Salt-Stress-Induced Ion Transport Contributes to K+/Na+ Homeostasis in Roots of Ping’ou Hybrid Hazelnut
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials and Hydroponic Culture
2.2. Salt Treatment and Sampling
2.3. Ion Concentration Analysis
2.4. Non-Invasive Micro-Test Technology
2.5. Measurements of Ion Flux under Salt Stress
2.6. Measurements of Ion Flux under Transport Inhibitor (Pharmacological Experiments)
2.7. Statistical Analysis
3. Results
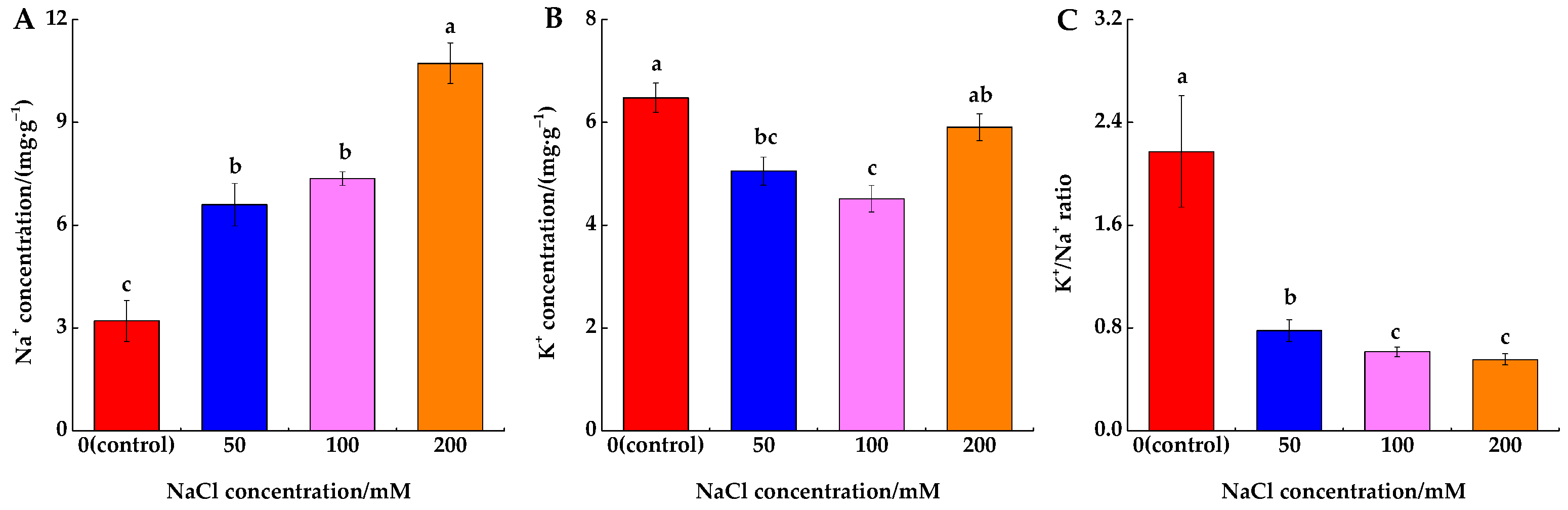
3.1. NaCl-Induced Ion Cncentration
3.2. NaCl-Induced Ion Fluxes
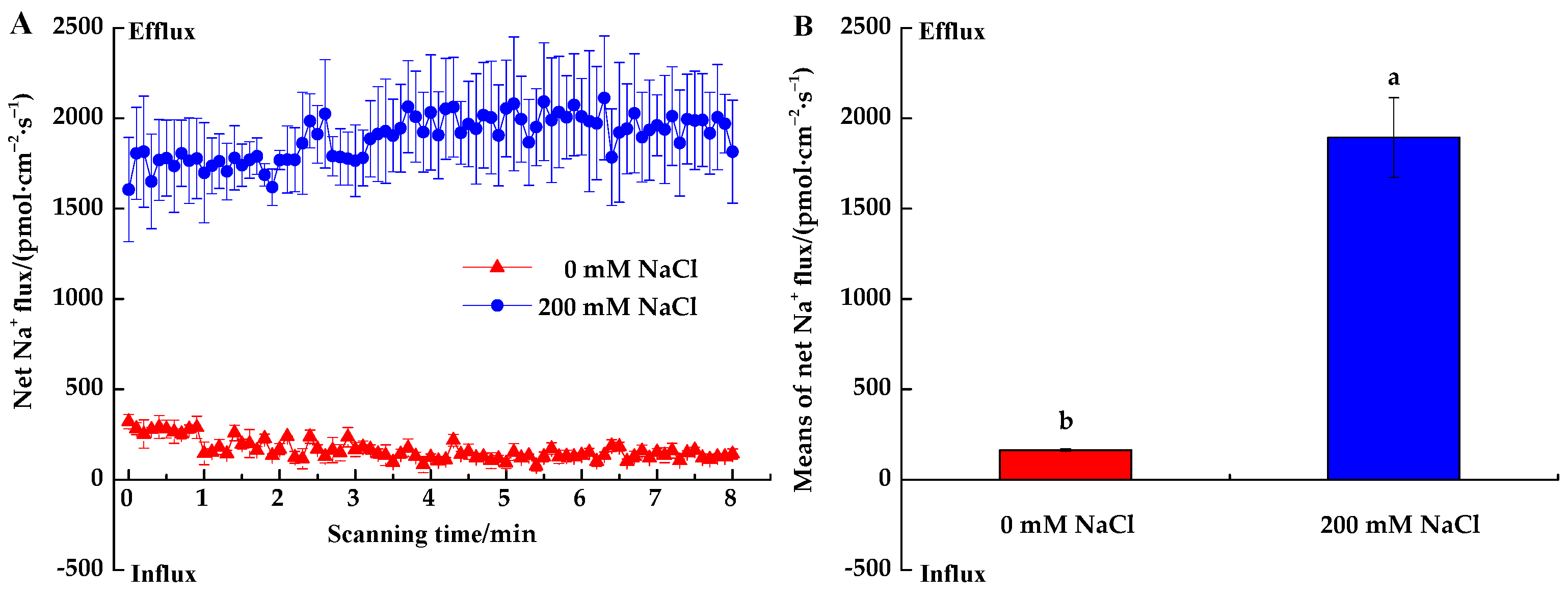
3.2.1. Na+ Fluxes
3.2.2. K+ Fluxes
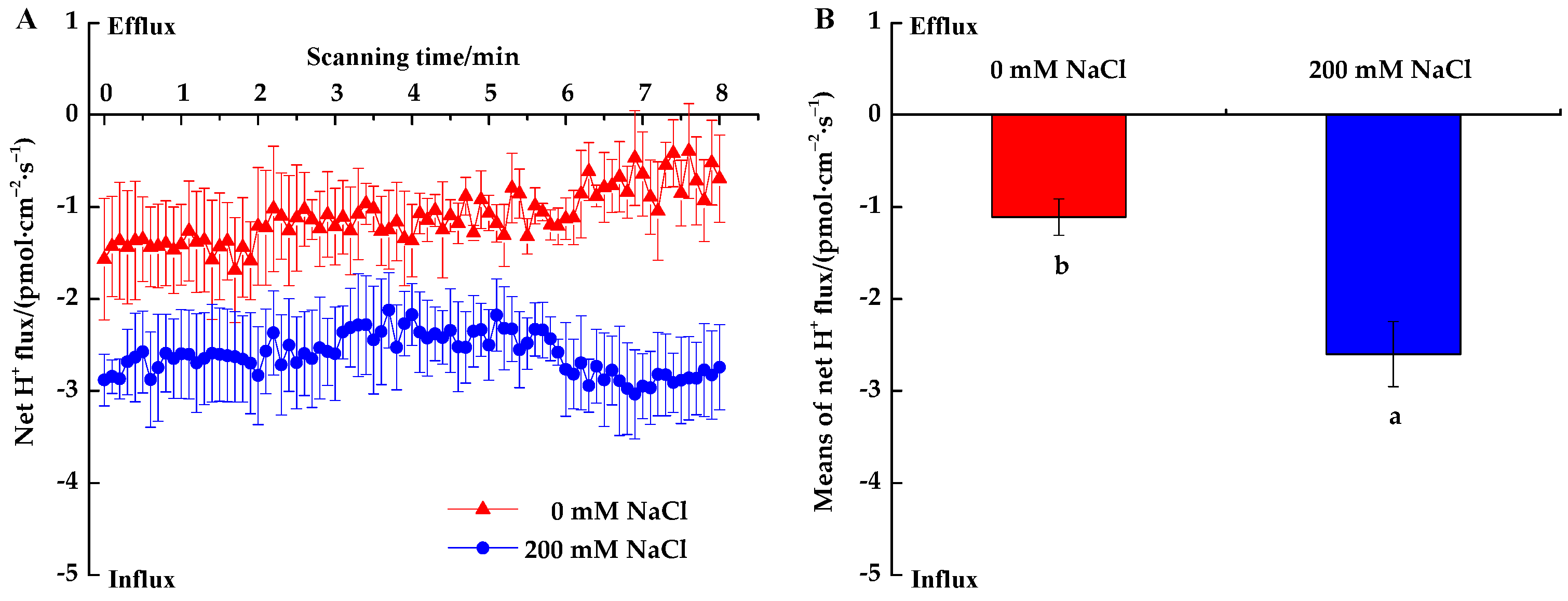
3.2.3. H+ Fluxes
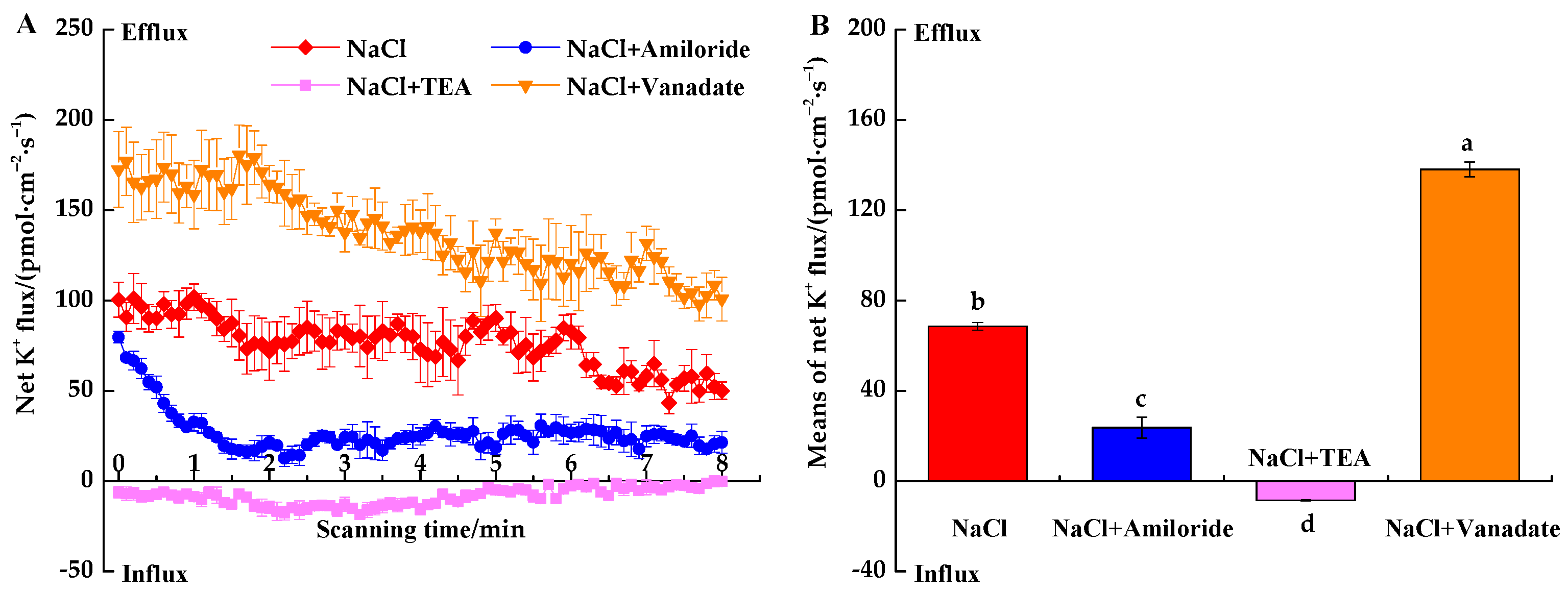
3.3. Effects of Transport Inhibitors on Ion Fluxes under NaCl Stress
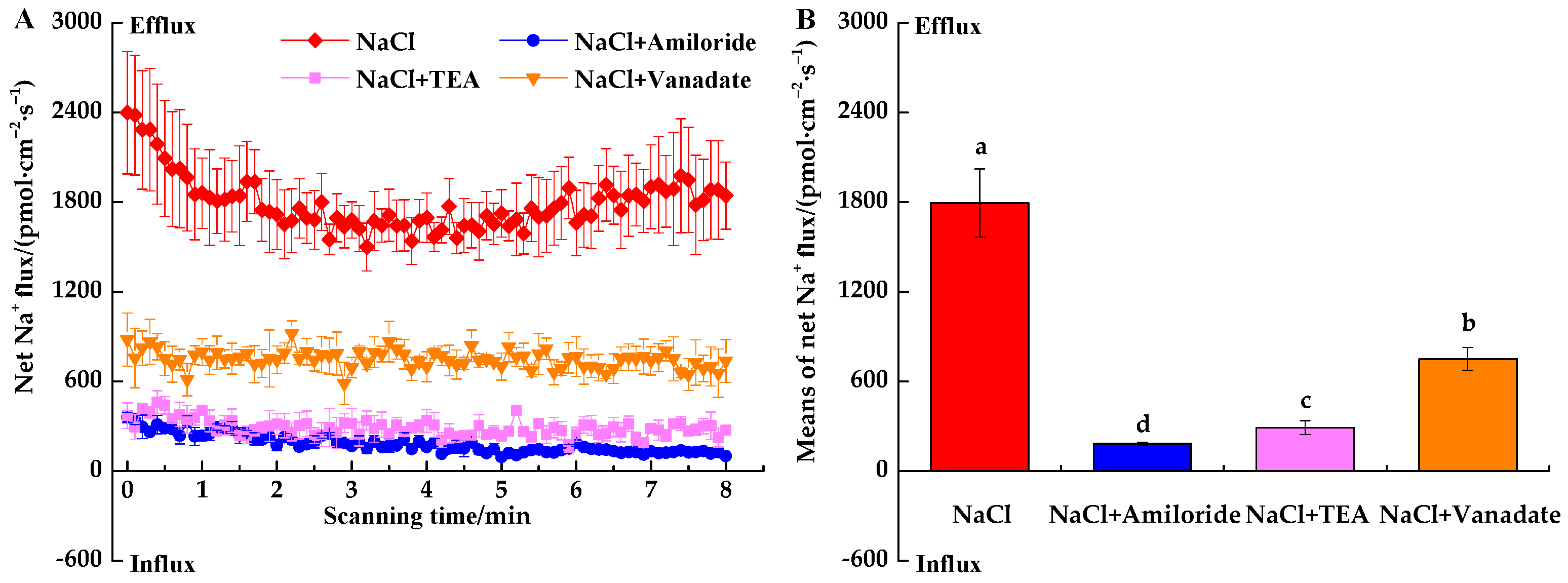
3.3.1. Inhibitor-Induced Na+ Fluxes under NaCl Stress
3.3.2. Inhibitor-Induced K+ Fluxes under NaCl Stress
3.3.3. Inhibitor-Induced H+ Fluxes under NaCl Stress
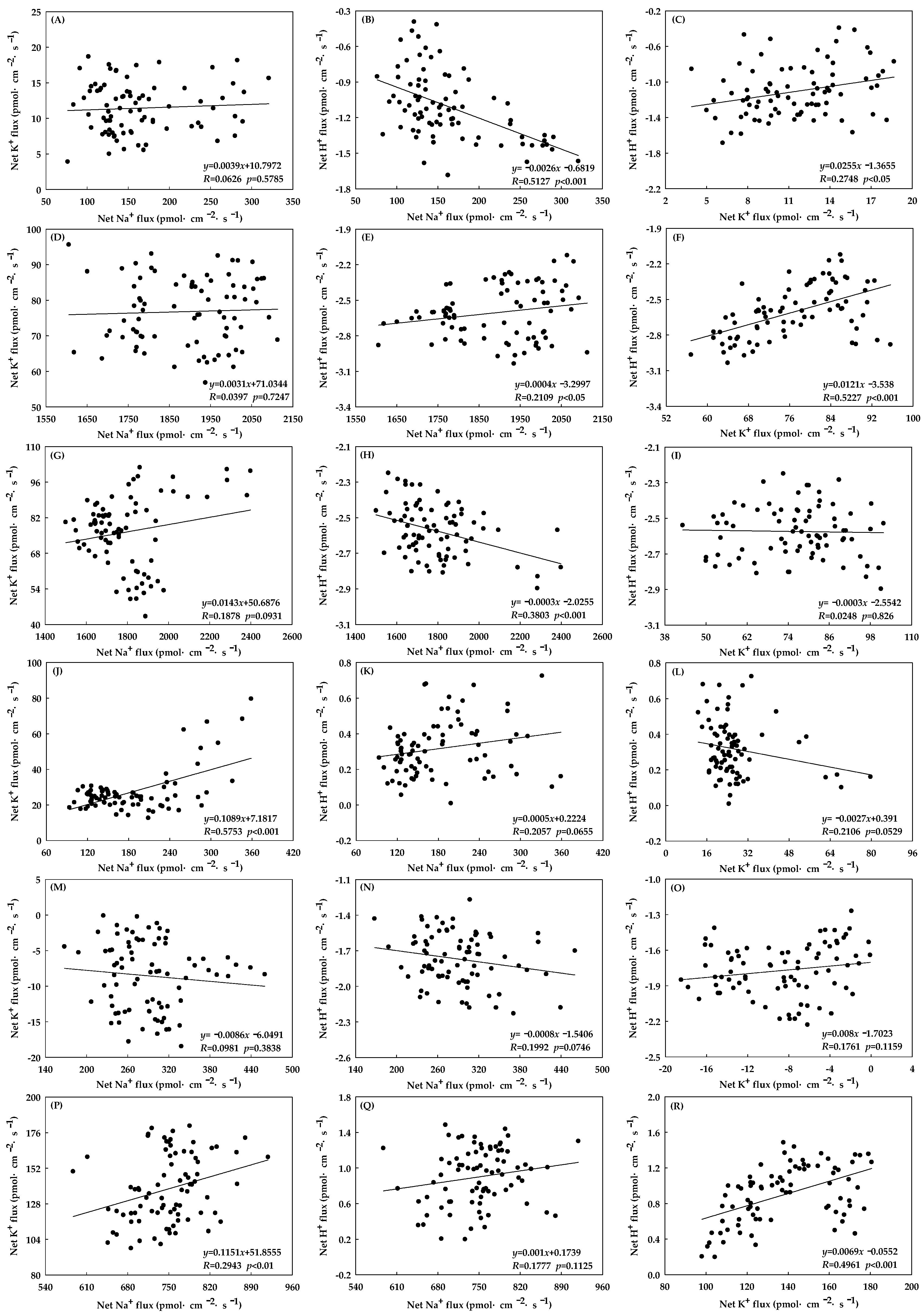
3.4. Relationships among Na+, K+ and H+ Fluxes
4. Discussion
4.1. Salinity Effects on Root Ion Concentration and Ratio
4.2. Salinity Effects on Root Na+, K+ and H+ Fluxes
4.3. Responses of Ion Fluxes to Inhibitors under NaCl Stress
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.Y.; Liu, Y.Q.; Duan, H.R.; Yin, X.X.; Cui, Y.N.; Chai, W.W.; Song, X.; Flowers, T.J.; Wang, S.M. SsHKT1 1 is coordinated with SsSOS1 and SsNHX1 to regulate Na+ homeostasis in Suaeda salsa under saline conditions. Plant Soil 2020, 449, 117–131. [Google Scholar] [CrossRef]
- Tang, X.Q.; Zhang, H.L.; Shabala, S.; Li, H.Y.; Yang, X.Y.; Zhuang, H.X. Tissue tolerance mechanisms conferring salinity tolerance in a halophytic perennial species Nitraria sibirica pall. Tree Physiol. 2021, 41, 1264–1277. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, A.M.; Horie, T. Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Annu. Rev. Plant Biol. 2017, 68, 405–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.Q.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.Z.; Zhang, H.; Song, C.P.; Zhu, J.K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Ponce, K.S.; Meng, L.; Guo, L.; Leng, Y.; Ye, G. Advances in sensing, response and regulation mechanism of salt tolerance in rice. Int. J. Mol. Sci. 2021, 22, 2254. [Google Scholar] [CrossRef]
- Raddatz, N.; de los Ríos, L.M.; Lindahl, M.; Quintero, F.J.; Pardo, J.M. Coordinated transport of nitrate, potassium, and sodium. Front. Plant Sci. 2020, 11, 247. [Google Scholar] [CrossRef] [Green Version]
- EI Mahi, H.; Hormaeche, J.P.; De, L.A.; Villalta, I.; Espartero, J.; Arjona, F.G.; Fernández, J.L.; Bundó, M.; Mendoza, I.; Mieulet, D.; et al. A critical role of sodium flux via the plasma membrane Na+/H+ exchanger SOS1 in the salt tolerance of rice. Plant Physiol. 2019, 180, 1046–1065. [Google Scholar] [CrossRef] [Green Version]
- Demidchik, V.; Maathuis, F.J.M. Physiological roles of nonselective cation channels in plants: From salt stress to signaling and development. New Phytol. 2007, 175, 387–404. [Google Scholar] [PubMed]
- Wu, H.H.; Zhang, X.C.; Giraldo, J.P.; Shabala, S. It is not all about sodium: Revealing tissue specificity and signalling roles of potassium in plant responses to salt stress. Plant Soil 2018, 431, 1–17. [Google Scholar]
- Jegadeeson, V.; Kumari, K.; Pulipati, S.; Parida, A.; Venkataraman, G. Expression of wild rice Porteresia coarctata PcNHX1 antiporter gene (PcNHX1) in tobacco controlled by PcNHX1 promoter (PcNHX1p) confers Na+-specific hypocotyl elongation and stem-specific Na+ accumulation in transgenic tobacco. Plant Physiol. Biochem. 2019, 139, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Shabala, L.; Cuin, T.A.; Newman, I.A.; Shabala, S. Salinity-induced ion flux patterns from the excised roots of Arabidopsis sos mutants. Planta 2005, 222, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Rubio, F.; Nieves-Cordones, M.; Horie, T.; Shabala, S. Doing ‘business as usual’ comes with a cost: Evaluating energy cost of maintaining plant intracellular K+ homeostasis under saline conditions. New Phytol. 2019, 225, 1097–1104. [Google Scholar]
- Shabala, S. Ionic and osmotic components of salt stress specifically modulate net ion fluxes from bean leaf mesophyll. Plant Cell Environ. 2000, 23, 825–837. [Google Scholar]
- Maathuis, F.J.M.; Amtmann, A. K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ ratios. Ann. Bot. 1999, 84, 123–133. [Google Scholar]
- Köster, P.; Wallrad, L.; Edel, K.H.; Faisal, M.; Alatar, A.A.; Kudla, J. The battle of two ions: Ca2+ signalling against Na+ stress. Plant Biol. 2019, 21, 39–48. [Google Scholar]
- Munns, R.; Day, D.A.; Fricke, W.; Watt, M.; Arsova, B.; Barkla, B.J.; Bose, J.; Byrt, C.S.; Chen, Z.H.; Foster, K.Y.; et al. Energy costs of salt tolerance in crop plants. New Phytol. 2020, 225, 1072–1090. [Google Scholar] [CrossRef] [Green Version]
- Shabala, S.; Chen, G.; Chen, Z.H.; Pottosin, I. The energy cost of the tonoplast futile sodium leak. New Phytol. 2019, 225, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Li, N.Y.; Lu, Y.J.; Sun, H.M.; Shen, Z.D.; Jing, X.S.; Zhao, R.; Shen, X.; Chen, S.L. Extracellular ATP, hydrogen peroxide, calcium and nitric oxide mediate root ion fluxes in Bruguiera gymnorrhiza subjected to salt stress. J. Beijing For. Univ. 2014, 36, 16–22. (In Chinese) [Google Scholar]
- Zhu, Z.M.; Mao, G.L.; Xu, X.; Wang, S.; Zheng, R.; Yang, S.J. Effect of salt stress and inhibitor on uptake and transportation of Na+ and K+ in the root of Ningxia Lycium barbarum L. Agric. Res. Arid Areas 2017, 35, 140–145. (In Chinese) [Google Scholar]
- Kang, H.X.; Wu, G.Q.; Wei, M.; Li, S.J. The role of Na+/H+ antiporter in response of plant to abiotic stress. Plant Physiol. J. 2022, 58, 511–523. (In Chinese) [Google Scholar]
- Shabala, S.; Newman, I.A. Salinity effects on the activity of plasma membrane H+ and Ca2+ transporters in bean leaf mesophyll: Masking role of the cell wall. Ann. Bot. 2000, 85, 681–686. [Google Scholar] [CrossRef] [Green Version]
- Silvestri, C.; Bacchetta, L.; Bellincontro, A.; Cristofori, V. Advances in cultivar choice, hazelnut orchard management and nuts storage for enhancing product quality and safety: An overview. J. Sci. Food Agric. 2021, 101, 27–43. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, L.J.; Zhao, T.T. High genetic variability and complex population structure of the native Chinese hazelnut. Braz. J. Bot. 2018, 41, 687–697. [Google Scholar] [CrossRef]
- Luo, D.; Shi, Y.J.; Song, F.H.; Mahmut, A.; Song, Z.J.; Ling, J.X.; Zuo, C. Evaluation of fruit economic traits of Corylus heterophylla × C. avellane. J. Northeast Forest. Univ. 2020, 48, 45–49. (In Chinese) [Google Scholar]
- Zhuang, Q.W.; Wu, S.X.; Yang, Y.; Niu, Y.X.; Yan, Y.Y. Spatiotemporal characteristics of different degree of salinized cultivated land in Xinjiang in recent ten years. J. Univ. Chin. Acad. Sci. 2021, 38, 341–349. (In Chinese) [Google Scholar]
- Morton, M.J.L.; Awlia, M.; Al-Tamimi, N.; Saade, S.; Pailles, Y.; Negrao, S.; Tester, M. Salt stress under the scalpel-dissecting the genetics of salt tolerance. Plant J. 2019, 97, 148–163. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Shi, Y.J.; Song, F.H.; Li, J.C. Effects of salt stress on growth, photosynthetic and fluorescence characteristics, and root architecture of Corylus heterophylla × C. avellan seedlings. Chin. J. Appl. Ecol. 2019, 30, 3376–3384. (In Chinese) [Google Scholar]
- Luo, D.; Shi, Y.J.; Song, F.H. Physiological responses of seedlings of Ping’ou hybrid hazelnut to salt stress and their evaluation of salt tolerance. Chin. J. Ecol. 2023, 42, 1–8. (In Chinese) [Google Scholar]
- Luo, D.; Wu, Z.B.; Song, F.H.; Shi, Y.J. Ion flux characteristics of root and their response to ion transport inhibitors of Corylus heterophylla × C. avellana seedlings under salt stress. Plant Physiol. J. 2023, 59, 889–898. (In Chinese) [Google Scholar]
- Luo, D.; Wu, Z.B.; Shi, Y.J.; Song, F.H. Effects of salt stress on leaf anatomical structure and ion absorption, transportation and distribution of three Ping’ou hybrid hazelnut seedlings. Acta Ecol. Sin. 2022, 42, 1876–1888. (In Chinese) [Google Scholar]
- Zhang, L.; Jia, Z.G.; Ma, Q.H.; Wang, G.X. Effects of saline-alkali stresses on the growth and endogenous hormone contents in leaves of hybrid hazelnut Liaozhen 3. For. Res. 2015, 28, 394–401. (In Chinese) [Google Scholar]
- Tang, X.Q.; Yang, X.Y.; Li, H.Y.; Zhang, H.X. Maintenance of K+/Na+ balance in the roots of Nitraria sibirica Pall. in response to NaCl stress. Forests 2018, 9, 601. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Chen, S.L.; Dai, S.X.; Wang, R.G.; Li, N.Y.; Shen, X.; Zhou, X.Y.; Lu, C.F.; Zheng, X.J.; Hu, Z.X.; et al. NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiol. 2009, 149, 1141–1153. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Zhang, H.X.; Liu, T.; Wu, H.W.; Ni, J.W.; Chen, Q.X. Study on ion metabolism characteristics of Elaeagnus angustifolia seedlings under NaCl stress. For. Res. 2016, 29, 140–146. (In Chinese) [Google Scholar]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.D.; Wang, C.; Ma, Z.H.; Hou, R.F.; Gao, Q.; Chen, Q. Effect of short term salt stress on the absorption of K+ and accumulation of Na+, K+ in seedlings of different wheat varieties. Acta Ecol. Sin. 2011, 31, 2822–2830. (In Chinese) [Google Scholar]
- Huang, Y.; Cao, H.S.; Yang, L.; Chen, C.; Shabala, L.; Xiong, M.; Niu, M.L.; Liu, J.; Zheng, Z.H.; Zhou, L.J.; et al. Tissue-specific respiratory burst oxidase homologue-dependent H2O2 signaling to the plasma membrane H+-ATPase confers potassium uptake and salinity tolerance in Cucurbitaceae. J. Exp. Bot. 2019, 70, 5879–5893. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.D.; Liu, A.Q.; Li, H.T.; Yu, Y.Y.; Wei, Z.C.; Wang, J.N.; Zhou, L.L. Applications and advances of non-invasive micro-test technology in plant physiological-ecology research. Chin. J. Appl. Environ. Biol. 2017, 23, 175–182. (In Chinese) [Google Scholar]
- Chen, T.X.; Wang, W.L.; Xu, K.; Xu, Y.; Ji, D.H.; Chen, C.S.; Xie, C.T. K+ and Na+ transport contribute to K+/Na+ homeostasis in Pyropia haitanensis under hypersaline stress. Algal Res. 2019, 40, 101526. [Google Scholar] [CrossRef]
- Wang, Z.; Hong, Y.C.; Zhu, G.T.; Li, Y.M.; Niu, Q.Y.; Yao, J.J.; Hua, K.; Bai, J.J.; Zhu, Y.F.; Shi, H.Z.; et al. Loss of salt tolerance during tomato domestication conferred by variation in a Na+/K+ transporter. EMBO J. 2020, 39, e103256. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, H.X.; Chen, Q.X.; Yang, X.Y. Responses of apical ion fluxes to NaCl stress in Elaeagnus angustifolia seedlings. Chin. J. Plant Ecol. 2017, 41, 489–496. (In Chinese) [Google Scholar]
- Sun, J.; Dai, S.X.; Wang, R.G.; Chen, S.L.; Li, N.Y.; Zhou, X.Y.; Lu, C.F.; Shen, X.; Zheng, X.J.; Hu, Z.X. Calcium mediates root K+/Na+ homeostasis in poplar species differing in salt tolerance. Tree Physiol. 2009, 29, 1175–1186. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Golldack, D.; Zhao, C.S.; Bohnert, H.J. The expression of HAK-type K+ transporters is regulated in response to salinity stress in common ice plant. Plant Physiol. 2002, 129, 1482–1493. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.H.; Pottosin, I.I.; Cuin, T.A.; Fuglsang, A.T.; Tester, M.; Jha, D.; Zepeda-Jazo, I.; Zhou, M.X.; Palmgren, M.G.; Newman, I.A.; et al. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt stressed barley. Plant Physiol. 2007, 145, 1714–1725. [Google Scholar] [CrossRef] [Green Version]
- Cuin, T.A.; Betts, S.A.; Chalmandrier, R.; Shabala, S. A root’s ability to retain K+ correlates with salt tolerance in wheat. J. Exp. Bot. 2008, 59, 2697–2706. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.Q.; Luo, Z.; Dong, H.Z.; Eneji, A.E.; Li, W.J. Effects of non-uniform root zone salinity on water use, Na+ recirculation, and Na+ and H+ flux in cotton. J. Exp. Bot. 2012, 63, 2105–2116. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Li, Y.X.; Yuan, H.J.; Hu, J.; Wei, L.; Bao, A.K.; Zhang, J.L.; Wang, S.M. ZxSOS1 is essential for long-distance transport and spatial distribution of Na+ and K+ in the xerophyte Zygophyllum xanthoxylum. Plant Soil 2014, 374, 661–676. [Google Scholar] [CrossRef]
- Chen, C.X.; He, G.F.; Li, J.F.; Perez-Hormaeche, J.; Becker, T.; Luo, M.Q.; Wallrad, L.; Gao, J.P.; Li, J.; Pardo, J.M. A salt stress-activated GSO1-SOS2-SOS1 module protects the Arabidopsis root stem cell niche by enhancing sodium ion extrusion. EMBO J. 2023, 42, e113004. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, D.; Song, F.; Lu, M.; Shi, Y.; Ma, Q. Salt-Stress-Induced Ion Transport Contributes to K+/Na+ Homeostasis in Roots of Ping’ou Hybrid Hazelnut. Forests 2023, 14, 1651. https://doi.org/10.3390/f14081651
Luo D, Song F, Lu M, Shi Y, Ma Q. Salt-Stress-Induced Ion Transport Contributes to K+/Na+ Homeostasis in Roots of Ping’ou Hybrid Hazelnut. Forests. 2023; 14(8):1651. https://doi.org/10.3390/f14081651
Chicago/Turabian StyleLuo, Da, Fenghui Song, Mingyan Lu, Yanjiang Shi, and Qinghua Ma. 2023. "Salt-Stress-Induced Ion Transport Contributes to K+/Na+ Homeostasis in Roots of Ping’ou Hybrid Hazelnut" Forests 14, no. 8: 1651. https://doi.org/10.3390/f14081651
APA StyleLuo, D., Song, F., Lu, M., Shi, Y., & Ma, Q. (2023). Salt-Stress-Induced Ion Transport Contributes to K+/Na+ Homeostasis in Roots of Ping’ou Hybrid Hazelnut. Forests, 14(8), 1651. https://doi.org/10.3390/f14081651




