Abstract
In urban forestry plantations are implemented in different cities of the world for social and environmental benefits. Bauhinia retusa and Bauhinia variegata are important species and to be used as large-scale plantation programs in urban forestry which might solve or mitigate urban, social, and environmental issues such as improving the physical & mental health of residents, food and nutrition security, increasing urban biodiversity, cooling the neighboring, preventing soil erosion, flooding, and mitigating greenhouse gas emissions and air pollution. The present study was conducted with the aim of producing quality planting material for B. retusa and B. variegata in the nursery for afforestation programs. Seeds of B. retusa and B. variegata were collected from the natural habitats to assess seed germination and seedling growth. Seeds were stored in different types of containers at room temperature and later on exposed to 15, 20, and 25 °C in seed germinator. Seeds were further sown in polythene bags according to the seed size, seed coat color, seed sowing depth, the orientation of seeds, and the result of the emergence of seedlings, their growth, and biomass were estimated. A two-way analysis of variance was calculated to estimate the variation among the studied parameters. Results revealed that a constant 25 °C temperature was considered best for seed germination of both the Bauhinia species. Polybags were found the most suitable for storing the Bauhinia seeds among the storage containers. The seedling emergence and growth were maximum in yellow color and large seeds. In B. retusa, seedling emergence, and growths were the maximum in seeds sown at a horizontal position and in B. variegata at an upright position. Seedling emergence, length, and biomass were recorded the maximum when seeds of B. retusa were sown at 4 cm depth and B. variegata seeds were sown at 2 cm depth. The study recommends that the yellow color seed that has to be sown at 2 cm to 4 cm depth with upright and horizontal positions is considered best for the production of quality planting stock of both studied Bauhinia species.
1. Introduction
Bauhinia belongs to the family Fabaceae and is a large genus of flowering plants that naturally occur in subtropical forest ecosystems with various forms such as trees, shrubs, and climbers [1]. Fifteen species of Bauhinia are generally grown in India [2]. Bauhinia retusa Roxb. and Bauhinia variegata L. are ecologically and economically important species. Farmers of the Himalayan region grow both species in different agroforestry systems [3], and harvested for good quality fodder during the lean period, particularly in the summer season [4]. Farmers harvest the foliage of these trees to feed their cows, ox, and buffaloes. The local inhabitants of the Himalayan region collect B. variegata flower buds for making vegetables and pickles. Consequently, seed formation is declining and reducing the natural regeneration of the species [5]. The pods of both Bauhinia species are dehiscent, the seeds are flat and the epigeal type of germination is has occurred [5]. The new seedling in nature is hindered due to the over-exploitation of fodder and firewood. B. variegata contains many secondary metabolites in their flowers, flower buds, stems, roots, bark, seeds, and leaves and is used as a medicine for the prevention of various diseases [6,7]. Both Bauhinia species are most suitable for avenue plantation in urban areas. They produce beautiful flowers and leaves which are prerequisites for aesthetic plantation. Due to nitrogen fixation ability, both tree species are also good for the improvement of the soil quality and rehabilitation of land in urban areas [4]. The planting of trees in urban forestry programs provides different direct and indirect benefits. The direct benefits are in terms of economic as well as social well-being and indirect benefits as the environmental conditions of the region are modified. Thus, afforestation or plantation programs of any species, quality planting material is required. The quality of planting material grown in the nursery depends on the seed quality, time of sowing, and methods of sowing [8].
The emergence of the seedling is a remarkable process that is resolved by proper timing, location of emergence, and burial depth in the soil [5]. The seeds buried at different soil depths may experience unusual environmental situations such as oxygen, carbon dioxide, temperature, water, and nutrition deficiency affecting seed emergence and seedling growth [9,10,11]. Seed germination depends on several ecological conditions, i.e., light, temperature, and moisture. The color of the seed coat is a trait associated with water assimilation in seeds [12,13]. The water imbibition rate of several legume species depends on the seed coat color [12]. The seed coat development is related to the kind of genotypes, but environmental conditions such as photoperiod influence the color of the seed coat [12]. Bewley and Black [14] reported that the green seeds of Salsola volkensii Asch. & Schweinf. (Amaranthaceae) did not have dormancy, while the non-green seeds showed dormancy. The orientation of seed sowing, especially in large-seeded species, influences seedling emergence both on the forest floor and in the nursery. Prasad and Nautiyal [15] reported that the seedling emergence in B. retusa is affected by seed orientation in the nursery seed bed. Planting seeds of these species in nurseries recorded variable germination, while low emergence from the forest floor. Seed size is a heritable trait and is used to improve the performance of seeds for the future. A significant relationship between seed size and weight on seed germination and seedling growth in different forest tree species was observed [16,17,18]. Seed sowing depth plays a significant role in germination and seedling growth [19,20]. The seeds buried at shallower depths have the chance to be taken by predators. Seeds buried deeply spend extra reserves of food materials in elongation of stem for their shoot apices to emerge at the soil surface than seeds sown at shallower depths. However, seeds disclosed to air on the soil surface or forest litter are disposed of and lose viability due to rapid moisture loss [9]. Thus, the maximum emergence of a seedling is dependent on the sowing depth and orientation of the seeds. Seed sowing in shallow or too deep with an incorrect position would delay the emergence and growth of seedlings. An assessment of the relative impact of temperature, seed size, and sowing position on seed germination and seedling growth of B. retusa and B. variegata is of high significance when focussing on the production of good quality seedlings in short durations in the nursery. Therefore, the present investigation was conducted to find the answer to the following questions (i) Does the temperature, seed size, color of the seed coat, and period of storage influence the seed germination of B. retusa and B. variegata ? and (ii) Do the seed orientation (positioning) seed depth of sowing affects the seedling emergence, growth, and biomass accumulation of both Bauhinia species?
2. Materials and Methods
Mature pods of B. retusa and B. variegata, which were hard, flat, and brown in appearance, were collected from the trees by direct climbing from the Nagni area of Tehri district located, between 30° 16′15″ N latitude and 78°22′01″ E longitude, at an altitude of 890 m a.s.l. After seed collection, all pods were kept in sunlight for dehiscence till the seeds separated from the pod. Healthy seeds were separated and stored at room temperature (average 22 °C) for further study.
Thirty seeds (three replicates of ten seeds each) of both Bauhinia species were dipped in distilled water for 24 h before placing them into Petri plates (12 cmdiameter.) containing Whatman No.1 filter paper (two layers) and placed in pre-fixed temperatures. The temperatures used were 15, 20, and 25 °C in the seed germinator under a completely randomized design. The humidity in the germinator was pre-fixed at 70.0%. The emergence of the radicle was considered an indicator of the germination of seeds. The period for germination was 28 days, and five seedlings were selected from each replicate to measure the radicle and plumule length with the help of a meter scale in centimeters (cm). Mean Germination Time (MGT) was calculated as per the formula given by Ellis and Roberts [21].
Germination Index (GI) was estimated as per the formula given by Kendrick and Frankland [22].
Three different types of storage containers, i.e., polybags, canes, and cotton bags, were used for storing the seeds of both Bauhinia species. Four hundred seeds were retained in each type of container at room temperature for one year in the Laboratory, College of Forestry, Ranichauri, Tehri Garhwal, Uttarakhand, to evaluate the storage behavior of Bauhinia seeds.
Ninety seeds were placed in three replicates (30 seeds each) in Petri dishes lined with Whatman No. 1 filter paper, at 25 °C, after 3, 6, 9, and 12 months of storage in each type of container. When the radicle had emerged, seeds were considered to germinate. Germination percentage, MGT, GI, radicle, and plumule length were calculated as per the above-given methods.
Grading of seeds was executed based on their size and seed coat color to assess the effect of the seed size and seed coat color on germination, survival, and growth of seedlings. Seeds of both species were sown in the nursery in pre-filled poly bags with a mixture of a 1:2:1 ratio of sand, soil, and Farm Yard Manure (FYM). A hundred seeds of each species, five replicates (20 seeds each) were sown in the poly bags according to seed size (large and small), seed coat color (brown and yellow), seed sowing depth (2, 4, and 6 cm), and seed orientation (micropylar up, micropylar down and horizontal radicle end at 90° in respect to soil surface). The emergence of seedlings was recorded every day for 30 days or one week further if germination continued. Weeding and watering in the poly bags were executed manually when desirable till 12 months. Twenty seedlings of each treatment, in each of the five replications, were randomly tagged to assess 12 months growth, and morphological traits viz shoot length (cm), collar diameter (mm), number of leaves per seedling, root length (cm), shoot dry weight (g), root dry weight (g), leaves dry weight (g), and total dry weight (g) measured as per the standard procedures.
Statistical analysis: Standard derivation (±SD) and two-way analysis of variance (ANOVA) were calculated to analyze the effects of different seed sizes, seed coat color, and sowing treatments on germination, shoot length, root length, shoot dry weight, root dry weight, leaves dry weight and total dry weight of both Bauhinia species by using the statistical software WASP version 1.0 (online software package), ICAR, GOA, India, and the level of significant were tested at p < 0.01 and p < 0.05. Duncan’s multiple range tests were used to determine significant differences between different treatments at a 5% level.
3. Results
Seeds of two Bauhinia species were tested for germination at constant temperatures were 15, 20, and 25 °C. Significant variation (p < 0.05) was found in seed germination at different constant temperatures. The radicle and plumule growth were also significantly (p < 0.05) different at different constant temperature regimes. Maximum germination, radicle, and plumule length were recorded at 25 °C, and the lowest germination percent, radicle, and plumule length were recorded at 15° C in both species (Table 1).

Table 1.
Effects of temperatures on germination, radicle, and plumule growth (Mean ± SD) of two Bauhinia species (Mean values followed by the same letter within the column are not significant (p < 0.05) among the temperature treatments).
Significant variation (p < 0.05) was found in seed germination after the seed was stored in different types of containers. After one year of storage of B. retusa seeds, a maximum of 50.0% germination was recorded in seeds stored in a cotton bag, and a minimum of 43.0% was in polybags. While B. variegata seeds showed the highest longevity in terms of germination in polybags and the lowest in cotton bags (Table 2). The radicle and plumule growth of both species were also recorded and found that plumule and radicle growth were significantly (p < 0.05) reduced after increasing the period of storage. The type of containers also significantly (p < 0.05) influenced the radicle and plumule growth of B. retusa (Table 3).

Table 2.
Germination percentage (Mean ± SD) of Bauhinia retusa and B. variegata seeds stored on different types of containers (Mean values followed by the same letter within the row are not significant (p < 0.05) among the types of containers).

Table 3.
Radicle and plumule growth (Mean ± SD) of Bauhinia retusa and B. variegata seeds stored in different types of containers (Mean values followed by the same letter within the column are not significant (p < 0.05) among the types of containers.
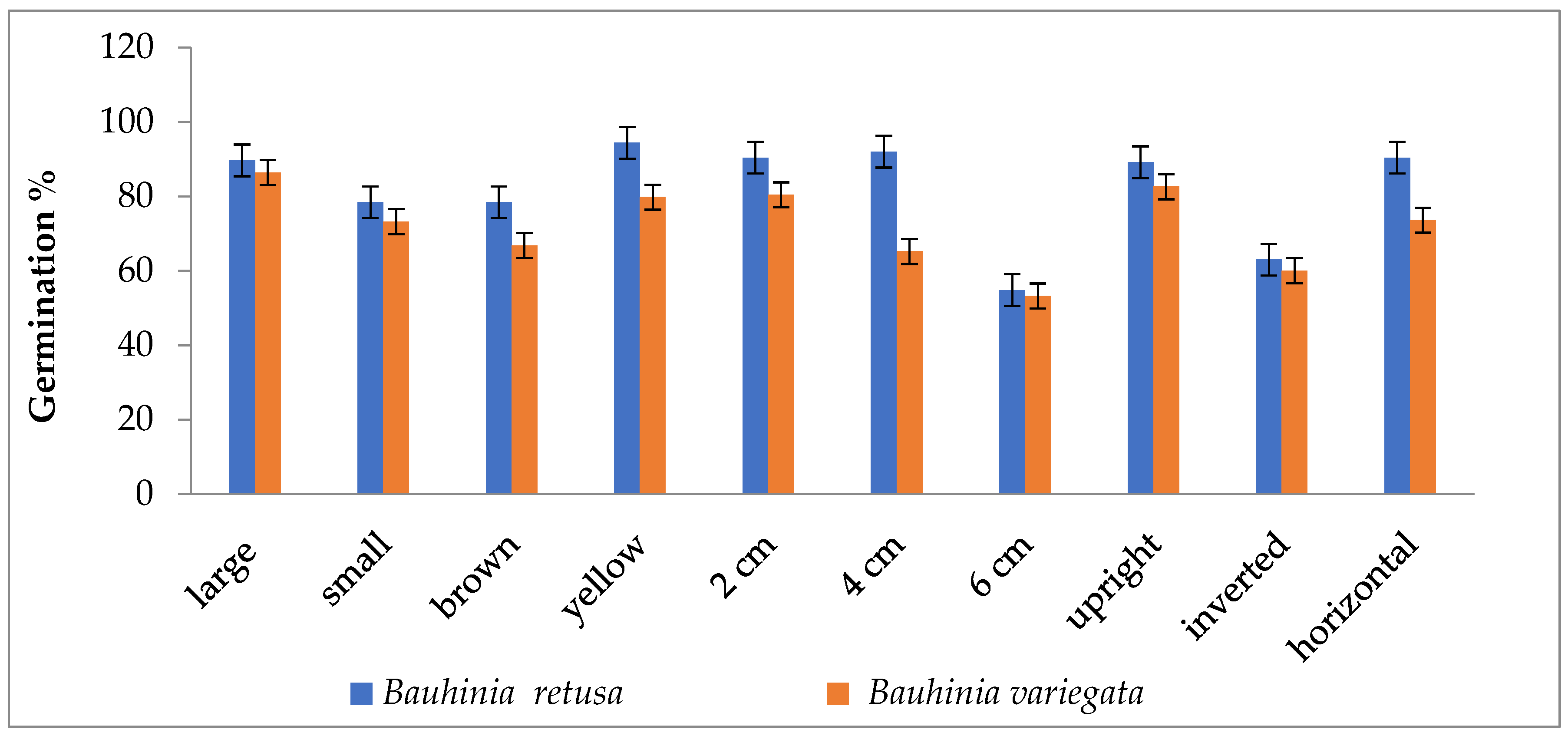
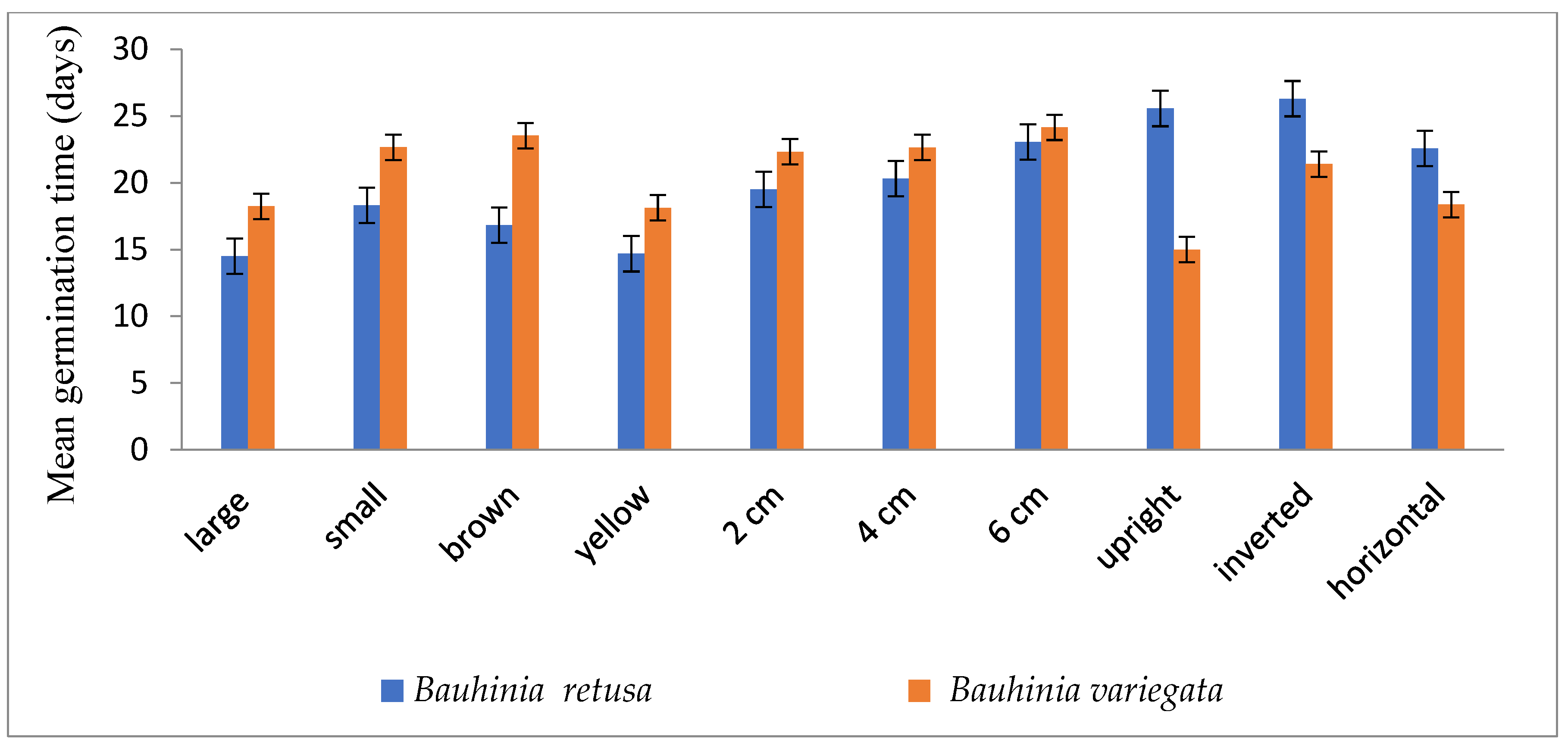
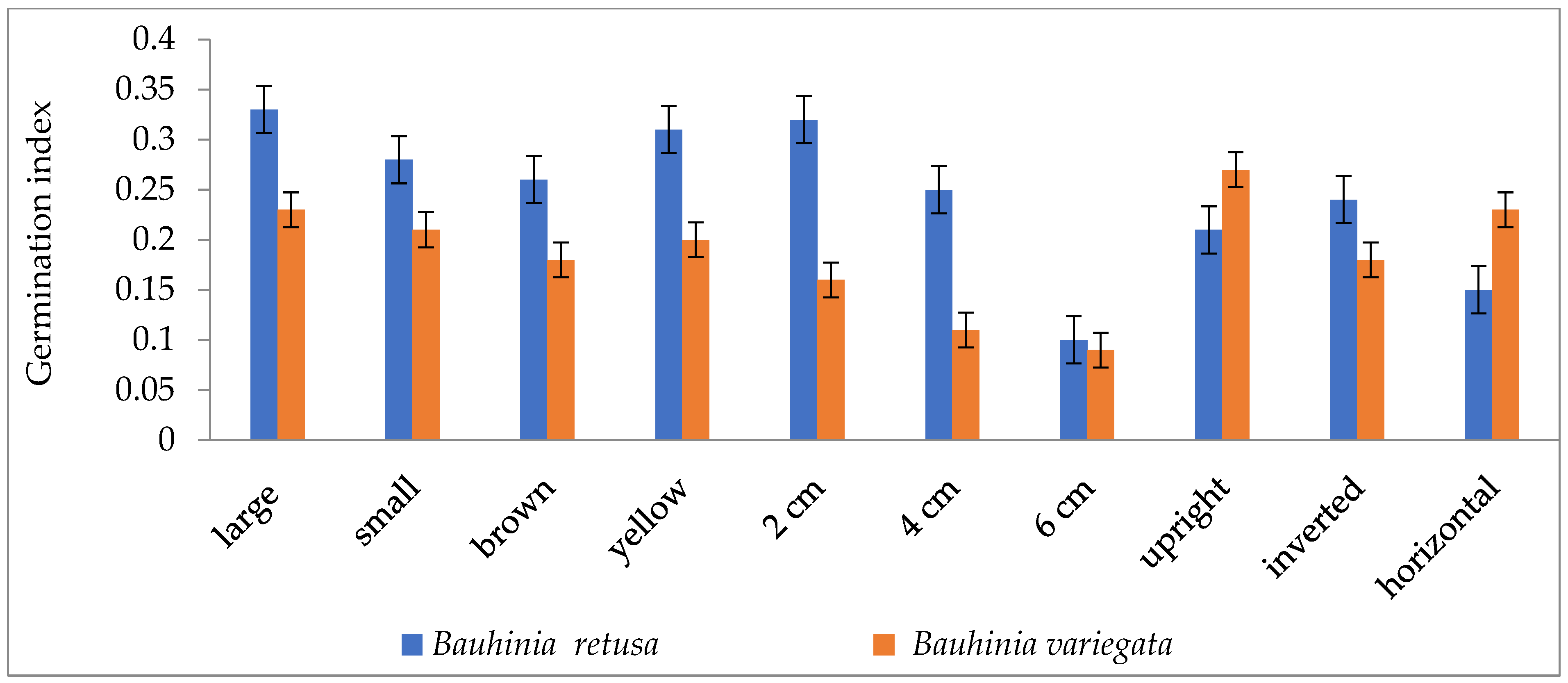
Seed germinations were significantly (p < 0.05) influenced by different seed sizes, colors, and seed-sowing treatments in B. retusa. Large-size seeds have a higher germination percentage than small-size seeds in both species (Figure 1). The value of MGT was 14.5 days for large-size seeds and 18.3 days for small-size seeds (Figure 2). GI values were 0.33 and 0.28 for large and small-size seeds, respectively (Figure 3). Similarly to the results of B. retusa, the value of MGT was the maximum (22.66 days) for small-size seeds and the minimum (18.23 days) for large-size seeds in B. variegata (Figure 2). The maximum and minimum (0.23 and 0.21) GI values were found for large and small-size seeds, respectively (Figure 3). On average, yellow color seeds produced a maximum of 94.4% and 78.4% germination in B. retusa and B. variegata, respectively. While the minimum of 79.6% and 66.8% germination were recorded in brown color seed coats, respectively in both species (Figure 1). MGT and GI were recorded as the maximum in yellow color seeds as compared to brown color seeds (Figure 2 and Figure 3).
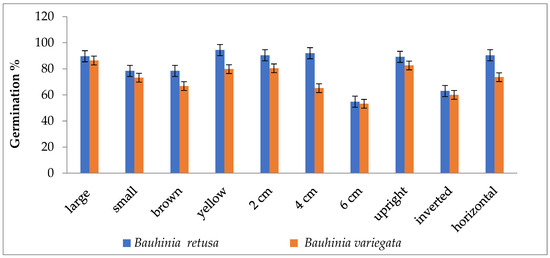
Figure 1.
Germination percentage of Bauhinia retusa and B. variegata in different seed sizes, seed coat color, sowing depth, and sowing orientation (vertical bars representing ± S.D).
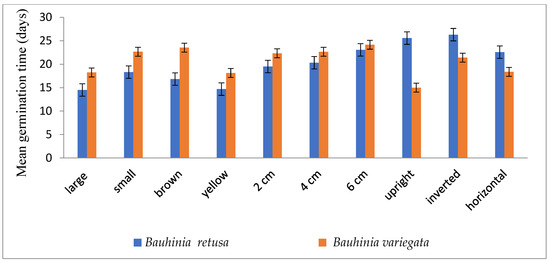
Figure 2.
Mean germination time of Bauhinia retusa and B. variegata in different seed sizes, seed coat color, sowing depth, and sowing orientation (vertical bars representing ± S.D).
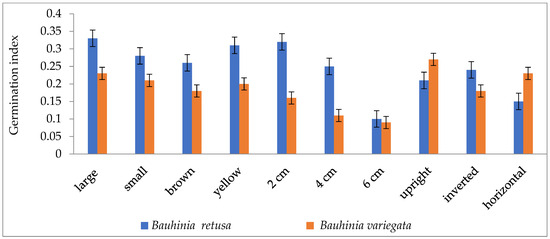
Figure 3.
Germination index of Bauhinia retusa and B. variegata in different seed sizes, seed coat color depth, and sowing orientation (vertical bars representing ± S.D).
Seeds that were sown at 4 cm depth recorded a maximum of 92.0 and 82.4% emergence of seedlings and a minimum of 54.8 and 60.0% at 6 cm depth, respectively, for B. retusa and B. variegata (Figure 1). The MGT was the highest (23.0 and 24.15 days) at 6 cm depth, and the lowest (19.5 and 22.32 days) at 2 cm depth for both species (Figure 2). GI values were the highest (0.32 and 0.27) at 2 cm depth and the lowest (0.10 and 0.18) at 6 cm depth (Figure 3).
The seeds of B. retusa sown in different orientations, upright, inverted, and horizontal revealed that the horizontal sowing orientation had the highest (90.40%) germination and the lowest (63.0%) observed at inverted seed sowing orientation (Figure 1). The MGT was observed as the highest (21.4 days) in an inverted position, and the lowest (15.0 days) in an upright position (Figure 2). GI values were highest (0.20) in an inverted position and lowest (0.16) in a horizontal position (Figure 3).
Shoot length, collar diameter, number of leaves per plant, and root length were recorded as maximum (20.24 cm, 3.31 mm 2.60, and 29.56 cm, respectively) in the seedlings produced by large size seeds, and the minimum (17.77 cm, 2.75 mm, 2.28 and 15.75 cm, respectively) in small size seeds, after 12 months of growth of B. retusa. The large seeds also produced higher biomass as compared to the smaller size seeds. The dry weight of shoot, root, leaves, total dry weight, and root/shoot dry weight ratio wasnoted as the maximum (0.54 g, 1.78 g, 0.50 g, 2.83 g, and 3.29 g, respectively) in large, and the minimum (0.43 g, 1.36 g, 0.47 g, 2.261 g, and 3.16 g) in small size seeds (Table 4).

Table 4.
Growth attributes and their biomass in different sizes, sowing orientation, seed color, and sowing depth after 12 months of growth in B. retusa (Mean values followed by the same letter within the column are not significant (p < 0.05) among the sowing conditions/treatments).
Length of the shoot, collar diameter, number of leaves per plant, root length, and dry weight of shoot, root, and leaves in B. retusa was the maximum at the upright position, and the minimum observed in the inverted position (Table 4). However, in B. variegata seedling growth and biomass production were recorded as the maximum in the horizontal position, and the minimum in the inverted position (Table 5).

Table 5.
Growth attributes and their biomass in different sizes, sowing orientation, seed color, and sowing depth, after 12 months of growth in B. variegata (Mean values followed by the same letter within the column are not significant (p < 0.05) among the sowing conditions /treatments).
The yellow color seeds had gained the maximum growth attributes and biomass allocations as compared to the brown color seeds in both Bauhinia species. Shoot length, collar diameter, root length, number of leaves per plant, and biomass production were recorded as the maximum at 2 cm depth in both species, except collar diameter and number of leaves per plant, which were found at 4 cm depth in B. retusa. Large seeds produced the maximum growth attributes and biomass after 12 months as compared to the small size seeds (Table 4 and Table 5).
The ANOVA was significantly (p < 0.05, 0.01) different among various treatments. Collar diameter was significantly (p < 0.01) affected by seed size, seed coat color, and sowing orientation treatments, except sowing depth in B. variegata, while in B. retusa sowing depth significantly affected the collar diameter. Seed sizes significantly (p < 0.01) enhanced the number of leaves per plant in B. variegata. However, the number of leaves was significantly influenced by the seed size and sowing depth in B. retusa (Table 6 and Table 7).

Table 6.
Two-way analysis of variance (ANOVA) for seed germination, growth attributes, and biomass allocation of B. retusa under different seed sizes, color, sowing depths, and sowing orientations.

Table 7.
Two-way analysis of variance (ANOVA) for seed germination, growth attributes, and biomass allocation of B. variegata under different seed sizes, colors, sowing depths, and sowing orientation.
4. Discussion
Seeds of Bauhinia species exhibited optimum germination at 25 °C and decreases at 20 °C and 15 °C temperature regimes. Low germination at a lower temperature is attributed to protein denaturation [23,24]. The natural occurrence of these tree species is extended from the tropical to the subtropical region in Garhwal Himalaya and the optimum temperature occurs during the rainy season in these areas [25] oscillating between 25 and 35 °C, which is the most suitable for natural regeneration. In both study species, the GI decreased, whereas MGT increased with increasing constant temperature regimes, as also recorded for Albizia lebbek (L.) Benth. of the family Fabaceae [26], and Celtis australis L. of the family Cannabaceae [27].
The quicker and more consistent germination in both Bauhinia species was achieved at 25 °Ctemperature regimes. Similar results were reported for Dalbergia sissoo Roxb. (Fabaceae) [28], and Terminalia spp. (Combretaceae) [29]. The rate of germination in most plant species seem to be strongly temperature dependent, but it is also related to other external factors: light, humidity, and seed coat width [12,30]. Zangoie et al. [31] conducted a study to examine the effects of temperature on seed germination of Ferula assafoetida L. (Umbelliferae) and revealed that the seed germination was optimum at 25 °C but not significantly higher as compared to the 20 °C temperature. A temperature range of 25 to 30 °C was required to obtain uniform and maximum germination in twelve indigenous and eight exotic multipurpose leguminous species [32]. The rate of germination and germination capacity increased with increasing temperature from 13 °C to 33 °C with the optimum at 28 °C [33]. Thus, studies on seed germination indicated that a certain range of temperature gives the highest percentage of germination; however, the optimum temperature requirement of each species is different for their germination, signifying a possible genetic control [34].
In the case of observations regarding seed storage under the present investigation, the seeds of the two Bauhinia species stored at room temperature, in different types of containers, resulted in reasonable viability and germination of seeds which indicates that storage at room temperature under climatic conditions of the present study site is effective for storage of Bauhinia seeds for one year. In an earlier study, Singh et al. [35] also observed that Casuarina equisetifolia L. (Casuarinaceae) seeds could be stored well under ambient conditions. Abdul-Banki and Anderson [36] reported that the low germination capacity and viability in seeds stored in cloth bags were caused due to changes in the physiochemical condition of seeds, chiefly the metabolism of seeds owing to the decrease in moisture content. The changes in seed metabolism are earlier reported as one of the major factors for low seed germination and viability [37]. We also observed that the germination and viability percentage of both Bauhinia species were recorded higher in polythene bags under room temperature and lower viability was found in cotton bags. The results of our study are at par with that reported for Toona ciliata M. Roem (Meliaceae) [38]. The seeds stored in cotton bags resulted in the loss of seed viability due to the loss of seed moisture during storage, which further affected the longevity of the seeds. A seed package that is moisture-proof or moisture-resistant would have extra importance in prolonging the viability and vigor of the seeds [23].
Seeds of Bauhinia that were sown with radicle downwards had higher germination percentages and produced larger seedlings than seeds that were sown with radicle facing upward and horizontal orientations. Contrary to the present study, seeds of B. retusa [15], B. vahlli Wight and Arnott, and B. racemosa Lamk. [39] had resulted in higher germination percentage, faster rates of germination, and seedling growth when sown with the radicle end facing upward direction in the soil surface. Similarly, the earliest onset of emergence in Quercus leucotrichophora A. Camus (Fagaceae) was recorded for seeds sown with radicle end-up [40]. In contrast, seeds of Pinus roxburghii Sarg. (Pinaceae) [41] and Shorea robusta Gaertn (Dipterocarpaceae) [42] had reported higher germination for seeds sown with radicle end downward. Swaminathan et al. Moreover, [43] recorded the maximum germination in Derris indica Lam. Bennet (Fabaceae) seeds when sown in a vertical position with the radicle end downwards compared to any other position. Singh et al. [18] reported that seed emergence of Cinnamomum tamala (Buch.-Ham.) T. Nees & Eberm. (Lauraceae) was higher in upright seed orientation as compared to the seeds sown in an inverted or horizontal orientation.
Krishna et al. [44] reported that purple color seeds have significant differences in seed germination and speed of germination over the other seed coat colors in Erythrina indica Lam. (Fabaceae). These findings are in agreement with the results of the present study in which seed coat color had significantly influenced the germination, MGT, and GI, as well as growth attribute and biomass allocation in both Bauhinia species. Liu et al. [45] reported that the seeds having yellow seed coats of Prunus armeniaca L. (Rosaceae) had the highest germination percentage. Bonner et al. [46] also examined that seed coat color was pretentious for indicating the germination in Gleditsia triacanthos L. (Fabaceae) seeds. In the present study, the seeds of both Bauhinia species with a yellow seed coat color were found to be suitable for the production of quality seedlings in the nursery.
The size of an individual seed may be influenced by the physical condition of parent plants and affects the regeneration process of the population [47], seedling biomass [47], and seedling survival [48,49]. Germination of seeds is essential for the production of plants and to obtain the optimal number of seedlings for the regeneration of a species and afforestation/reforestation in their natural habitat [50]. Therefore, it is important to identify the consequences of seed size on germination and seedling growth of Bauhinia species for facilitating its large-scale plantations. The large-size seeds augment the emergence, and survival growth of the seedlings and biomass allocation as compared to the small-size seeds in both Bauhinia species. Based on the present study, large size seeds of both Bauhinia species supplemented the emergence, growth, and pertaining biomass of the seedlings compared to the small size seeds. Baraloto et al. [51] reported that the larger-seeded Eperua grandiflora (Aubl.) Bailland Vouacapoua americana Aubl. (both Caesalpiniaceae) produced larger seedlings as compared to small size seeds. Reich et al. [52] also reported that the seed mass significantly influences the emergence of seedlings and their survival in stressful environmental conditions and enhance flexibility in shoot/root allometry, speed of germination, and overall growth.
Temperature, moisture, oxygen, and soil conditions are essential for seed germination, emergence, and seedling recruitment [53,54] and these factors vary under different burial depths and influence the emergence and seeding establishment. Seeds sown at deep depth take more time for seedling emergence [55]. Ahirwar et al. [56] revealed the emergence of seedlings in Butea frondosa Roxb. Ex willd (Fabaceae) seeds were higher when sown at 2 cm depth and the emergence of seedlings was gradually reduced as the sowing depth was increased. Seed germination and other growth characteristics in Vicia faba L. (Fabaceae) decreased with increasing sowing depth [57]. The result of an earlier study showed that the upper soil layer had produced a higher seedling emergence rate, which indicates the favorable micro-habitat, light, water, nutrient, and pathogen for the germination of seeds of Azadirachta indica A. Juss. (Meliaceae) [17]. Seeds buried deeply resulted in delayed emergence of seedlings than those that were shallowly buried because the seeds buried in deep lost maximum reserves of nutrients in elongation of the shoot to come in the apices to reach and emerge in the soil surface as compared to a seed buried in shallower depths [58,59,60].
The seeds sown in an upright situation revealed detrimental effects on the emergence and seedling growth in our observations. The germination was postponed or suppressed by the upright positioning of seeds owing to the requirement of extra energy for the emergence of plumule from the soil when sown in this position. The epigeal type of seed emergence in Hardwickia binata Roxb. (Fabaceae) used additional energy for hypocotyl development [61]. Mahgoub [62] also reported similar results in Delonix regia (Bojer) Raf. and Vachellia nilotica (L.) P.J.H.Hurter & Mabb (both Fabaceae). The unusual morphological development of seedlings was the manifestation of the chemical substances or physiological changes due to the transposition of the seedling’s strength [63].
The production of seedlings and nursery management are very important parts of most plantation programs which facilitate the landholders and farming communities with high-quality planting material [64,65]. B. retusa and B. variegata are ecologically and economically important species of Indian Himalaya, with large potential in afforestation or forest plantation programs. The improvement in the germination rate of forest species for production purposes is more crucial keeping allowance for the fact that forest tree seeds are less available commercially, are difficult to procure, and have low rates of germination [66,67,68]. Therefore, the initial step toward addressing these problems is to improve our knowledge and understanding of the major factors which affect seed germination and seedling growth [69]. However, the present study investigated the effects of temperature, seed size, and sowing position on germination and seedling growth of B. retusa and B.. seeds, as well as the effects of storage containers on seed viability with the aim to provide assistance for quality seedling production. However, the changes in seed moisture during storage may also be addressed. The moisture content plays an important role in seed physiology in two ways. First, the ratio of vicinal water in the tissue of the seed to the bulk water may serve as the indicator of the expected metabolic activity of the seed which includes development, resting, and germination. Therefore, the absolute moisture content does not specifically dominate the physiological activity. Second, the extra dehydration may switch off the reserve material’s production irreversibly and the senescence may be initiated that continues at varying pace till the availability of seed reserve [70]. Furthermore, apart from seed traits, the seedling emergence from the soil is also influenced by different environmental factors like light, temperature, pH, and other soil properties [71] and the initiation of seed germination generally needs specific soil conditions [72]. For example, seed germination occurs only in the condition when surface soil water conditions are optimum [73]. Thus, the monitoring of changes in seed moisture during storage and consideration of soil characteristics remains the limitation under the present investigation which may be the point of consideration for further studies while keeping the other factors like seed storage container, temperature, seed size, sowing position etc as constant.
5. Conclusions
The results of the present study indicate that the large-size seed had more germination percentage, as well as seedling growth and biomass production in both Bauhinia species. Yellow seed coat color is the best indicator for the maximum germination and seedling growth and biomass in both Bauhinia species. Seedling emergence of B. retusa and B. variegata was higher when sown at 4 cm and 2 cm soil depth, respectively. While their seedling growth characters and biomass attributes were the highest at 2 cm depth. Similarly, seed sowing orientation showed the highest emergence of seedlings at the upright position in B. retusa and the horizontal position in B. variegata with their growth and biomass. Thus, it may be rational to take into consideration, the size, appearance, sowing depth, and direction of sowing of the seeds in B. retusa and B. variegata for the production of good quality seedlings to continue metabolic integrity and sub-cellular management even under an unfavorable situation.
Author Contributions
N.Y. experimented, data collection, and prepared the first draft of the manuscript. B.S. and C.S.D. analysed data and preparation of the first manuscript. V.P.K. and B.S. designed the research and supervised the research. B.S., D.R., V.P.K. and M.K. reviewed and modified the manuscript. M.K.R. and T.A. used software to analyse the data. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data obtained during the investigation in this study are included in the article.
Acknowledgments
The authors are thankful to the College of Forestry, V.C.S.G Uttarakhand University of Horticulture and Forestry, Ranichauri, Tehri Garhwal, Uttarakhand for providing all the facilities.
Conflicts of Interest
The authors declared as an apparent no conflict of interest.
References
- Schmid, R. Plant Systematics: A Phylogenetic Approach; Photo Gallery of Vascular Plants. Taxon 2007, 56, 1316. Available online: https://www.jstor.org/stable/25065934?origin=crossref (accessed on 9 August 2023).
- Khare, P.; Kamal, K.; Sharma, D.K. Historical aspects, medicinal uses, phytochemistry and pharmacological review of Bauhinia variegata. Asian J. Pharm. Pharmacol. 2018, 4, 546–562. [Google Scholar] [CrossRef]
- Mishra, A.; Nautiyal, S.; Nautiyal, D.P. Growth characteristics of some indigenous fuelwood and fodder tree species of sub-tropical Garhwal Himalayas. Indian For. 2009, 135, 373–379. [Google Scholar]
- Albert, S.; Sharma, B. Comparative foliar micromorphological studies of some Bauhinia (Leguminosae) species. Turk. J. Bot. 2013, 37, 5. [Google Scholar] [CrossRef]
- Nagar, A.; Khanduri, V.P.; Singh, B.; Riyal, M.K.; Singh, I. Altitudinal variation in morphometric traits of pod, seed, and seedling growth of Bauhinia variegata L. in Garhwal Himalaya. Ann. Silvic. Res. 2022, 47, 85–94. [Google Scholar]
- Gautam, S. Bauhinia variegata Linn: All-purpose utility and medicinal tree. ENVIS For. Bull. 2012, 12, 61–64. [Google Scholar]
- Al-Snafi, A.E. The pharmacological importance of Bauhinia variegata, a review. Int. J. Pharma Sci. Res. 2013, 4, 160–164. [Google Scholar]
- Pillai, P.K.C.; Viswanath, S.C.; Hrideek, T.K.; Jiji, A.H. Seed characteristics and germination behaviour of Bauhinia malabarica Roxb. In Horticultural Crops; Baimey, H.K., Hamamouch, N., Kolombia, Y.A., Eds.; IntechOpen-London: London, UK, 2020. [Google Scholar]
- Borchert, M.I.; Davis, F.W.; Michaelson, J.; Oyler, L.D. Interaction of factors affecting seedling recruitment of blue oak (Quercus douglassii) in California. Ecology 1989, 70, 389–404. [Google Scholar] [CrossRef]
- Armstrong, W.D.M. Root growth and metabolism under oxygen deficiency. In Plant Roots: The Hidden Half; Waisel, Y., Eshel, A., Kafkafi, U., Eds.; Mercel Dekker: New York, NY, USA, 2002; pp. 729–761. [Google Scholar]
- Guo, C.-R.; Wang, Z.L.; Lu, J.-Q. Seed germination and seedling development of Prunusarmeniaca under different burial depths in the soil. J. For. Res. 2010, 21, 492–496. [Google Scholar] [CrossRef]
- De Souza, F.H.D.; Marcos-Filho, J. The seed coat as a modulator of seed-environment relationships in Fabaceae. Revta Bras. Bot São Paulo. 2001, 24, 365–375. [Google Scholar] [CrossRef]
- Ertekin, M.; Kirdar, E.R. Effects of seed coat colour on seed characteristics of honeylocust (Gleditsia triacanthos). Afr. J. Agric. Res. 2010, 5, 2434–2438. [Google Scholar]
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination; Plenum Press: New York, NY, USA, 1994; p. 221. [Google Scholar]
- Prasad, P.; Nautiyal, A.R. Influence of seed orientation on seedling emergence in Bauhinia retusa Ham. Ex. Roxb. Seed Sci. Technol. 1995, 20, 70–73. [Google Scholar]
- Gera, M.; Gera, N.; Ginwal, H.S. Seed source variation in germination and early growth among ten indigenous population of Dalbergia sissoo Roxb. India J. For. 1999, 125, 1190–1197. [Google Scholar]
- Uniyal, A.K.; Singh, B.; Todaria, N.P. Effects of seed size, sowing orientation and depth on germination and seedling growth in Neem, Azadirachta indica. Seed Technol. 2007, 29, 68–75. [Google Scholar]
- Singh, B.; Rawat, J.M.S.; Pandey, V. Influence of sowing depth and orientation on germination and seedling emergence of Cinnamomum tamala Nees. J. Environ. Biol. 2017, 38, 271. [Google Scholar] [CrossRef]
- Vander, W.S.B. A model of caching depth: Implication for scatter hoarders and plant dispersal. Am. Nat. 1993, 141, 217–232. [Google Scholar]
- Chen, H.; Maun, M.A. Effect of sand burial depth on seed germination and seedling emergence of Cirsium pitcher. Plant Ecol. 1999, 140, 53–60. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. The quantification of aging and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Kendrick, R.E.; Frankland, B. Photo control of germination in Amaranthus caudatus. Planta 1981, 85, 326–329. [Google Scholar] [CrossRef]
- Harrington, J.E. Problems of seed storage. In Seed Ecology; Heydecker, W., Ed.; Butterworths: London, UK, 1973; pp. 251–263. [Google Scholar]
- Simon, E.W.; Minchin, A.; Mcmenamin, M.M.; Smith, J.M. The low-temperature limit for seed germination. New Phytol. 1976, 77, 301–311. [Google Scholar] [CrossRef]
- Singh, S.P.; Phartyal, S.S.; Rosbakh, S. Tree seed traits’ response to monsoon climate and altitude in Indian subcontinent with particular reference to the Himalayas. Ecol. Evol. 2017, 7, 7408–7419. [Google Scholar] [CrossRef] [PubMed]
- Todaria, N.P.; Singh, B.; Kumar, R. Seed source variation and germination studies in Albizia lebbek from Garhwal Himalaya. J. Tree Sci. 2002, 21, 27–34. [Google Scholar]
- Singh, B.; Bhatt, B.P.; Prasad, P. Variation in seed and seedling traits of Celtis australis, a multipurpose tree, in Central Himalaya, India. Agrofor. Syst. 2006, 67, 115–122. [Google Scholar] [CrossRef]
- Singh, B.; Bhatt, B.P. Provenance variation in pod, seed and seedling traits of Dalbergia sissoo (Roxb. ex dc), Central Himalaya, India. Trop. Agric. Res. Ext. 2008, 11, 39–44. [Google Scholar] [CrossRef]
- Chauhan, S.; Bhatt, B.P.; Todaria, N.P. Germination behavior of Terminalia species influenced by temperature, light and seed maturity in Garhwal Himalaya. J. Plant Biol. 2002, 29, 208–209. [Google Scholar]
- Smýkal, P.; Vernoud, V.; Blair, M.W.; Soukup, A.; Thompson, R.D. The role of the testa during development and in establishment of dormancy of the legume seed. Front. Plant Sci. 2014, 17, 351. [Google Scholar]
- Zangoie, M.S.; Parsa, M.; Mahmoodi, M.; Jami, A.-A.; Sanjari, G. Effect of temperature regimes on seed germination asafoetida (Ferula assafoetida L.). Plant Breed. Seed Sci. 2014, 69, 59–67. [Google Scholar] [CrossRef]
- Teketay, D. Germination ecology of twelve indigenous and eight exotic multipurpose leguminous species from Ethiopia. For. Ecol. Manag. 1996, 80, 209–223. [Google Scholar] [CrossRef]
- López, U.J.; Mendoza, H.A.J.; Jasso Mata, J.; Gómez, G.A. Morphological variation of plants and the influence of pH of irrigation water on twelve population Pinusgregii. Madera Y Bosques 2000, 6, 81–94. [Google Scholar]
- Khullar, P.; Thapliyal, R.C.; Beniwal, B.S.; Vakshasya, R.K.; Sharma, A. Forest Seed; FRI Publication: Dehradun, India, 1991; p. 412. [Google Scholar]
- Singh, B.G.; Mahadeevan, N.P.; Manimuthu, L. The effect of storage temperature on the viability of Casusarina equistifolia Forester and Forester. In Seed and Nursery Technology of Forest Trees; New Age Publications: New Delhi, India, 1999; pp. 183–187. [Google Scholar]
- Abdul-Banki, A.A.; Anderson, J.D. Relationship between decarboxylation of glutamic acid and vigour in soybean seeds. Crop Sci. 1973, 13, 222–226. [Google Scholar]
- Carrera-Castaño, G.; Calleja-Cabrera, J.; Pernas, M.; Gómez, L.; Oñate-Sánchez, L. An updated overview on the regulation of seed germination. Plants 2020, 9, 703. [Google Scholar] [CrossRef] [PubMed]
- Chand, G. Seed Storage and Viability of Toona Ciliata and Shorea robusta. Master’s Thesis, Parmar University of Horticulture, Solan, India, 1994; pp. 125–136. [Google Scholar]
- Prasad, P.; Nautiyal, A.R. Effect of orientation of seed placement in soil on seedling emergence in two Bauhinia species: Bauhinia vahlii WightetArn. and Bauhinia racemosa Lam. Seed Sci. Technol. 2003, 31, 183–186. [Google Scholar] [CrossRef]
- Rawat, D.C.S.; Nautiyal, A.R. Physiology of germination in Quercus. II. Effect of seed orientation in Quercus leucotrichophora. Seed Res. 1998, 26, 183–186. [Google Scholar]
- Thapiyal, R.C. Influence of seed orientation on seedling emergence in Chir plant Pinusroxburghii. Indian J. Exp. Biol. 1979, 17, 119–120. [Google Scholar]
- Sharma, M.M.; Purohit, A.N. Seedling survival and seed germination under natural and laboratory conditions in Shorea robusta. Seed Sci. Technol. 1980, 8, 283–287. [Google Scholar]
- Swaminathan, C.; Rai, V.; Suresh, R.S.; Sivaganam, K. Improving seed germination of Derris indica by vertical showing. J. Trop. For. Sci. 1993, 6, 152–158. [Google Scholar]
- Krishna, A.; Ganiger, B.S.; Rathod, R. Effect of seed weight and seed coat colour on germination and vigour of Forest tree Erythrina indica (Lam). Karnataka J. Agric. Sci. 2005, 18, 208–209. [Google Scholar]
- Liu, H.; Zhang, Z. Effect of mast seedling and rodent abundance on seed predation and dispersal by rodents in Prunus armeniaca (Rosaceae). For. Ecol. Manag. 2007, 242, 511–517. [Google Scholar] [CrossRef]
- Bonner, F.T.; Vozzo, J.A.; Elam, W.W.; Land, S.B.J. Tree Seed Technology Training Course; Instructional Manual, Southern Forest Experimental Station, U.S. Department of Agriculture: New Orleans, Louisiana, 1994; p. 160. [Google Scholar]
- Bonfil, C. The effects of seed size, cotyledon reserves, and herbivory on seedling survival and growth in Quercus rugosa and Q. laurina (Fagaceae). Am. J. Bot. 1998, 85, 79–87. [Google Scholar] [CrossRef]
- Tripathi, R.S.; Khan, M.L. Effects of seed weight and microsite characteristics on germination and seedling fitness in two species of Quercus in a subtropical wet hill forest. Oiko 1990, 57, 289–296. [Google Scholar] [CrossRef]
- Vera, M.L. Effects of altitude and seed size on germination and seedling survival of heathland plants in north Spain. Plant Ecol. 1997, 133, 101–106. [Google Scholar] [CrossRef]
- Abiyu, A.; Teketay, D.; Glatzel, G.; Gratzer, G. Seed production, seed dispersal and seedling establishment of two Afromontane tree species in and around a church forest: Implications for forest restoration. For. Ecosyst. 2016, 3, 1–10. [Google Scholar] [CrossRef]
- Baraloto, C.; Forget, P.M.; Goldberg, D.E. Seed mass, seedling size, and neotropical tree seedling establishment. J. Ecol. 2005, 93, 1156–1166. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J.; Tjoelker, M.G. Seed mass effects on germination and growth of diverse European Scots pine populations. Can. J. For. Res. 1994, 24, 306–320. [Google Scholar] [CrossRef]
- Michael, P.D.; Paul, B.C. Effects of wetting and drying on seed germination and seedling emergence of bull thistle, Cirsium vulgare (Savi) Ten. Can. J. Bot. 2000, 78, 1545–1551. [Google Scholar]
- Kos, M.; Poschlod, P. Correlation of inter-specific variation in germination response to water stress in a semi-arid savannah. Basic Appl. Ecol. 2008, 9, 645–652. [Google Scholar] [CrossRef]
- Li, T.S. Effect of seedling depth and soil texture on seedling emergence and root shape of American ginseng. Korean J. Ginseng Sci. 1997, 21, 115–118. [Google Scholar]
- Ahirwar, R.K. Effect of sowing depth on seed germination of Bute afrondosa(Roxb.). Int. Res. J. Biol. Sci. 2015, 4, 45–47. [Google Scholar]
- Ali, S.A.; Idris, A.Y. Effect of seed size and sowing depth on germination and some growth parameters of faba bean (Vicia faba L.). Agric. Biol. Sci. J. 2015, 1, 1–5. [Google Scholar]
- Guo, K.; Li, R.; Werger, M.J.A. Effect of acorn burying depth on germination, seedling emergence and development of Quercus aliena var. acuteserrata. Acta Bot. Sin. 2001, 43, 974–978. [Google Scholar]
- Guo, C.; Yang, J.; Lu, D.; Zhao, L. Impacts of burial and insect infection on germination and seedling growth of acorns of Quercus variabilis. For. Ecol. Man. 2009, 258, 1497–1502. [Google Scholar] [CrossRef]
- Proctor, J.T.; Sullivan, J.A. Effect of seeding depth on seedling growth and dry matter partitioning in American ginseng. J. Ginseng Res. 2013, 37, 254. [Google Scholar] [CrossRef]
- Masilamani, P.; Singh, G.B.; Chinnusamy, C.; Annadurai, K. Influence of seed orientation and depth of sowing on germination and vigour of Anjan (HardwickiabinataRoxb.). Trop. Agric. Res. Extension 1999, 2, 76–78. [Google Scholar]
- Mahgoub, S. The influence of positioning of seed during sowing on seed germination of some tree species in relation to germination type and seed shape. In Proceedings of the IUFRO Symposium on Innovations in Tropical Tree Seed Technology, Tampere, Finland, 6–12 August; 1995; pp. 149–159. [Google Scholar]
- BennetClark, T.A.; Younis, A.F.; Esnault, R. Geotropic behavior of roots. J. Exp. Bot. 1959, 10, 69–86. [Google Scholar] [CrossRef]
- Gregorio, N.O.; Herbohn, J.L.; Harrison, S.R. The potential role of nurseries in improving access to high quality planting stock and promoting appropriate silvicultural systems to improve the productivity of smallholder tree farms in Leyte, Philippines. In ACIAR Smallholder Forestry Project–Redevelopment of a Timber Industry Following Extensive Land Clearing: Proceedings from the End-of-Project Workshop; Harrison, S.R., Herbohn, J.L., Suh, J., Mangaoang, E., Vanclay, J., Eds.; ACIAR Smallholder Forestry Project: Ormoc, Phillipines, 2004; pp. 269–278. [Google Scholar]
- Allen, K.S.; Harper, R.W.; Bayer, A.; Brazee, N.J. A review of nursery production systems and their influence on urban tree survival. Urban For. Urban Green 2017, 21, 183–191. [Google Scholar] [CrossRef]
- Kumar, A.; Memo, M.; Mastinu, A. Plant behaviour: An evolutionary response to the environment? Plant Biol. 2020, 22, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.J.; Atkinson, C.J.; Bengough, A.G.; Else, M.A.; Fernandez-Fernandez, F.; Harrison, R.J.; Schmidt, S. Contributions of roots and rootstocks to sustainable, intensified crop production. J. Exp. Bot. 2013, 64, 1209–1222. [Google Scholar] [CrossRef]
- Seidel, S.J.; Gaiser, T.; Kautz, T.; Bauke, S.L.; Amelung, W.; Barfus, K.; Ewert, F.; Athmann, M. Estimation of the impact of precrops and climate variability on soil depth differentiated spring wheat growth and water, nitrogen and phosphorus uptake. Soil Till. Res. 2019, 195, 104427. [Google Scholar] [CrossRef]
- Chaichi, M.; Nemati, A.; Dadrasi, A.; Heydari, M.; Hassanisaadi, M.; Yousefi, A.R.; Baldwin, T.C.; Mastinu, A. Germination of Triticum aestivum L.: Effects of Soil–Seed Interaction on the Growth of Seedlings. Soil Syst. 2022, 6, 37. [Google Scholar] [CrossRef]
- Adams, C.A.; Rinne, R.W. Moisture Content as a Controlling Factor in Seed Development and Germination. In International Review of Cytology; Bourne, G.H., Danielli, J.F., Jeon, K.W., Eds.; Academic Press, 1980; Volume 68, pp. 1–8. [Google Scholar] [CrossRef]
- Rizzardi, M.; Luiz, A.R.; Roman, E.S.; Vargas, L. Effect of Cardinal Temperature and Water Potential on Morning Glory (Ipomoea triloba) Seed Germination. Planta Daninha 2009, 27, 13–21. [Google Scholar] [CrossRef]
- Ahn, J.; Oh, S.; Kang, Y.J.; Kim, K.; Moon, S.-K.; Moon, B.; Myung, S.; Kim, M.-S.; Lee, Y.K.; Ko, K. Effect of Oak Tree Sawdust Fermentation Period on Peanut Seed Germination, Seedling Biomass, and Morphology. Horticulturae 2021, 7, 182. [Google Scholar] [CrossRef]
- Wang, X.; Wu, R.; Shen, T.; Li, Z.; Li, C.; Wu, B.; Jiang, H.; Zhao, G. An R2R3-MYB Transcription Factor OsMYBAS1 Promotes Seed Germination under Different Sowing Depths in Transgenic Rice. Plants 2022, 11, 139. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).