Compensatory Growth and Physiological Protective Mechanisms of Populus talassica Kom. × Populus euphratica Oliv. in Response to Leaf Damage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Study Site
2.2. Plant Material and Experimental Treatments
2.3. Measurement of Growth Indexes
2.4. Measurement of Leaf Morphological Indexes
2.5. Measurements of Chlorophyll Content
2.6. Measurements of Photosynthetic Parameters
2.7. Measurements of Stomatal Indexes
2.8. Measurements of Anatomical Indexes
2.9. Measurements of Biochemical Indexes
2.10. Statistical Analysis
3. Result
3.1. Damage Treatment Effects on Growth Indicators
3.2. Damage Treatment Effects on Leaf Growth Indicators
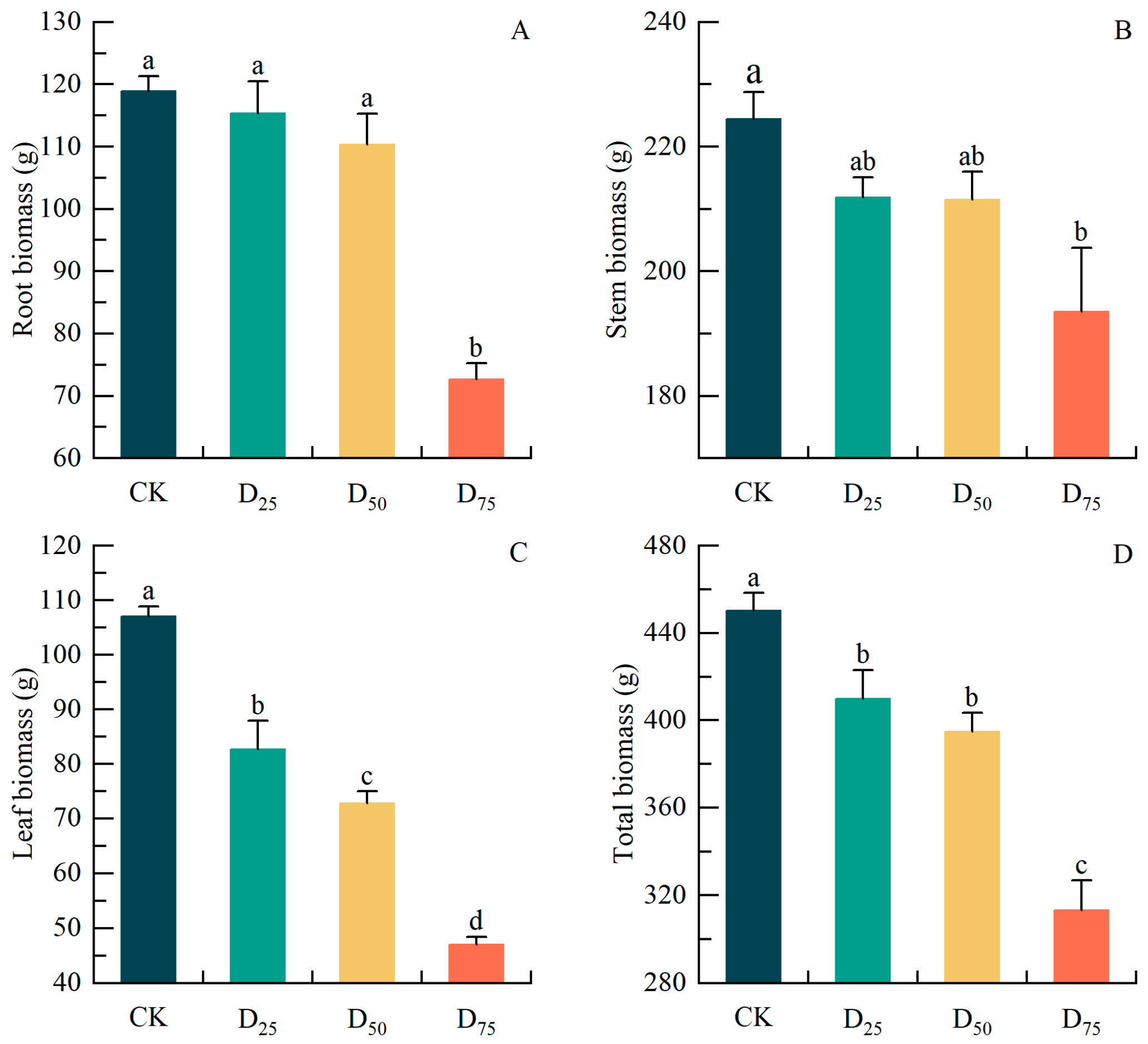
3.3. Damage Treatment Effects on Biomass
3.4. Damage Treatment Effects on R/S, QI and CI
3.5. Damage Treatment Effects on Chlorophyll Contents
3.6. Damage Treatment Effects on Photosynthetic Parameters
3.7. Damage Treatment Effects on Stomatal Characteristics
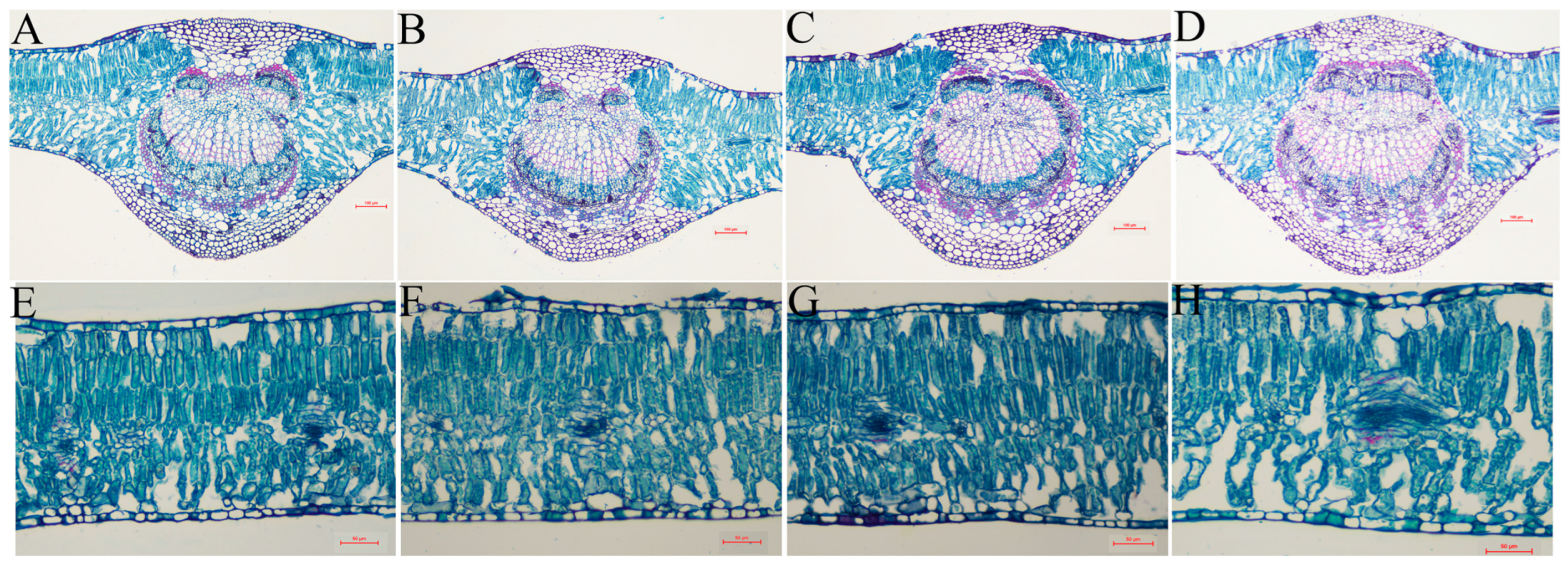
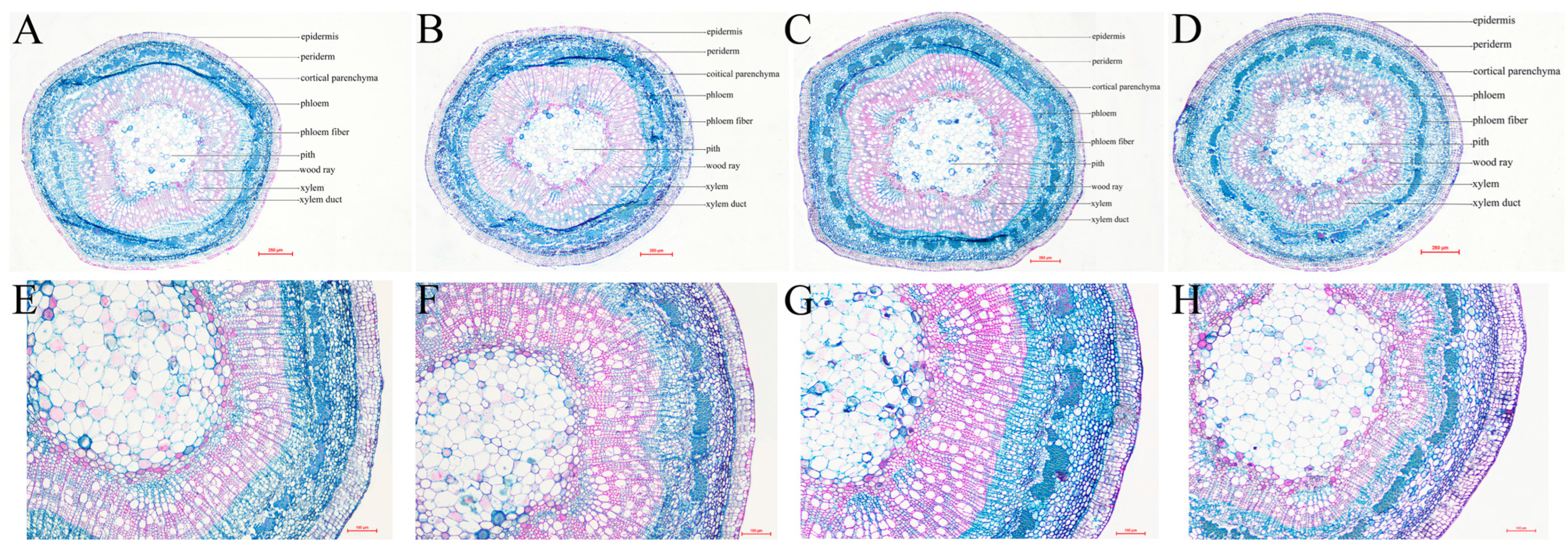
3.8. Damage Treatment Effects on Anatomical Structure of Stems and Leaves
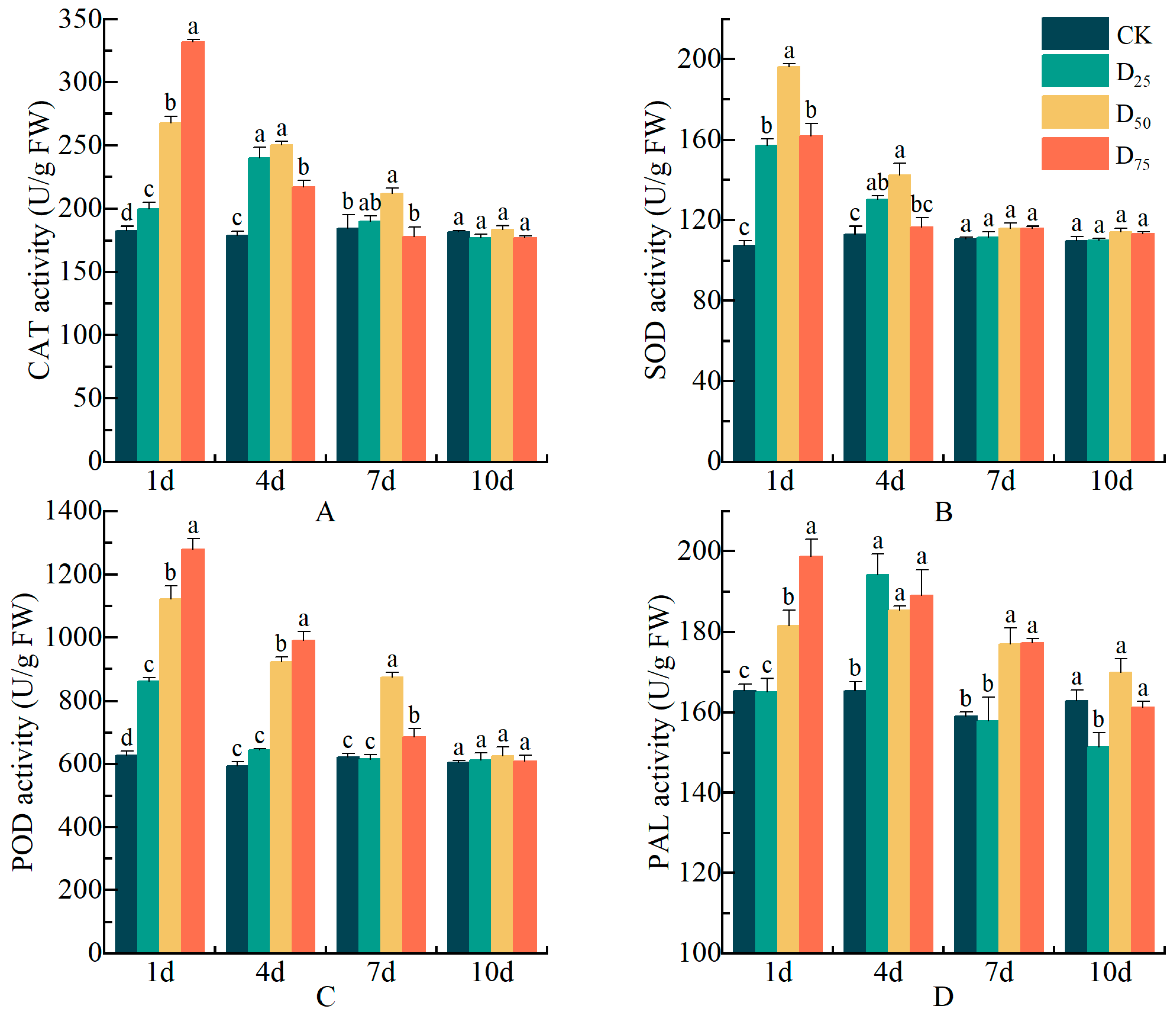
3.9. Damage Treatment Effects on Enzyme Activities
4. Discussion
4.1. Effects of Leaf Damage on Growth and Biomass of P. talassica × P. euphratica
4.2. Effects of Leaf Damage on Physiological Characteristics of P. talassica × P. euphratica
4.3. Effects of Leaf Damage on Anatomical Structures of P. talassica × P. euphratica
4.4. Effects of Leaf Damage on Defense Enzyme Activities of P. talassica × P. euphratica
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Q.; Jin, Y.; Guo, X.; Xu, M.; Wei, G.; Lu, X.; Tang, Z. Metabolomic responses to the mechanical wounding of Catharanthus roseus’ upper leaves. PeerJ 2023, 11, e14539. [Google Scholar] [CrossRef]
- Béland, J.M.; Bauce, É.; Cloutier, C.; Berthiaume, R.; Hébert, C. Accuracy of defoliation estimates from aerial and ground surveys in a boreal forest during an outbreak of the hemlock looper, Lambdina fiscellaria (Guenée). Forests 2022, 13, 1120. [Google Scholar] [CrossRef]
- Ferretti, M.; Ghosh, S.; Gottardini, E. Stem radial growth is negatively related to tree defoliation and damage in conifers, northern Italy. Front. For. Glob. Chang. 2021, 4, 775600. [Google Scholar] [CrossRef]
- Eyles, A.; Pinkard, E.A.; Mohammed, C. Shifts in biomass and resource allocation patterns following defoliation in Eucalyptus globulus growing with varying water and nutrient supplies. Tree Physiol. 2009, 29, 753–764. [Google Scholar] [CrossRef]
- Wang, N.; Ji, T.; Liu, X.; Li, Q.; Sairebieli, K.; Wu, P.; Song, H.; Wang, H.; Du, N.; Zheng, P.; et al. Defoliation significantly suppressed plant growth under low light conditions in two Leguminosae species. Front. Plant Sci. 2022, 12, 777328. [Google Scholar] [CrossRef]
- Helbig, C.E.; Müller, M.G.; Landgraf, D. Effects of leaf loss by artificial defoliation on the growth of different Poplar and Willow Varieties. Forests 2021, 12, 1224. [Google Scholar] [CrossRef]
- Alcorn, P.J.; Bauhus, J.; Smith, R.G.B.; Thomas, D.; James, R.; Nicotra, A. Growth response following green crown pruning in plantation-grown Eucalyptus pilularis and Eucalyptus cloeziana. Can. J. Forest Res. 2008, 38, 770–781. [Google Scholar] [CrossRef]
- Willaume, M.; Pagès, L. How periodic growth pattern and source/sink relations affect root growth in oak tree seedlings. J. Exp. Bot. 2006, 57, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Corona, C.; Leeper, A.C.; LaMontagne, J.M. Differential defoliation and mortality of white spruce and balsam fir by eastern spruce budworm. Forest Ecol. Manag. 2022, 508, 120042. [Google Scholar] [CrossRef]
- Quentin, A.G.; O’Grady, A.P.; Beadle, C.L.; Mohammed, C.; Pinkard, E.A. Interactive effects of water supply and defoliation on photosynthesis, plant water status and growth of Eucalyptus globulus Labill. Tree Physiol. 2012, 32, 958–967. [Google Scholar] [CrossRef]
- Eyles, A.; Smith, D.; Pinkard, E.A.; Smith, L.; Corkrey, R.; Elms, S.; Beadle, C.; Mohammed, C. Photosynthetic responses of fild-grown Pinus radiata trees to artificial and aphid-induced defoliation. Tree Physiol. 2011, 31, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Gleason, S.M.; Ares, A. Photosynthesis, carbohydrate storage and survival of a native and an introduced tree species in relation to light and defoliation. Tree Physiol. 2004, 24, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Zhao, W.L.; Gao, X.W. Communication between plants: Induced resistance in poplar seedlings following herbivore infestation, mechanical wounding, and volatile treatment of the neighbors. Entomol. Exp. Appl. 2013, 149, 110–117. [Google Scholar] [CrossRef]
- Wang, Q.W.; Jia, L.Y.; Shi, D.L.; Wang, R.F.; Lu, L.N.; Xia, J.J.; Sun, k.; Feng, H.Q.; Li, X. Effects of extracellular ATP on local and systemic responses of bean (Phaseolus vulgaris L) leaves to wounding. Biosci. Biotechol. Biochem. 2018, 83, 417–428. [Google Scholar] [CrossRef]
- Su, M.; Zhang, M.; Liu, Y.; Han, Z. Abscisic acid, paclobutrazol, and salicylic acid alleviate salt stress in Populus talassica × Populus euphratica by modulating plant root architecture, photosynthesis, and the antioxidant defense system. Forests 2022, 13, 1864. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Su, M.X.; Han, Z.J.; Shi, J.Y. Evaluation of salt and drought tolerances of Populus talassica × Populus euphratica seedlings using leaf anatomical structures and physiological processes. Pak. J. Bot. 2023, 55, 1205–1214. [Google Scholar] [CrossRef]
- Belsky, A.J.; Carson, W.P.; Jensen, C.L.; Fox, G.A. Overcompensation by plants: Herbivoreo ptimization of red herring? Evol. Ecol. 1993, 7, 109–121. [Google Scholar] [CrossRef]
- Yang, Z.J.; Wu, X.H.; Grossnickle, S.C.; Chen, L.H.; Yu, X.X.; EI-Kassaby, Y.A.; Feng, J.L. Formula fertilization promotes Phoebe bournei robust seedling cultivation. Forests 2020, 11, 781. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, M.; Qi, L.; Sun, X.; Li, Q.; Liu, X.; Wang, N.; Zwiazek, J.J.; Zhang, W.; Guo, W.; et al. Shading reduced the compensation and enhancement effects of soil nutrition on the growth of cotyledon-damaged Quercus acutissima seedlings. Plant Soil 2022, 482, 665–678. [Google Scholar] [CrossRef]
- Martínez-Sancho, E.; Vásconez Navas, L.K.; Seidel, H.; Dorado-Liñán, I.; Menzel, A. Responses of contrasting tree functional types to air warming and drought. Forests 2017, 8, 450. [Google Scholar] [CrossRef]
- Fan, X.; Cao, X.; Zhou, H.; Hao, L.; Dong, W.; He, C.; Xu, M.; Wu, H.; Wang, L.; Chang, Z.; et al. Carbon dioxide fertilization effect on plant growth under soil water stress associates with changes in stomatal traits, leaf photosynthesis, and foliar nitrogen of bell pepper (Capsicum annuum L.). Environ. Exp. Bot. 2020, 179, 104203. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, L.; Gao, C.; Liao, D.; Long, L.; Qiu, J.; Wei, H.; Deng, Q.; Zhou, Y. A comparative study on the leaf anatomical structure of Camellia oleifera in a low-hot valley area in Guizhou Province, China. PLoS ONE 2022, 17, e0262509. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, C.; Meng, Z.; Li, Y.; Abid, M.A.; Askari, M.; Wang, P.; Wang, Y.; Sun, G.; Cai, Y.; et al. Leveraging Atriplex hortensis choline monooxygenase to improve chilling tolerance in cotton. Environ. Exp. Bot. 2019, 162, 364–373. [Google Scholar] [CrossRef]
- Peskin, A.V.; Winterbourn, C.C. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1). Clin. Chim. Acta 2000, 293, 157–166. [Google Scholar] [CrossRef]
- Rösler, J.; Krekel, F.; Amrhein, N.; Schmid, J. Maize phenylalanine ammonia-lyase has tyrosine ammonia-lyase activity. Plant Physiol. 1997, 113, 175–179. [Google Scholar] [CrossRef]
- Fukushima, M.; Kanzaki, M.; Hara, M.; Ohkubo, T.; Preechapanya, P.; Choocharoen, C. Secondary forest succession after the cessation of swidden cultivation in the montane forest area in Northern Thailand. For. Ecol. Manag. 2008, 255, 1994–2006. [Google Scholar] [CrossRef]
- Li, C.; Barclay, H.; Huang, S.; Roitberg, B.; Lalonde, R.; Xu, W.; Chen, Y. Modelling the stand dynamics after a thinning induced partial mortality: A compensatory growth perspective. Front. Plant Sci. 2022, 13, 1044637. [Google Scholar] [CrossRef]
- Quijano-Medina, T.; Covelo, F.; Moreira, X.; Abdala-Roberts, L. Compensation to simulated insect leaf herbivory in wild cotton (Gossypium hirsutum): Responses to multiple levels of damage and associated traits. Plant Biol. 2019, 21, 805–812. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Z.; Wang, C. Defoliation-induced tree growth declines are jointly limited by carbon source and sink activities. Sci. Total Environ. 2021, 762, 143077. [Google Scholar] [CrossRef]
- Jansen, M.; Zuidema, P.A.; van Ast, A.; Bongers, F.; Malosetti, M.; Martínez-Ramos, M.; Núñez-Farfán, J.; Anten, N.P.R. Heritability of growth and leaf loss compensation in a long-lived tropical understorey palm. PLoS ONE 2019, 14, e0209631. [Google Scholar] [CrossRef]
- Iván, D.C.; Rosalinda, T.; Juan, N. Ecotypic variation in growth responses to simulated herbivory: Trade-off between maximum relative growth rate and tolerance to defoliation in an annual plant. AoB Plants 2015, 7, plv015. [Google Scholar]
- Powers, S.J.; Peacock, L.; Yap, M.L.; Brain, P. Simulated beetle defoliation on willow genotypes in mixture and monotype plantations. Ann. Appl. Biol. 2006, 148, 27–38. [Google Scholar] [CrossRef]
- Ambebe, T.F.; Mendi, A.G.; Shidiki, A.A. Influence of simulated defoliation and N fertilization on compensatory growth of Gmelina arborea seedlings. J. Appl. Life Sci. Int. 2020, 3, 59–66. [Google Scholar] [CrossRef]
- Wang, N.; Song, M.; Zhang, Y.; Liu, X.; Wu, P.; Qi, L.; Song, H.; Du, N.; Wang, H.; Zheng, P.; et al. Physiological responses of Quercus acutissima and Quercus rubra seedlings to drought and defoliation treatments. Tree Physiol. 2023, 43, 737–750. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, T.J.; Li, B.H.; Liang, W.J.; Zhang, Q.L.; Cai, M.L.; Peng, C.L. Strong response of stem photosynthesis to defoliation in Mikania micrantha highlights the contribution of phenotypic plasticity to plant invasiveness. Front. Plant Sci. 2021, 12, 638796. [Google Scholar] [CrossRef] [PubMed]
- Sweet, G.B.; Wareing, P.F. Role of plant growth in regulating photosynthesis. Nature 1966, 210, 77. [Google Scholar] [CrossRef]
- Li, W.H.; Luo, J.N.; Tian, X.S.; Peng, C.L.; Zhou, X.Y. Patterns of defoliation and their effect on the plant growth and photosynthetic characteristics of Ipomoea cairica. Weed Biol. Manag. 2012, 12, 40–46. [Google Scholar] [CrossRef]
- Raza, M.A.; Gul, H.; Yang, F.; Ahmed, M.; Yang, W. Growth rate, dry matter accumulation, and partitioning in soybean (Glycine max L.) in response to defoliation under high-rainfall conditions. Plants 2021, 10, 1497. [Google Scholar] [CrossRef]
- Aguirre, N.M.; Grunseich, J.M.; Lima, A.F.; Davis, S.; Helms, A.M. Plant communication across different environmental contexts suggests a role for stomata in volatile perception. Plant Cell Environ. 2023, 46, 2017–2030. [Google Scholar] [CrossRef]
- Jordi, W.; Schapendonk, A.; Davelaar, E.; Stoopen, G.M.; Pot, C.S.; De Visser, R.; Van Rhijn, J.A.; Gan, S.; Amasino, R.M. Increased cytokinin levels in transgenic PSAG12-IPT tobacco plants have large direct and indirect effects on leaf senescence, photosynthesis and N partitioning. Plant Cell Environ. 2000, 23, 279–289. [Google Scholar] [CrossRef]
- Õunapuu-Pikas, E.; Sellin, A. Plasticity and light sensitivity of leaf hydraulic conductance to fast changes in irradiance in common hazel (Corylus avellana L.). Plant Sci. 2020, 290, 110299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, J.; Ge, X.; Cao, D.; Hu, J. Leaf size development differences and comparative transcriptome analyses of two Poplar Genotypes. Genes 2021, 12, 1775. [Google Scholar] [CrossRef] [PubMed]
- Holloway-Phillips, M. Illuminating photosynthesis in the mesophyll of diverse leaves. Plant Physiol. 2019, 180, 1256–1258. [Google Scholar] [CrossRef]
- Knapp, A.; Carter, G. Variability in leaf optical properties among 26 species from a broad range of habitats. Am. J. Bot. 1998, 85, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.N.; John, G.P.; Scoffoni, C.; Sack, L. How does leaf anatomy influence water transport outside the xylem? Plant Physiol. 2015, 168, 1616–1635. [Google Scholar] [CrossRef]
- Hillabrand, R.M.; Hacke, U.G.; Lieffers, V.J. Defoliation constrains xylem and phloem functionality. Tree Physiol. 2019, 39, 1099–1108. [Google Scholar] [CrossRef]
- Stratton, L.; Goldstein, G.; Meinzer, F.C. Stem water storage capacity and efficiency of water transport: Their functional significance in a Hawaiian dry forest. Plant Cell Environ. 2000, 23, 99–106. [Google Scholar] [CrossRef]
- Shafqat, W.; Mazrou, Y.S.A.; Sami-ur-Rehman; Nehela, Y.; Ikram, S.; Bibi, S.; Naqvi, S.A.; Hameed, M.; Jaskani, M.J. Effect of three water regimes on the physiological and anatomical structure of stem and leaves of different citrus rootstocks with distinct degrees of tolerance to drought stress. Horticulturae 2021, 7, 554. [Google Scholar] [CrossRef]
- Shah, A.Z.; Ma, C.; Zhang, Y.; Zhang, Q.; Xu, G.; Yang, G. Decoyinine induced resistance in rice against small brown planthopper Laodelphax striatellus. Insects 2022, 13, 104. [Google Scholar] [CrossRef]
- Andersone, U.; Samsone, I.; Ievinsh, G. Neodiprion sertifer defoliation causes long-term systemic changes of oxidative enzyme activities in Scots pine needles. Anthropod-Plant Interact. 2009, 3, 209–214. [Google Scholar] [CrossRef]
- Arimura, G.; Kost, C.; Boland, W. Herbivore-induced, indirect plant defences. BBA-Mol. Cell Biol. Lipids 2005, 1734, 91–111. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Shen, Y.; Zhang, Z. Effects of mechanical damage and herbivore wounding on H2O2 metabolism and antioxidant enzyme activities in hybrid poplar leaves. J. Forestry Res. 2009, 20, 156–160. [Google Scholar] [CrossRef]
- Lucija, L.; Ivana, R.R.; Ivan, L.; Nenad, P.; Ivan, S.; Mia, M.; Ana, J.T.; Tamara, J.; Lukrecija, B. Are foliar nutrition status and indicators of oxidative stress associated with tree defoliation of four mediterranean forest species? Plants 2022, 11, 3484. [Google Scholar]
- Song, Y.; Simard, S.; Carroll, A.; Mohn, W.W.; Zeng, R.S. Defoliation of interior Douglas-fir elicits carbon transfer and stress signalling to ponderosa pine neighbors through ectomycorrhizal networks. Sci. Rep. 2015, 5, 8495. [Google Scholar] [CrossRef] [PubMed]
- Hara-Nishimura, I.; Matsushima, R. A wound-inducible organelle derived from endoplasmic reticulum: A plant strategy against environmental stresses? Curr. Opin. Plant Biol. 2003, 6, 583–588. [Google Scholar] [CrossRef]




| Treatment | Damage Treatment |
|---|---|
| CK | no defoliation treatment |
| D25 | cutting 1 leaf per 4 leaves |
| D50 | cutting 1 leaf per 2 leaves |
| D75 | cutting 3 leaves per 4 leaves |
| Treatment | Plant Height Growth (cm) | Ground Diameter Growth (cm) | Crown Width Growth (cm) | |||
|---|---|---|---|---|---|---|
| 0–30 d | 30–60 d | 0–30 d | 30–60 d | 0–30 d | 30–60 d | |
| CK | 12.180 ± 0.697 a | 3.400 ± 0.499 b | 0.969 ± 0.090 b | 0.971 ± 0.090 a | 15.460 ± 0.544 a | 9.680 ± 0.711 a |
| D25 | 11.500 ± 1.114 a | 5.640 ± 0.898 a | 1.491 ± 0.070 a | 1.004 ± 0.070 a | 16.730 ± 0.892 a | 3.910 ± 0.859 b |
| D50 | 11.080 ± 0.708 a | 2.800 ± 0.042 b | 1.105 ± 0.104 b | 0.974 ± 0.042 a | 8.700 ± 0.293 b | 4.520 ± 0.590 b |
| D75 | 8.180 ± 0.491 b | 2.300 ± 0.257 b | 0.652 ± 0.035 c | 0.568 ± 0.061 b | −4.200 ± 0.110 c | 3.020 ± 0.200 b |
| Days After Treatment (d) | Treament | Chl a Content (mg/g) | Chl b Content (mg/g) | Total Chl Content (mg/g) |
|---|---|---|---|---|
| 5 d | CK | 2.118 ± 0.006 ab | 0.795 ± 0.026 ab | 2.913 ± 0.021 ab |
| D25 | 2.242 ± 0.073 a | 0.852 ± 0.039 a | 3.094 ± 0.084 a | |
| D50 | 2.045 ± 0.075 b | 0.738 ± 0.039 bc | 2.783 ± 0.114 b | |
| D75 | 1.843 ± 0.013 c | 0.678 ± 0.007 c | 2.520 ± 0.013 c | |
| 10 d | CK | 2.040 ± 0.052 bc | 0.743 ± 0.015 ab | 2.783 ± 0.067 bc |
| D25 | 1.972 ± 0.017 c | 0.703 ± 0.024 b | 2.675 ± 0.037 c | |
| D50 | 2.145 ± 0.010 a | 0.789 ± 0.004 a | 2.934 ± 0.006 a | |
| D75 | 2.122 ± 0.023 ab | 0.758 ± 0.012 a | 2.879 ± 0.033 ab | |
| 30 d | CK | 1.431 ± 0.002 bc | 0.519 ± 0.009 ab | 1.950 ± 0.010 bc |
| D25 | 1.509 ± 0.028 ab | 0.556 ± 0.015 a | 2.065 ± 0.043 ab | |
| D50 | 1.553 ± 0.011 a | 0.559 ± 0.010 a | 2.112 ± 0.010 a | |
| D75 | 1.367 ± 0.051 c | 0.497 ± 0.024 b | 1.863 ± 0.075 c | |
| 60 d | CK | 1.555 ± 0.081 b | 0.546 ± 0.035 b | 2.101 ± 0.115 b |
| D25 | 1.899 ± 0.055 a | 0.708 ± 0.011 a | 2.607 ± 0.065 a | |
| D50 | 1.540 ± 0.041 b | 0.544 ± 0.012 b | 2.085 ± 0.053 b | |
| D75 | 1.756 ± 0.008 a | 0.698 ± 0.002 a | 2.454 ± 0.007 a |
| Treatment | Stomatal Length (μm) | Stomatal Width (μm) | Stomatal Area (μm2) | Stomatal Density (number/mm2) | Stomatal Aperture (μm) | Proportion of Opened Stomata (%) | Stomatal Shape Index | Stomatal Area Index |
|---|---|---|---|---|---|---|---|---|
| CK | 26.755 ± 0.197 a | 14.273 ± 0.204 b | 264.358 ± 3.051 b | 171.200 ± 2.133 a | 7.684 ± 0.219 c | 0.585 ± 0.112 c | 1.173 ± 0.007 a | 0.045 ± 0.017 a |
| D25 | 26.495 ± 0.363 a | 14.312 ± 0.358 b | 266.592 ± 3.920 b | 154.000 ± 2.813 b | 8.351 ± 0.139 b | 0.631 ± 0.112 b | 1.144 ± 0.083 b | 0.041 ± 0.010 a |
| D50 | 25.544 ± 0.348 b | 15.499 ± 0.367 a | 286.558 ± 5.597 a | 157.200 ± 1.890 b | 9.472 ± 0.368 a | 0.650 ± 0.192 a | 1.103 ± 0.088 c | 0.045 ± 0.017 a |
| D75 | 27.314 ± 0.310 a | 15.215 ± 0.117 a | 281.428 ± 4.735 a | 151.200 ± 2.048 b | 8.536 ± 0.289 b | 0.500 ± 0.017 d | 1.132 ± 0.073 b | 0.042 ± 0.013 a |
| Treatment | Upper Epidermis Thickness (μm) | Leaf Thickness (μm) | Palisade Tissue Thickness (μm) | Spongy Tissue Thickness (μm) | Palisade/Spongy | Leaf Structure Tightness |
|---|---|---|---|---|---|---|
| CK | 12.782 ± 0.115 c | 294.944 ± 1.417 c | 135.483 ± 0.707 c | 125.255 ± 0.653 a | 1.082 ± 0.009 c | 0.459 ± 0.002 c |
| D25 | 12.958 ± 0.100 c | 297.447 ± 0.530 bc | 137.116 ± 0.579 c | 124.994 ± 0.364 a | 1.097 ± 0.006 c | 0.461 ± 0.002 bc |
| D50 | 13.714 ± 0.133 b | 299.563 ± 1.144 b | 140.512 ± 0.775 b | 125.517 ± 0.628 a | 1.120 ± 0.008 b | 0.469 ± 0.004 b |
| D75 | 14.081 ± 0.125 a | 310.119 ± 1.379 a | 151.972 ± 0.897 a | 120.861 ± 0.305 b | 1.257 ± 0.007 a | 0.490 ± 0.003 a |
| Treatment | Epidermi Thickness (μm) | Cortica Thickness (μm) | Phloem Thickness (μm) | Xylem Thickness (μm) | Pith Diameter (μm) |
|---|---|---|---|---|---|
| CK | 10.703 ± 0.189 b | 115.900 ± 3.051 a | 167.899 ± 1.959 b | 227.513 ± 2.012 b | 747.603 ± 4.088 b |
| D25 | 10.898 ± 0.239 ab | 119.139 ± 1.005 a | 166.229 ± 1.520 b | 229.445 ± 2.751 b | 743.546 ± 8.542 b |
| D50 | 11.531 ± 0.167 a | 119.364 ± 1.169 a | 191.663 ± 2.656 a | 345.070 ± 3.861 a | 931.236 ± 2.349 a |
| D75 | 11.335 ± 0.239 ab | 114.504 ± 1.543 a | 148.681 ± 2.189 c | 226.013 ± 1.698 b | 756.657 ± 8.045 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, M.-X.; Han, Z.-J.; Liu, Y.; Zhao, Z.; Wu, J.-J. Compensatory Growth and Physiological Protective Mechanisms of Populus talassica Kom. × Populus euphratica Oliv. in Response to Leaf Damage. Forests 2023, 14, 1713. https://doi.org/10.3390/f14091713
Su M-X, Han Z-J, Liu Y, Zhao Z, Wu J-J. Compensatory Growth and Physiological Protective Mechanisms of Populus talassica Kom. × Populus euphratica Oliv. in Response to Leaf Damage. Forests. 2023; 14(9):1713. https://doi.org/10.3390/f14091713
Chicago/Turabian StyleSu, Meng-Xu, Zhan-Jiang Han, Ying Liu, Zhen Zhao, and Jia-Ju Wu. 2023. "Compensatory Growth and Physiological Protective Mechanisms of Populus talassica Kom. × Populus euphratica Oliv. in Response to Leaf Damage" Forests 14, no. 9: 1713. https://doi.org/10.3390/f14091713
APA StyleSu, M.-X., Han, Z.-J., Liu, Y., Zhao, Z., & Wu, J.-J. (2023). Compensatory Growth and Physiological Protective Mechanisms of Populus talassica Kom. × Populus euphratica Oliv. in Response to Leaf Damage. Forests, 14(9), 1713. https://doi.org/10.3390/f14091713






