schoelkopf, J.; Zoppe, J.O. Correction to: Mineral-based composition with deliquescent salt as flame retardant for melamine–urea–formaldehyde (MUF)-bonded wood composites. Wood Sci. Technol. 2021, 55, 1529–1530. [Google Scholar]
- Luo, J.; Zhang, J.; Gao, Q.; Mao, A.; Li, J. Toughening and enhancing melamine–urea–formaldehyde resin properties via in situ polymerization of dialdehyde starch and microphase separation. Polymers 2019, 11, 1167. [Google Scholar] [PubMed]
- Li, Y.; Yang, W.; Li, J.; Yuan, Y.; Zhang, H.; Wang, F.; Cui, Y. Dynamic self-test scheme and authentication protocol for improving robustness of strong PUF. Microelectron. J. 2023, 139, 105864. [Google Scholar]
- Cao, M. Study on the Synthesis Mechanism of Phenol-Formaldehyde Resin and Phenol-Urea-Formaldehyde Co-Condensation Resin. Ph.D. Thesis, Nanjing Forestry University, Nanjing, China, 2017. [Google Scholar]
- Wang, R.; Zhang, Z.; Chen, R.; Zhao, L.; Wang, C.; Chu, F. Synthesis of phenol-urea-formaldehyde resin and its reaction mechanism. Chem. Ind. For. Prod. 2018, 38, 101–109. [Google Scholar]
- Yi, D.; Liu, M.; Zhou, G.; Xu, Z.; Peng, Y.; Shi, X.; Zhu, J.; Bi, Y. Preparation and characterization of phenol-melamine-formaldehyde aerogels. J. Funct. Mater. 2017, 48, 4141–4144. [Google Scholar]
- Xiang, X.; Liu, E.; Xie, H.; Tian, Y.; Wu, Y.; Wu, Z.; Zhu, Y. Highly stable performance of supercapacitors using microporous carbon derived from phenol-melamine-formaldehyde resin. J. Solid State Electr. 2012, 16, 2661–2666. [Google Scholar]
- Wang, F.; Liu, J.; Lu, W. Flame retardant properties of Chinese fir modified by PMUF resin and boron compounds. J. Northeast. Univ. 2017, 45, 53–56. [Google Scholar]
- Mamatha, B.S.; Sujatha, D.; Uday, D.N. Synthesis of PUMF resin for the manufacture of plywood. Chem. Sci. Trans. 2019, 8, 36–42. [Google Scholar]
- Yang, S.; Fu, Y.; Yuan, T. Synthesis of lignin-phenol-urea-formaldehyde co-polymer resin adhesive. J. Cent. South Univ. For. Technol. 2021, 41, 130–137+167. [Google Scholar]
- Wu, Z.; Zhang, B.; Zhou, X.; Li, L.; Yu, L.; Liao, J.; Du, G. Influence of single/collective use of curing agents on the curing behavior and bond strength of soy protein-melamine-urea-formaldehyde (SMUF) resin for plywood assembly. Polymers 2019, 11, 1995. [Google Scholar] [PubMed]
- Fan, D.; Qin, T.; Chu, F. A soy flour-based adhesive reinforced by low addition of MUF resin. J. Adhes. Sci. Technol. 2011, 25, 323–333. [Google Scholar] [CrossRef]
- Gao, Q.; Shi, S.Q.; Zhang, S.; Li, J.; Wang, X.; Ding, W.; Wang, J. Soybean meal-based adhesive enhanced by MUF resin. J. Appl. Polym. Sci. 2012, 125, 3676–3681. [Google Scholar] [CrossRef]
- Lee, J.; Park, B.D. Thermal cure kinetics of cold-setting melamine–urea–formaldehyde resins with high melamine content. J. Therm. Anal. Calorim. 2023, 148, 6407–6422. [Google Scholar]
- Zhang, B.; Wu, Z.; Liang, J.; Yu, L.; Xi, X.; Lei, H.; Du, G. Effects of polyethylene glycol on the flexibility of cold-setting melamine-urea-formaldehyde resin. Eur. J. Wood Wood Prod. 2022, 80, 975–984. [Google Scholar]
- Wibowo, E.S.; Park, B.D. Enhancing adhesion of thermosetting urea-formaldehyde resins by preventing the formation of H-bonds with multi-reactive melamine. J. Adhes. 2022, 98, 257–285. [Google Scholar] [CrossRef]
- Xu, S.; Xiao, H.; Chen, Y.; Li, J.; Jiang, K.; He, X.; Zhang, J.; Jiang, Y.; Huang, X.; Xie, J.; et al. Preparation and thermal degradation property analysis of the tea-based melamine-modified urea–formaldehyde (TMUF) resin. J. Therm. Anal. Calorim. 2020, 146, 1845–1852. [Google Scholar] [CrossRef]
- Xu, S.; Li, J.; Luo, X.; Wang, Y.; Zhang, J.; Xiao, H.; Cheng, Y. Preparation and thermal degradation of melamine-modified urea-formaldehyde resin from walnut shell. J. Mater. Sci. Eng. 2021, 39, 826–831. [Google Scholar]
- Sun, G.; Sun, H.; Liu, Y.; Zhao, B.; Zhu, N.; Hu, K. Comparative study on the curing kinetics and mechanism of a lignin-based-epoxy/anhydride resin system. Polymer 2007, 48, 330–337. [Google Scholar]
- Li, R.; Gutierrez, J.; Chung, Y.; Frank, C.; Billington, S.; Sattely, E. A lignin-epoxy resin derived from biomass as an alternative to formaldehyde-based wood adhesives. Green Chem. 2018, 20, 1459–1466. [Google Scholar]
- Hasan, A.; Fatehi, P. Stability of kaolin dispersion in the presence of lignin-acrylamide polymer. Appl. Clay Sci. 2018, 158, 72–82. [Google Scholar] [CrossRef]
- Li, C.; Ye, H.; Ge, S.; Yao, Y.; Ashok, B.; Hariram, N.; Liu, H.; Tian, H.; He, Y.; Guo, G. Fabrication and properties of antimicrobial flexible nanocomposite polyurethane foams with in situ generated copper nanoparticles. J. Mater. Res. Technol. 2022, 19, 3603–3615. [Google Scholar]
- Li, T.; Xie, X.; Du, G. Formation of methylolureas under alkaline condition: A theoretical study. Asian J. Chem. 2013, 25, 8317–8323. [Google Scholar]
- Siahkamari, M.; Emmanuel, S.; Hodge, D.B.; Nejad, M. Lignin-glyoxal: A fully biobased formaldehyde-free wood adhesive for interior engineered wood products. ACS Sustain. Chem. Eng. 2022, 10, 3430–3441. [Google Scholar]
- Li, T.; Xie, X.; Du, G. A theoretical study on the water-mediated asynchronous addition between urea and formaldehyde. Chin. Chem. Lett. 2013, 24, 85–88. [Google Scholar] [CrossRef]
- Li, T.; Wang, H.; Liang, J. Specific solvation effects on the formation of urea anion in alkaline solution. Prog. React. Kinet. Mech. 2014, 39, 115–121. [Google Scholar] [CrossRef]
- Liang, J.; Li, Q.; Wu, Z.; Du, G.; Li, T.; Lei, H.; Li, L. Competitive polycondensation of model compound melamine-urea-formaldehyde (MUF) resin system by 13CNMR. J. Bioresour. Bioprod. 2020, 5, 60–66. [Google Scholar] [CrossRef]


| Synthesis Scheme | MUF | LMUF |
|---|---|---|
| Step 1 | F + U1 + M1, pH = 9.0, 90 °C | F + U1 + M1 + Lignin, pH = 9.0, 90 °C |
| Step 2 | pH = 5.0–5.2, 60 min | pH = 5.0–5.2, 60 min |
| Step 3 | +M2, pH = 9.0 | +M2, pH = 9.0 |
| Step 4 | +U2, pH = 9.0, 45 °C | +U2, pH = 9.0, 45 °C |
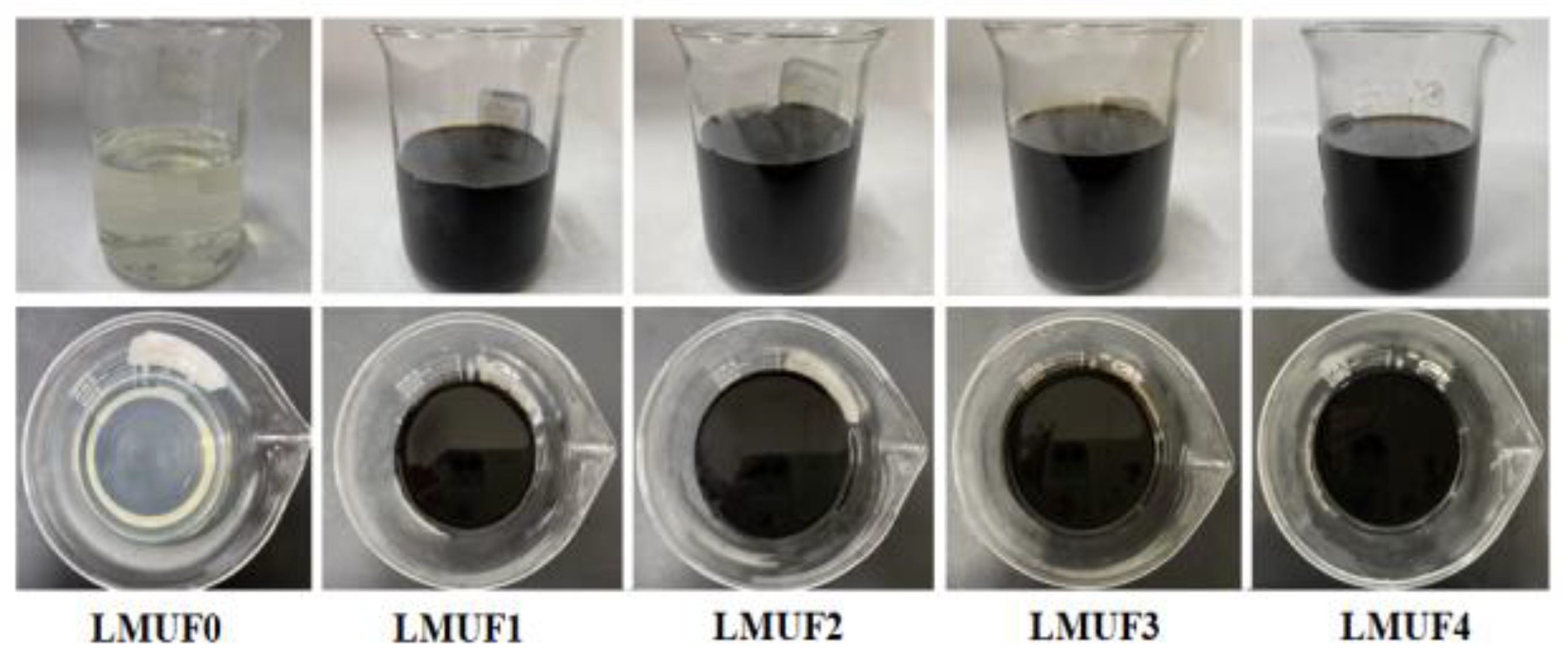
| Adhesives | Solid Content /% | Viscosity /mPa·s | Free Formaldehyde /% | Pot Life /d |
|---|---|---|---|---|
| LMUF0 | 51.8 | 68.4 | 0.12 | 90 |
| LMUF1 | 52.7 | 88.6 | 0.08 | 41 |
| LMUF2 | 53.1 | 107.9 | 0.06 | 36 |
| LMUF3 | 53.3 | 232.6 | 0.06 | 23 |
| LMUF4 | 54.8 | 412.4 | 0.07 | 19 |
| Structures | Chemical Shifts/ppm | MUF | LMUF2 |
|---|---|---|---|
| -NH-CH2-NH- | 46–48 | 11.2 | 10.5 |
| -N(CH2-)-CH2-NH- | 54–56 | 6.1 | 2.2 |
| CH3OH/-CH2OCH3 | 49–50 | ||
| M-NH-CH2OH/U-NH-CH2OH | 63–65 | 48.8 | 44.3 |
| -NHCH2OCH2(NH-/-OH) | 67–70 | 19.0 | 19.3 |
| -NHCH2OCH3 | 72–73 | 4.3 | 5.8 |
| M-N(CH2-)-CH2-O-CH2-N(CH2-)-U | 74–75 | 9.8 | 10.3 |
| M-NH-CH2-O-CH2-N(CH2-)-U | 77–78 | ||
| HOCH2OH | 82.4–82.5 | 0.8 | 0.2 |
| HOCH2OCH2OH | 86.2–86.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Yu, L.; Li, L.; Liang, J.; Wu, Z.; Xu, X.; Zhong, X.; Gong, F. Melamine–Urea–Formaldehyde Resin Adhesive Modified with Recycling Lignin: Preparation, Structures and Properties. Forests 2023, 14, 1625. https://doi.org/10.3390/f14081625
Li D, Yu L, Li L, Liang J, Wu Z, Xu X, Zhong X, Gong F. Melamine–Urea–Formaldehyde Resin Adhesive Modified with Recycling Lignin: Preparation, Structures and Properties. Forests. 2023; 14(8):1625. https://doi.org/10.3390/f14081625
Chicago/Turabian StyleLi, De, Liping Yu, Lifen Li, Jiankun Liang, Zhigang Wu, Xiaoxue Xu, Xiao Zhong, and Feiyan Gong. 2023. "Melamine–Urea–Formaldehyde Resin Adhesive Modified with Recycling Lignin: Preparation, Structures and Properties" Forests 14, no. 8: 1625. https://doi.org/10.3390/f14081625
APA StyleLi, D., Yu, L., Li, L., Liang, J., Wu, Z., Xu, X., Zhong, X., & Gong, F. (2023). Melamine–Urea–Formaldehyde Resin Adhesive Modified with Recycling Lignin: Preparation, Structures and Properties. Forests, 14(8), 1625. https://doi.org/10.3390/f14081625







