Abstract
To prepare and manage urban greenspace for a hotter future, we must select trees that can tolerate or acclimate to high temperatures. Here, we compared tolerance and acclimation to high temperatures among nine urban tree species in Japan. Saplings were exposed to heat from different times (early and mid-summer) during the growing season in a greenhouse. With the exception of Ginko, heating in early summer did not affect whole-tree health, suggesting that most urban tree species may be able to acclimate to higher temperatures during the early growing season. However, continued exposure to higher temperatures, as well as heating from mid-summer, had negative effects on tree health, leading to wilting/browning, especially for evergreen broadleaved species whose leaves mature slowly. Cornus florida, Styrax japonicus and Morella rubra were the most vulnerable to heating, such that all heated saplings had died by the end of summer. At the leaf level, leaf maturation of the deciduous species and Morella was negatively affected by heating, whereas that of Eurya emarginata and Euonymus japonicas were not affected. These two species also showed heat tolerance, having a higher T50 (temperature where leaf quantum yield declined to 50% of maximum value due to heat stress) compared to other species, as well as heat acclimation, where T50 was higher for the heated saplings compared to the control. Our results indicate that, while some species that cannot recover from heat damage in early summer could die, others can acclimate to sustained high temperatures, as well as to late summer heat. As heatwaves are expected to become more frequent and severe due to global warming, tree species need to be screened individually to assess their ability to tolerate or acclimate to high temperature.
1. Introduction
The current progression of global climate change is faster than any previous climate shift in Earth’s history [1,2]. Impacts of global warming on trees include growth reduction and mortality due to drought [3,4,5], as well as range shifts, local extinctions of species [6] and changes in leafing and flowering phenology [7,8] due to increasing temperatures. We can expect that most tree species will be unable to migrate or adapt quickly enough to climate change. Species’ persistence, therefore, depend on the capacity of individuals to tolerate or acclimate to extreme environmental conditions, such as heatwaves and droughts, and survive [6].
Urban greenspace mitigates the negative effects of global warming on our society by buffering heat island effects and controlling stormwater runoff [9,10]. Such beneficial functions will be at risk if urban greenspace is not managed sustainably and tree health not maintained [11]. As part of the social adaptation to climate change, it is imperative that we prepare urban greenspaces for higher temperatures due to global warming [12,13]. For example, plant species that are susceptible to high temperatures need to be replaced by more tolerant species [14,15]. Species that originate in warmer regions, such as tropical and sub-tropical species, however, tend to have a low tolerance for temperature fluctuation [16,17] and may not be able to survive in temperate climates. Careful assessment of urban tree species’ tolerance and acclimation to high temperatures is needed to prepare urban greenspace for a hotter future [18].
Several observational studies have found adaptation and acclimation of plant traits to high temperatures at global as well as at regional scales, e.g., [19,20,21], including the urban-rural environmental gradient, e.g., [22]. On the other hand, responses to experimental warming can be variable among species [23]. Some studies found a decline or no change in photosynthetic rates in response to leaf warming [24,25,26], while others report partial to full acclimation of photosynthesis as well as respiration in response to warming, e.g., [25,27,28]. Reponses to heat can also vary between sun and shade leaves of the same species, with sun leaves having higher tolerance than shade leaves in some, but not all, species [29]. Under high temperatures, leaf photosynthetic rate decreases due to stomatal closure, as well as to decreasing rates of enzymatic reactions. Extreme heat can cause irreversible, biochemical damage to proteins comprising photosynthetic enzymes and membranes [20,30,31,32].
In urban areas of the warm-temperate zone, surface temperatures near the pavement can exceed 50 °C in summer [9,11]. When exposed to high temperatures, plants maintain leaf temperature by transpirational cooling or thermoregulation [33]. Urban trees are often water-limited, and increasing transpiration due to heat could exacerbate water stress [34]. Stomatal closure to avoid water stress, on the other hand, could cause overheating in the short term due to a lack of thermoregulation [35], or carbon starvation and death in the long term [36,37]. Heat stress can have negative effects on both leaf photosynthesis and respiration [38,39,40]. Both critical and optimal temperatures for photosynthesis and respiration tend to increase with decreasing latitude from cool-temperate to tropical regions, suggesting that species from warm regions are more adapted to and tolerant of heat stress [20,21,41]. However, thermal safety margins, defined as the difference between the critical temperature and expected temperature rise due to global warming, are narrowest at mid-latitude regions (20–50°), suggesting that species in temperate regions, where most urban areas are located, are at the greatest risk of damage due to extreme heatwave events [41].
Trees may acclimate to extreme heat by sustaining transpirational cooling and/or increasing leaf thermal tolerance [42]. This response could occur at different times during the growing season, and the effects of heat stress could be variable depending on the time of year relative to the leaf maturation and leaf habits of individual species. To investigate this question, we compared the effects of high temperatures on leaf maturation and photosynthesis among nine commonly used urban tree species in Japan. The effects of heat in relation to time of growing season and duration of heat exposure were assessed by heating potted saplings in a greenhouse from different times during the growing season (early and mid-summer). The effects of heat stress were assessed at the whole-tree and leaf levels to compare tolerance and acclimation potential among the nine species.
2. Materials and Methods
Four-year-old, potted saplings of five deciduous (Zelkova serrata (Thunb.), Ginko biloba L., Acer buergerianum Miq., Cornus florida L., Makino, Styrax japonicas Siebold et Zucc.) and four evergreen trees (Morella rubra Lour., Cinnamomum camphora (L.) J. Presl., Euonymus japonicas Thunb., and Eurya emarginata (Thunb.) Makino) were used in the experiment (Table 1). All saplings were planted in pots (height × diameter = 186 × 205 mm) filled with 5:3:2 mixtures of red ball earth, Kanuma pumice, and manure. All saplings were watered for 15 min at 5:00 a.m. and 7:00 p.m. each day.

Table 1.
Sizes of the potted saplings of each species used in the study. Mean ± one s.d. of all saplings are shown (n = 18 for each species). These were then sequentially moved to the heated area of the greenhouse, on 7 June and 9 July 2023, until the end of the experiment.
All saplings (18 of each species) were initially grown together under controlled conditions in the greenhouse at the Tsukuba Research Institute, Sumitomo Forestry Co. Ltd., in Tsukuba City, Ibaraki Prefecture (36.11° N, 140.02° E), from 3 April 2023. To observe effects of high temperature starting from different times during the growing season, randomly selected saplings of each species were moved to a heated area of the green house, where an electric heater (Chrester HEAT-R-101BSH, Comfort Co., Ltd., Tokyo, Japan) was used to maintain a higher temperature (hereafter: “heated” treatment). To ensure that all saplings were exposed equally to heating, the position of each pot relative to the heater were rotated periodically. To simulate heat exposure from early and mid-summer, six saplings were moved to the heated area on 7 June and 9 July, respectively, until the end of the experiment (14 September 2023). Mean and maximum temperatures during the experiment were 2 and 10 °C higher in the heated area compared to the control (Table 2).

Table 2.
Mean, maximum, minimum temperatures and relative humidity (RH) for control and heated saplings of nine urban tree species during the experiment.
2.1. Assessment of Sapling Appearance/Health
We assessed the appearance/health of each sapling during the experiment by visually scoring the percentage of leaf wilting/browning. On each measurement date, two observers visually scored each sapling in 10% increments of wilting/browning relative to its initial condition (0%), where −100% indicates a dead sapling (Figure 1).

Figure 1.
Potted saplings of Ginko used in the experiment showing levels of wilting/browning of leaves.
2.2. Measurement of Leaf Traits
To infer the effects of heat on leaf maturation, we measured leaf mass per area (LMA, g m−2) four times during the experiment (4 June, 4 July, 6 August and 14 September 2021). We randomly selected ten current-year leaves produced before June from each sapling. Leaf disks (8 mm in diameter) were punched out from each leaf and dried in a convection oven (DK 600, Yamato Kagaku, Tokyo, Japan) at 65 °C for 48 h before the measurement of mass.
To assess heat tolerance, we measured the quantum yield (QY) of dark-adapted leaves at temperatures ranging from 20 to 60 °C. Before each measurement, the saplings were watered and allowed to rehydrate in darkness overnight. Under complete darkness, we took leaf discs (15 mm in diameter) from one randomly selected, current-year leaf of each sapling. For each species, four leaf discs taken from different saplings were sealed in plastic bags with a moist cloth to keep humidity within the bag at equilibrium with leaf moisture. The sealed bags were immersed for 30 min in water heated to a set temperature (T) in a water bath consisting of a sous vide cooker (Felio F9575, Fujisho Co., Tokyo, Japan) and a 3L molybdenum pot. The bags were then removed from the water and cooled to room temperature for 15 min before measuring QY using a FluorPen FP110 (Photo System Instruments, Drásov, Czech Republic). QY is a fluorometric measurement of the maximum quantum yield of photosystem II, also referred to as the maximum–minimum chlorophyll fluorescence relative to maximum fluorescence (Fv/Fm). The amount of time for heating and cooling were based on a previous study by Hara et al. [43], so that we could determine if irreversible change had occurred. The heat-tolerance measurements were conducted on 3 June, 5−6 July, 6−7 August and 7−10 September 2021.
2.3. Statistical Analyses
For each species, a pattern of change in the LMA was assessed by comparing mean values to those of the previous measurement in a t-test. The LMA of the heated saplings were compared to that of the control in a t-test. To assess the leaf thermal tolerance of each species, measurements of QY from the four leaf disks were plotted in relation to T. We fit a logistic curve to the relationship using a non-linear least-squares regression to obtain the heat tolerance curve for each species:
where T50 is the temperature and b is the slope at the inflection point where QY is 50% of the initial maximum value, a. The 95% confidence intervals for the parameter estimates were obtained using the drc package in R (ver. 3.5.3, R Development Core Team).
Estimates of T50 in Equation (1) were considered significantly different between the heated and control saplings on the same measurement date, and between consecutive measurements if confidence intervals did not overlap. We chose this test because our sample sizes are limited, and it is a more conservative test than comparing non-linear regressions between pooled vs. individual regressions using dummy variables. For a given species, a significant difference in T50 between heated and control saplings was interpreted as heat acclimation.
3. Results
3.1. Sapling Health
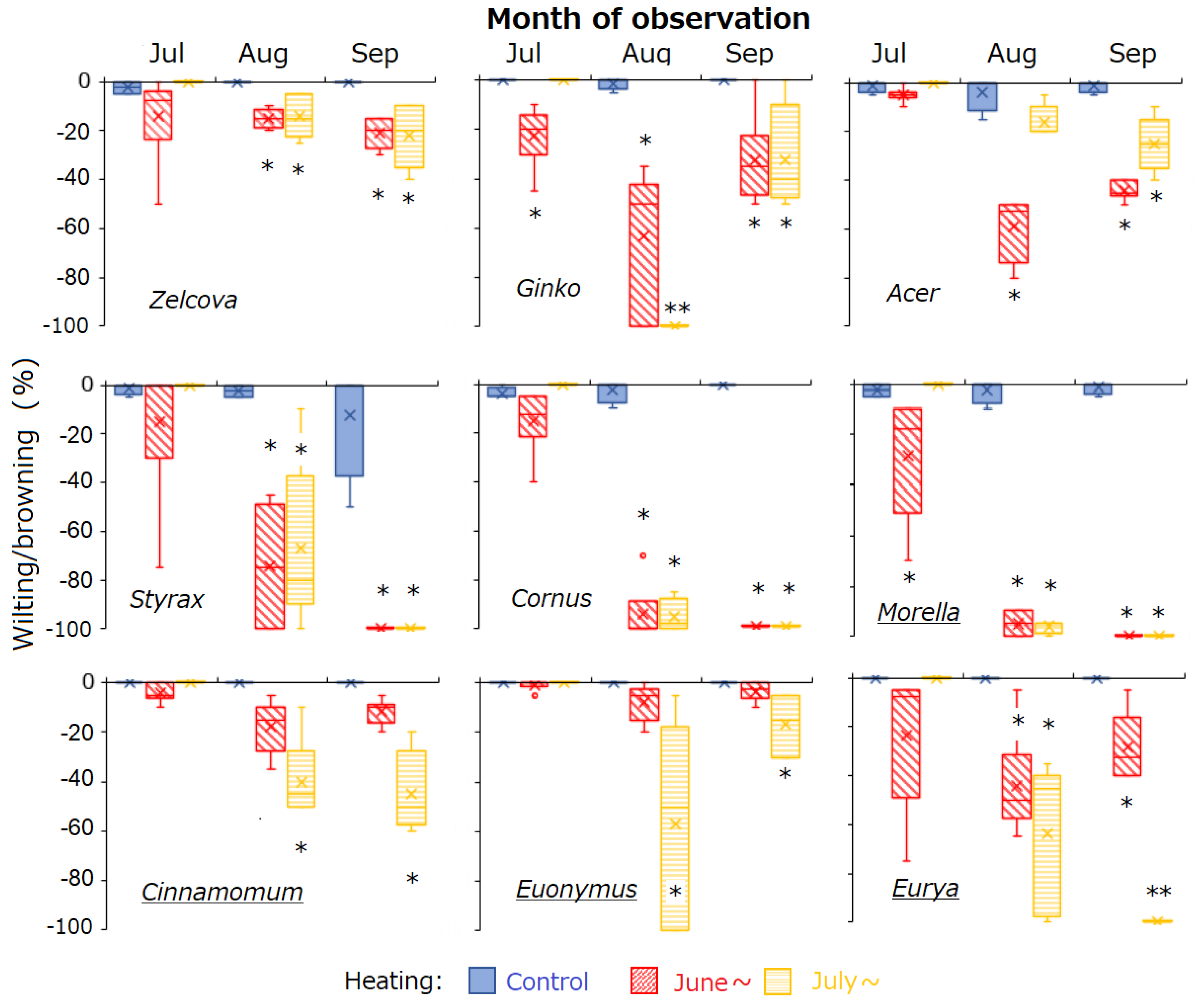
On 3 June, the initial measurement, all saplings were healthy (0% wilting/browning). Subsequently, all control saplings survived until the end of the experiment, whereas the heated saplings were negatively affected by heating. Among the nine species, Zelcova, Cinnamomum and Euonymus were the least affected by heating (Figure 2). In July, negative effects of heating from early summer were observed for Ginko and Morella, whose saplings heated from June showed more wilting/browning than the control. From August to September, Acer, Ginko and Eurya saplings heated from June recovered by producing new leaves, while Cornus, Styrax and Morella saplings continued to decline. For most species, heating from mid-summer (July) had similar effects on sapling health as heating from early summer. For Acer, saplings heated from July showed less wilting/browning than those heated from June, whereas for Cinnamomum, Euonymus and Eurya, effects of mid-summer heating were more severe. By August, wilting was so severe for Cornus, Styrax and Morella such that there were not enough leaves of heated saplings remaining for measurement of LMA and T50 and, by September, all heated individuals of these three species had died (wilting/browning = −100%).
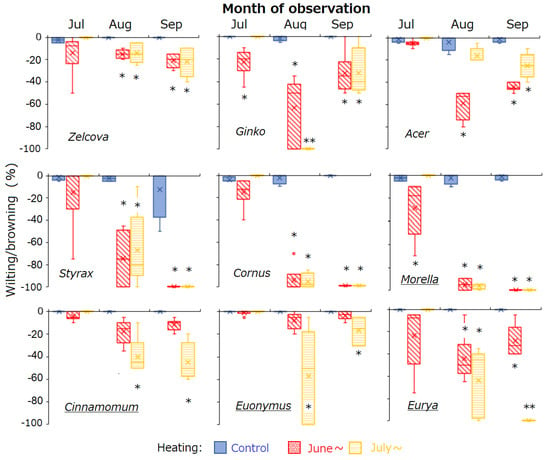
Figure 2.
Box–whisker plots showing seasonal change in wilting/browning of saplings of the nine urban tree species. Boxes and lines indicate the upper and lower quartiles and the median. Error bars indicate the upper and lower second quartiles. Bar colors indicate the control saplings and saplings heated from 7 June and 9 July 2023. Asterisks indicate significant difference relative to control (p < 0.05). Underlined species names indicate evergreen trees.
3.2. Leaf Maturation
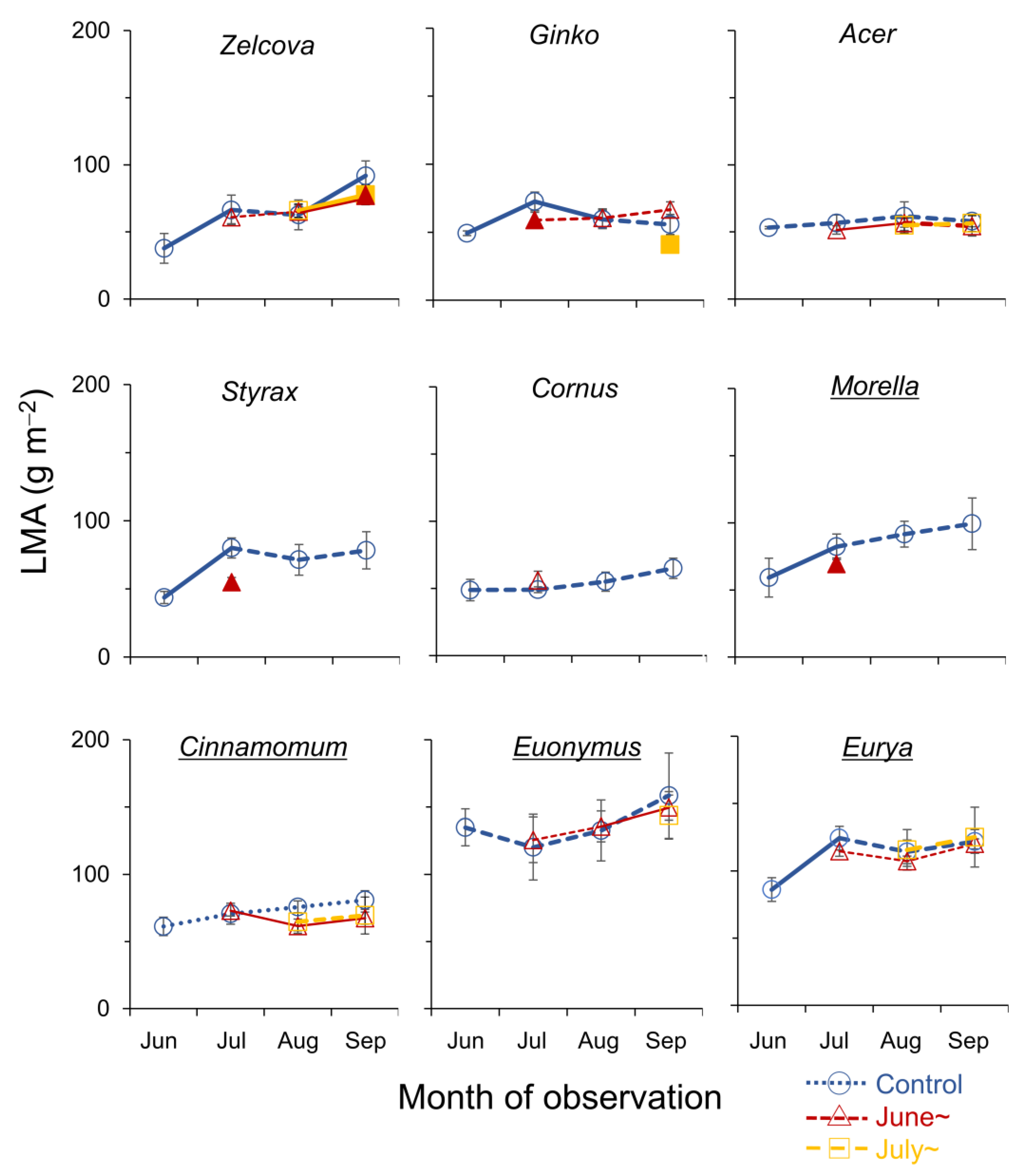
The LMA increased from June to July in five of the nine species reflecting leaf development (Figure 3). In July, the LMA of Styrax, Ginko and Morella saplings heated from June was smaller than the control, suggesting negative effects of heating from early summer on leaf maturation. On the other hand, for Cornus, Acer, Cinnamomum and Euonymus, the LMA did not change from June to September, suggesting that the leaves were fully developed by June. For these species, the LMA was not affected by heating, albeit for the surviving leaves for species with significant wilting/browning (see Figure 2). In August, no differences in the LMA between heated and control saplings were observed. In September, the LMA of Zelcova and Ginko saplings heated from July was smaller than the control. For Zelcova, the LMA of the control leaves had increased from August to September, suggesting negative effects of heating from mid-summer on leaf maturation in this species.
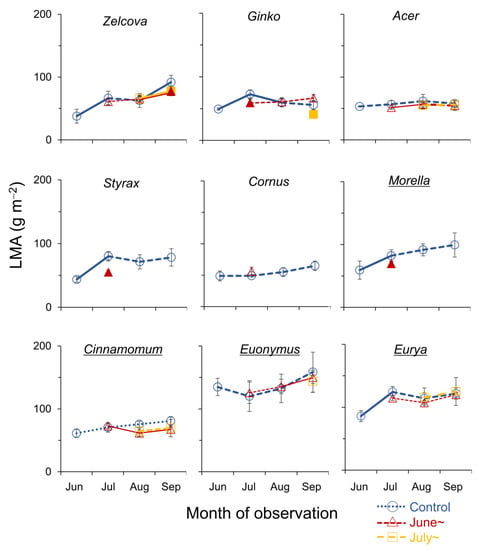
Figure 3.
Seasonal change in leaf mass per area (LMA, g m−2) of current-year leaves of the nine urban tree species. Symbols indicate control saplings (O), saplings heated from June (∆), and from July (□). Significant increase/decrease with season is indicated by solid lines (p < 0.05). Filled symbols of the heated saplings indicate significant difference from control (p < 0.05). Underlined species names indicate evergreen trees.
3.3. Heat Tolerance and Acclimation
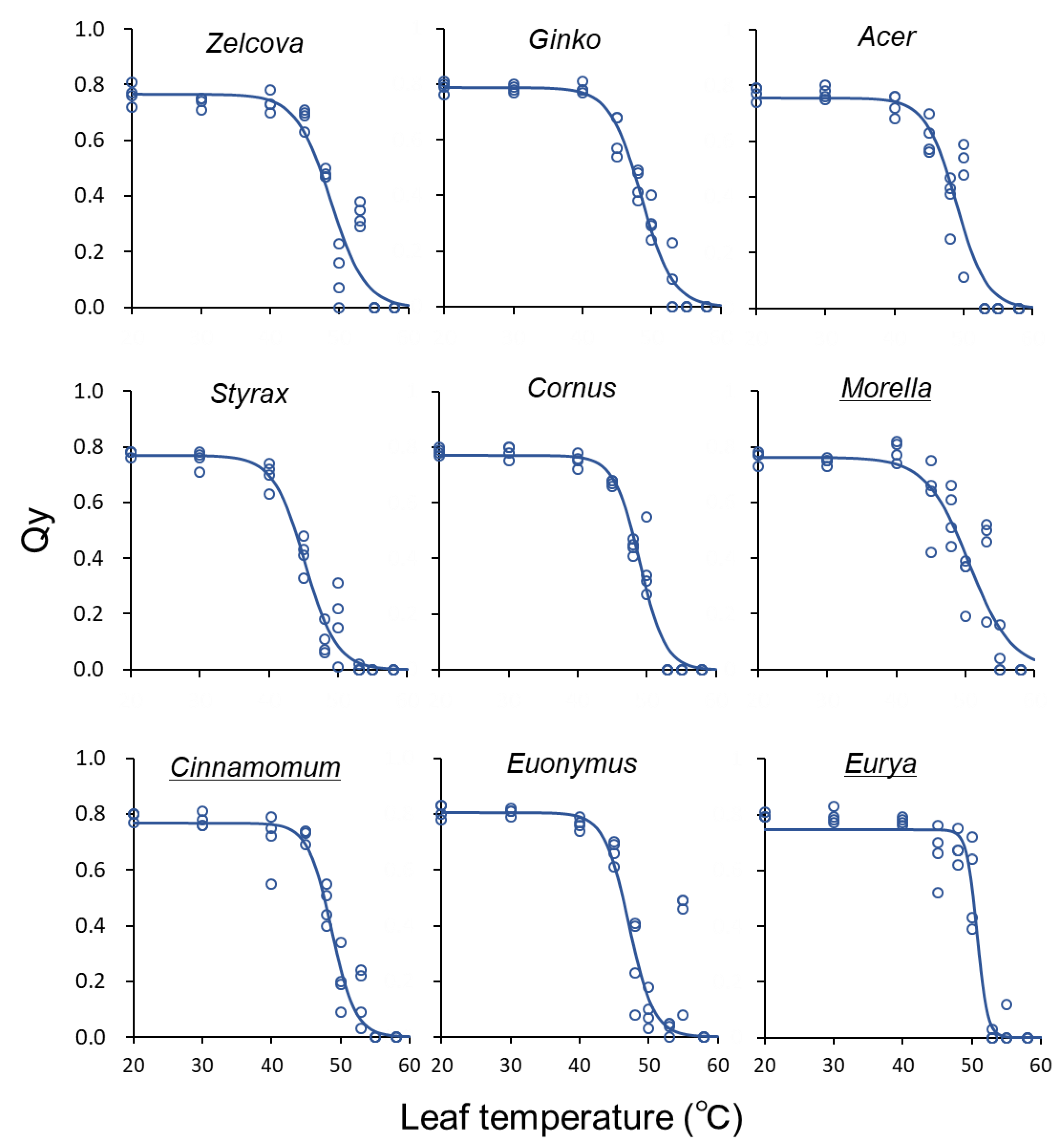
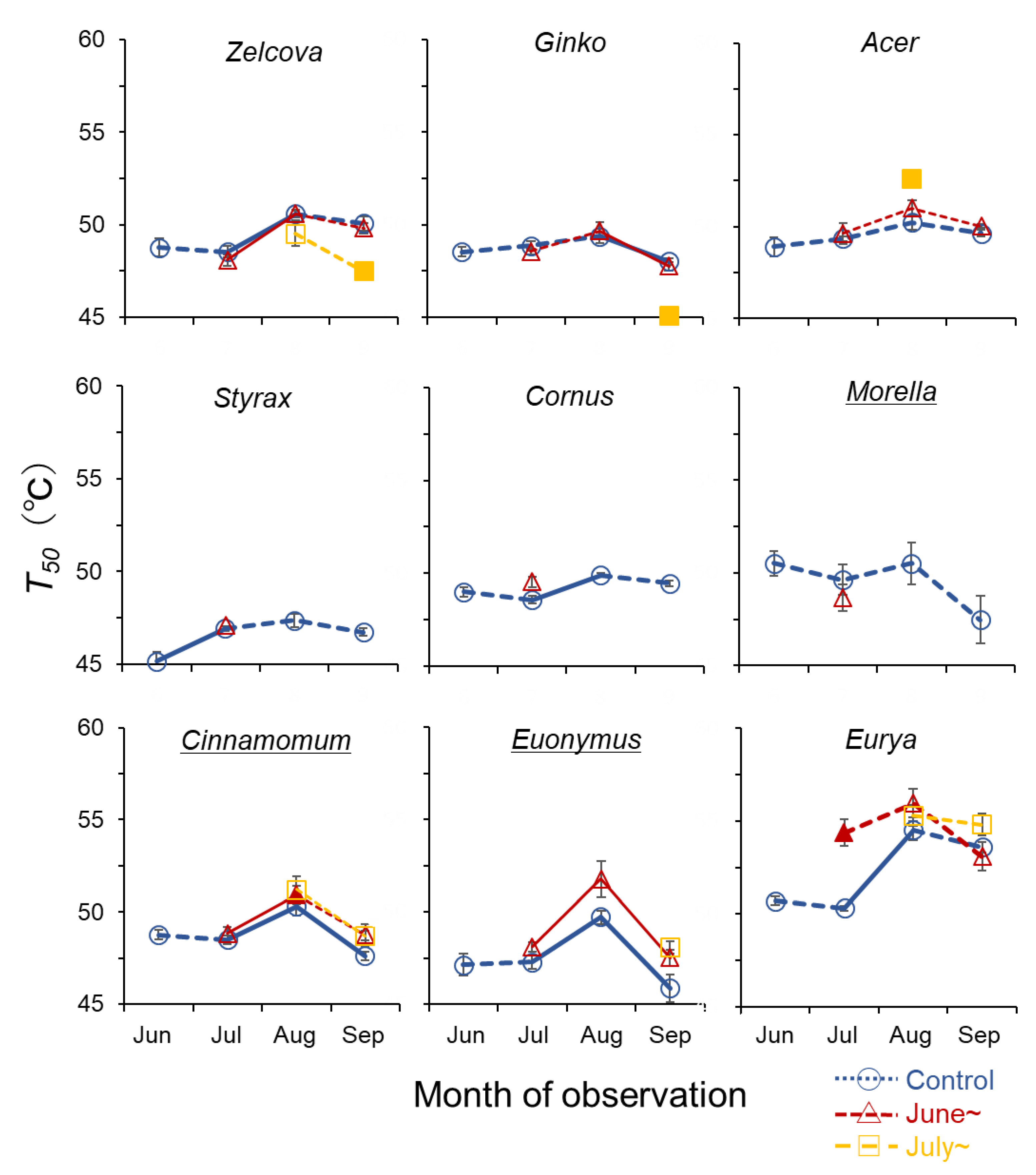
The QY of the deciduous species declined gradually between 40–50 °C, whereas for the evergreen species, QY tended to be sustained at higher temperatures and then decline rapidly (Figure 4). The highest T50 was observed for Eurya in August (54.5–55.3 °C), indicating a high heat tolerance for this species (Figure 5). For all species, T50 showed an increasing trend from June to August, after which it decreased in September following the seasonal temperature trend. The greatest increase was observed for Eurya, where T50 of the control saplings increased by more than 4 °C from July to August, indicating high acclimation potential to seasonal temperature increase. In July, the T50 of Eurya saplings heated from June was higher than the control, indicating a high acclimation potential to heating from early summer. In August, T50 of Acer saplings heated from July was higher than the control, suggesting acclimation to heating from mid-summer. In September, the T50 of Zelcova and Ginko saplings heated from July was lower than the control, suggesting negative effects of heating from mid-summer. Recall that for these two species, the LMA of saplings heated from July was also smaller than the control in September.
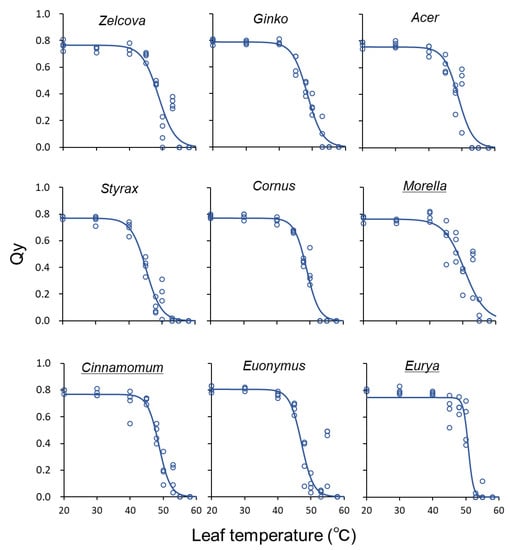
Figure 4.
Quantum yield (Qy) of current-year leaves in relation to leaf temperature for the nine urban tree species in June, before heating began. The temperature at the inflection point of the logistic regression curve is T50. Underlined species names indicate evergreen trees.
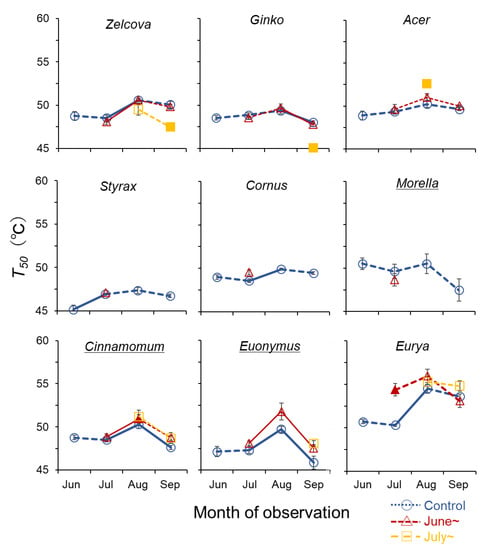
Figure 5.
Seasonal change in temperature at 50% loss of quantum yield (T50) of current-year leaves of the nine urban tree species. Symbols indicate control saplings (O), saplings heated from June (∆), and from July (□). Significant increase/decrease with season is indicated by solid lines (p < 0.05). Filled symbols of the heated saplings indicate significant difference from control (p < 0.05). Underlined species names indicate evergreen trees.
4. Discussion
Our results indicated that response of urban tree species to heating can vary depending on the species and leaf habit (deciduous/evergreen), as well as the timing and duration of high temperatures in relation to seasonal leaf development. While some species could not recover from damage due to heating from early summer and died, others were relatively less affected or subsequently recovered. We found that, with the exception of Ginko, most of the study species showed no immediate effects of heating from June, suggesting that initial symptoms of heat stress in early summer may be subtle. However, continued exposure to high temperature, as well as heating from July, had significant negative effects on tree appearance/health, leading to wilting/browning and, ultimately, to mortality in species such as Styrax, Cornus and Morella. Such species may need to be replaced by more tolerant or resilient species, such as Zelcova, Cinnamomum, Euonymus or Ginko, Acer, Eurya, respectively. In addition, for Cinnamomum, Euonymus and Eurya, heating from July had more severe effects on sapling health, while for Acer and other deciduous species that survived the heating treatment, the effects of mid-summer heating were less severe or similar to that of heating from early summer. This suggests that evergreen species from warm-temperate regions are not necessarily more heat-tolerant. Evergreen broadleaved species with slow leaf maturation require multiple growing seasons to acclimate to environmental change [44]. Although the LMA of the evergreen species in this study did not change after July, their leaves may still be developing physiologically.
Negative effects of heating in early summer on LMA were observed only for species whose LMA was increasing in June (Ginko, Styrax, Morella). This suggested that exposure to high temperatures from early summer may affect leaf maturation by limiting physiological functions and/or consuming photosynthate. Metabolic costs involved in increasing leaf-level heat tolerance, (e.g., the production of isoprenes, heat-stress proteins, etc., the strengthening of thylakoid membranes, and the regeneration of rubisco) may result in a tradeoff with leaf growth [45,46]. In contrast, species whose LMA was stable after June were not affected by subsequent heating. Note that this applies to the surviving leaves, because most species showed significant wilting/browning at the whole-tree level.
Evergreen species showed higher heat tolerance by sustaining photosynthetic capacity (QY) at higher temperatures than the deciduous species. The T50 estimates were higher than 50 °C for all four evergreen species. Three of the evergreen species (Cinnamomum, Euonymus, Eurya) also showed heat acclimation, such that T50 was 1–4 °C higher for the heated saplings compared to the control. In our experiment, heating increased mean temperature by 3 °C and maximum temperatures by 7 °C compared to the control. Our results indicate Euonymus and Eurya are able to acclimate to such high temperatures. Although some species maintain photosynthetic activity to temperatures as high as 40 °C [47], in many plants, irreversible biochemical damage occurs above 55 °C [46]. In this study, T50 values higher than 55 °C was observed only for Eurya saplings heated from June, suggesting that this species has the highest heat tolerance among the nine species examined. Eurya also showed the greatest acclimation both in response to seasonal temperature increases and to heating.
Global scale studies have found species’ heat tolerance to increase with decreasing latitude from temperate to tropical regions [21]. However, T50 values around 50–52 °C have been found for boreal conifers [48], as well as for tropical broadleaved trees, e.g., [29,49], suggesting that heat tolerance can be highly variable among species, regardless of their origin. Such variation among species in their response to heating may explain why previous studies of leaf warming produced such mixed results [24,25,26]. Up-regulation of photosynthesis under high temperature incurs higher overall metabolism [40]. On the other hand, species that respond to high temperature by down-regulating photosynthesis could sustain positive carbon balance by also down-regulating leaf respiration to maintain a low metabolism [50,51,52]. Species which realize high photosynthetic rates by increasing stomatal conductance, hence high transpirational cooling, could not survive if high temperatures co-occur with water deficits. Because physiological responses to heat vary among species, to predict more accurately how trees will respond to climate change, which involves not only higher temperatures but changes in precipitation pattern, we must assess the physiological responses of individual species’ leaf photosynthesis and respiration, as well as stomatal control and thermoregulation to high temperatures combined with water stress [53].
5. Conclusions
Heatwaves are becoming more frequent and severe worldwide, and could have adverse effects on tree health in the near future [54]. In urban areas, urban heat island effects are expected to become more extreme [55,56]. Social adaptation to climate change requires that we prepare urban greenspaces for higher temperatures due to global warming [18]. This involves long-term management and planning at various spatial scales, from replanting individual trees to redesigning entire cityscapes. The solution may not be as simple as selecting species from warmer regions for planting in hot urban environments. Our results imply that, although evergreen broadleaved species originating in warm-temperate to sub-tropical climates are generally more tolerant of high temperature than deciduous species, there are exceptions, such as Morella in this study. We also found that evergreen broadleaved species with slow leaf maturation may be more vulnerable to heatwaves in mid-summer and may require multiple growing seasons to acclimate to environmental change. Previous studies have found tropical species have narrower temperature ranges for physiological functioning [16], as well as lower plasticity of functional traits [22], suggesting lower acclimation potential to environmental fluctuation [57]. Kullberg et al. [58] found no evidence for temperature acclimation among six subtropical tree species planted along an urban temperature gradient in Miami. This suggests that saplings of evergreen broadleaved species may need to be raised under high temperatures over multiple growing seasons before being planted in hot urban environments. Proper management, such as sufficient watering, is also necessary for acclimation of trees to hot urban environments, because stomatal conductance controls acclimation of the thermal optimum for photosynthesis [58]. Watering could also mitigate the negative effects of heat stress, as water availability can increase the thermal safety margins of leaf physiological function via thermoregulation [59]. We contend that, in order to realize climate change adaptation of urban greenspaces, species, regardless of their origin, need to be screened individually to predict their tolerance and acclimation potentials to predicted future growing conditions. Our methods and results provide a scientific basis and methodology for assessing the tolerance and acclimation potential of urban tree species to high temperatures at the individual and leaf levels.
Author Contributions
H.R.I. and S.I. conceived and designed the study. S.I. prepared the plant material and N.O. and S.I. conducted the experiments. H.R.I. wrote the paper, which is based on N.O.’s master’s thesis written in Japanese. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from Sumitomo Forestry Inc. to Kobe University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available in a database at Kobe University.
Acknowledgments
We thank H. Ishii, M. Hioki, and the staff of Sumitomo Forestry Co., Ltd. for assistance with the experiments, and K. Kuroda, W. Azuma and members of the Laboratory of Forest Resources, Kobe Univ. for guidance and advice during research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, Rhythms, and Aberrations in Global Climate 65 Ma to Present. Science 2001, 292, 686–693. [Google Scholar] [CrossRef]
- Visser, M.E. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc. R. Soc. B Biol. Sci. 2008, 275, 649–659. [Google Scholar]
- Williams, A.P.; Allen, C.D.; Macalady, A.K.; Griffin, D.; Woodhouse, C.A.; Meko, D.M.; Swetnam, T.W.; Rauscher, S.A.; Seager, R.; Grissino-Mayer, H.D.; et al. Temperature as a potent driver of regional forest drought stress and tree mortality. Nature 2013, 3, 292–297. [Google Scholar] [CrossRef]
- McDowell, N.; Allen, C.D. Darcy’s law predicts widespread forest mortality under climate warming. Nat. Clim. Chang. 2015, 5, 669–672. [Google Scholar] [CrossRef]
- Carnicer, J.; Coll, M.; Ninyerola, M.; Pons, X.; Sánchez, G.; Peñuelas, J. Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proc. Natl. Acad. Sci. USA 2011, 108, 1474–1478. [Google Scholar] [CrossRef] [PubMed]
- Jump, A.S.; Penuelas, J. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar]
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Roy, D.B. Altered geographic and temporal variability in phenology in response to climate change. Glob. Ecol. Biogeogr. 2006, 15, 498–504. [Google Scholar] [CrossRef]
- Emmanuel, R.; Loconsole, A. Green infrastructure as an adaptation approach to tackling urban overheating in the Glasgow Clyde Valley Region, UK. Landsc. Urban Plan. 2015, 138, 71–86. [Google Scholar] [CrossRef]
- Caplan, J.S.; Galanti, R.C.; Olshevski, S.; Eisenman, S.W. Water relations of street trees in green infrastructure tree trench systems. Urban For. Urban Green. 2019, 41, 170–178. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Jiang, B.; Wen, Z.; Yang, N.; Li, L. Tree survival and growth are impacted by increased surface temperature on paved land. Landsc. Urban Plan. 2017, 162, 68–79. [Google Scholar] [CrossRef]
- Gill, S.E.; Handley, J.F.; Ennos, A.R.; Pauleit, S. Adapting cities for climate change: The role of the green infrastructure. Built Environ. 2007, 33, 115–133. [Google Scholar] [CrossRef]
- Demuzere, M.; Orru, K.; Heidrich, O.; Olazabal, E.; Geneletti, D.; Orru, H.; Bhave, A.G.; Mittal, N.; Feliu, E.; Faehnle, M. Mitigating and adapting to climate change: Multi-functional and multi-scale assessment of green urban infrastructure. J. Environ. Manag. 2014, 146, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Norton, B.; Bosomworth, K.; Coutts, A.; WIlliams, N.; Livesley, S.; Trundle, A.; Harris, R.; McEvoy, D. Planning for a Cooler Future: Green Infrastructure to Reduce Urban Heat; Victorian Centre for Climate Change Adaptation Research: Melbourne, Australia, 2013. [Google Scholar]
- Nitschke, C.R.; Nichols, S.; Allen, K.; Dobbs, C.; Livesley, S.J.; Baker, P.J.; Lynch, Y. The influence of climate and drought on urban tree growth in southeast Australia and the implications for future growth under climate change. Landsc. Urban Plan. 2017, 167, 275–287. [Google Scholar] [CrossRef]
- Cunningham, S.; Read, J. Comparison of temperate and tropical rainforest tree species: Photosynthetic responses to growth temperature. Oecologia 2002, 133, 112–119. [Google Scholar] [CrossRef]
- Perez, T.M.; Stroud, J.T.; Feeley, K.J. Themal trouble in the tropics. Science 2016, 351, 1392–1393. [Google Scholar] [CrossRef]
- Roy, S.; Davison, A.; Östberg, J. Pragmatic factors outweigh ecosystem service goals in street tree selection and planting in South-East Queensland cities. Urban For. Urban Green. 2017, 21, 166–174. [Google Scholar] [CrossRef]
- Yamaguchi, D.P.; Nakaji, T.; Hiura, T.; Hikosaka, K. Effects ofseasonal change and experimental warming on the temperaturedependence of photosynthesis in the canopy leaves of Quercus serrata. Tree Physiol. 2016, 36, 1283–1295. [Google Scholar] [CrossRef]
- Kumarathunge, D.P.; Medlyn, B.E.; Drake, J.E.; Tjoelker, M.G. Acclimation and adaptation components of the temperature dependence of plant photosynthesis at the global scale. New Phytol. 2019, 222, 768–784. [Google Scholar] [CrossRef]
- Crous, K.Y.; Uddling, J.; De Kauwe, M.G. Temperature responses of photosynthesis and respiration in evergreen trees from boreal to tropical latitudes. New Phytol. 2022, 234, 353–374. [Google Scholar] [CrossRef]
- Esperon-Rodriguez, M.; Rymer, P.D.; Power, S.A.; Challis, A.; Marchin, R.M.; Tjoelker, M.G. Functional adaptations and trait plasticity ofurban trees along a climatic gradient. Urban For. Urban Green. 2020, 54, 126771. [Google Scholar] [CrossRef]
- Carter, K.R.; Wood, T.E.; Reed, S.C.; Schwartz, E.C.; Reinsel, M.B.; Yang, X.; Cavaleri, M.A. Photosynthetic and respiratory acclimation of understory shrubs in response to in situ experimental warming of a wet tropical forest. Front. For. Glob. Chang. 2020, 3, 576320. [Google Scholar] [CrossRef]
- Doughty, C.E. An in situ leaf and branch warming experiment in the Amazon. Biotropica 2011, 43, 658–665. [Google Scholar] [CrossRef]
- Slot, M.; Rey-Sánchez, C.; Gerber, S.; Lichstein, J.W.; Winter, K.; Kitajima, K. Thermal acclimation of leaf respiration of tropical trees and lianas: Response to experimental canopy warming, and consequences for tropical forest carbon balance. Glob. Chang. Biol. 2014, 20, 2915–2926. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.R.; Cavaleri, M.A. Within-canopy experimental leaf warming induces photosynthetic decline instead of acclimation in two northern hardwood species. Front. For. Glob. Chang. 2018, 1, 11. [Google Scholar] [CrossRef]
- Reich, P.B.; Sendall, K.M.; Stefanski, A.; Wei, X.; Rich, R.L.; Montgomery, R.A. Boreal and temperate trees show strong acclimation of respiration to warming. Nature 2016, 531, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, C.A.; Norby, R.J.; Wullschleger, S.D. Acclimation of photosynthesis and respiration to simulated climatic warming in northern and southern populations of Acer saccharum: Laboratory and field evidence. Tree Physiol. 2000, 20, 87–96. [Google Scholar] [CrossRef]
- Slot, M.; Krause, G.H.; Krause, B.; Hernández, G.G.; Winter, K. Photosynthetic heat tolerance of shade and sun leaves of three tropical tree species. Photosynth. Res. 2019, 141, 119–130. [Google Scholar] [CrossRef]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Sage, R.F.; Kubien, D.S. The temperature response of C3 and C4 photosynthesis. Plant, Cell & Environment. Plant Cell Environ. 2007, 30, 1086–1106. [Google Scholar] [PubMed]
- Hikosaka, K.; Ishikawa, K.; Borjigidai, A.; Muller, O.; Onoda, Y. Temperature acclimation of photosynthesis: Mechanisms involved in the changes in temperature dependence of photosynthetic rate. J. Exp. Bot. 2006, 57, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Pallardy, S.G. Physiology of Woody Plants, 3rd ed.; Academic Press: San Diego, CA, USA, 2008; p. 464. [Google Scholar]
- Pataki, D.E.; McCarthy, H.R.; Livtak, E.; Pincetl, S. Transpiration of urban forests in the Los Angeles metropolitan area. Ecol. Appl. 2011, 21, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.C.; Read, J. Foliar temperature tolerance of temperate and tropical evergreen rain forest trees of Australia. Tree Physiol. 2006, 26, 1435–1443. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719. [Google Scholar] [CrossRef] [PubMed]
- Sevanto, S.; McDowell, N.; Dickman, L.T.; Pangle, R.; Pockman, W. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ. 2014, 37, 153–161. [Google Scholar] [CrossRef]
- Slot, M.; Kitajima, K. General patterns of acclimation of leaf respiration to elevated temperatures across biomes and plant types. Oecologia 2015, 177, 885–900. [Google Scholar] [CrossRef]
- Lombardozzi, D.L.; Bonan, G.B.; Smith, N.G.; Dukes, J.S.; Fisher, R.A. Temperature acclimation of photosynthesis and respiration: A key uncertainty in the carbon cycle–climate feedback. Geophys. Res. Lett. 2015, 42, 8624–8631. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar]
- O’Sullivan, O.S.; Heskel, M.A.; Reich, P.B.; Tjoelker, M.G.; Weerasinghe, L.K.; Penillard, A.; Zhu, L.; Egerton, J.J.G.; Bloomfield, K.J.; Creek, D.; et al. Thermal limits of leaf metabolism across biomes. Glob. Chang. Biol. 2017, 23, 209–223. [Google Scholar] [CrossRef]
- Drake, J.E.; Tjoelker, M.G.; Vårhammar, A.; Medlyn, B.E.; Reich, P.B.; Leigh, A.; Pfautsch, S.; Blackman, C.J.; López, R.; Aspinwall, M.J.; et al. Trees tolerate an extreme heatwave via sustained transpirational cooling and increased leaf thermal tolerance. Glob. Chang. Biol. 2018, 24, 2390–2402. [Google Scholar] [CrossRef]
- Hara, C.; Inoue, S.; Ishii, H.R.; Okabe, M.; Nakagaki, M.; Kobayashi, H. Tolerance and acclimation of photosynthesis of nine urban tree species to warmer growing conditions. Trees 2021, 35, 1793–1806. [Google Scholar] [CrossRef]
- Ishii, H.; Ohsugi, Y. Light acclimation potential and carry-over effects vary among three evergreen tree species with contrasting patterns of leaf emergence and maturation. Tree Physiol. 2011, 31, 819–830. [Google Scholar] [CrossRef]
- Law, R.; Crafts-Brandner, S.J. Inhibition and acclimation of photosynthesis to heat stress is closely correlated with activation of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Physiol. 1999, 120, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D. Effects of moderate heat stress on photosynthesis: Importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ. 2005, 28, 269–277. [Google Scholar] [CrossRef]
- Colombo, S.; Timmer, R. Limits of tolerance to high temperatures causing direct and indirect damage to black spruce. Tree Physiol. 1992, 11, 95–104. [Google Scholar] [CrossRef]
- Kunert, N.; Hajek, P.; Hietz, P.; Morris, H.; Rosner, S.; Tholen, D. Summer temperatures reach the thermal tolerance threshold of photosynthetic decline in temperate conifers. Plant Biol. 2022, 24, 1254–1261. [Google Scholar] [CrossRef]
- Krause, G.H.; Winter, K.; Krause, B.; Jahns, P.; Garcia, M.; Aranda, J.; Virgo, A. High-temperature tolerance of a tropical tree, Ficus insipida: Methodological reassessment and climate change considerations. Funct. Plant Biol. 2010, 37, 890–900. [Google Scholar] [CrossRef]
- Araki, M.G.; Gyokusen, K.; Kajimoto, T. Vertical and seasonal variations in temperature responses of leaf respiration in a Chamaecyparis obtusa canopy. Tree Physiol. 2017, 37, 1269–1284. [Google Scholar] [CrossRef]
- Atkin, O.K.; Tjoelker, M.G. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 2003, 8, 343–351. [Google Scholar] [CrossRef]
- Way, D.A.; Yamori, W. Thermal acclimation of photosynthesis: On the importance of adjusting our definitions and accounting for thermal acclimation of respiration. Photosynth. Res. 2014, 119, 89–100. [Google Scholar] [CrossRef]
- Cheesman, A.W.; Winter, K. Growth response and acclimation of CO2 exchange characteristics to elevated temperatures in tropical tree seedlings. J. Exp. Bot. 2013, 64, 3817–3828. [Google Scholar] [CrossRef]
- Millar, C.I.; Stephenson, N.L.; Stephens, S.L. Climate change and forests of the future: Managing in the face of uncertainty. Ecol. Appl. 2007, 17, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Estoque, R.C.; Murayama, Y. Monitoring surface urban heat islandformation in a tropical mountain city using Landsat data (1987–2015). ISPRS J. Photogramm. Remote Sens. 2017, 133, 18–29. [Google Scholar] [CrossRef]
- Youngsteadt, E.; Dale, A.G.; Terando, A.J.; Dunn, R.R.; Frank, S.D. Do cities simulate climate change? A comparison of herbivore response to urban and global warming. Glob. Chang. Biol. 2015, 21, 97–105. [Google Scholar] [CrossRef]
- Crous, K.Y. Plant responses to climate warming: Physiological adjustments and implications for plant functioning in a future, wamer world. Am. J. Bot. 2019, 106, 1049–1051. [Google Scholar] [CrossRef]
- Kullberg, A.T.; Slot, M.; Feeley, K.J. Thermal optimum of photosynthesis is controlled by stomatal conductance and does not acclimate across an urban thermal gradient in six subtropical tree species. Plant Cell Environ. 2023, 46, 831–849. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.M.; Berry, N.; Milner, K.V.; Leigh, A. Water availability influences thermal safety margins for leaves. Funct. Ecol. 2021, 35, 2179–2189. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).