The Occurrence and Genetic Variability of Tea Plant Necrotic Ring Blotch Virus in Fujian Province, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. TPNRBV Detection
2.3. Full-Genome Determination
2.4. Sequence Analysis
3. Results
3.1. Occurrence of TPNRBV in Three Tea-Growing Regions of Fujian
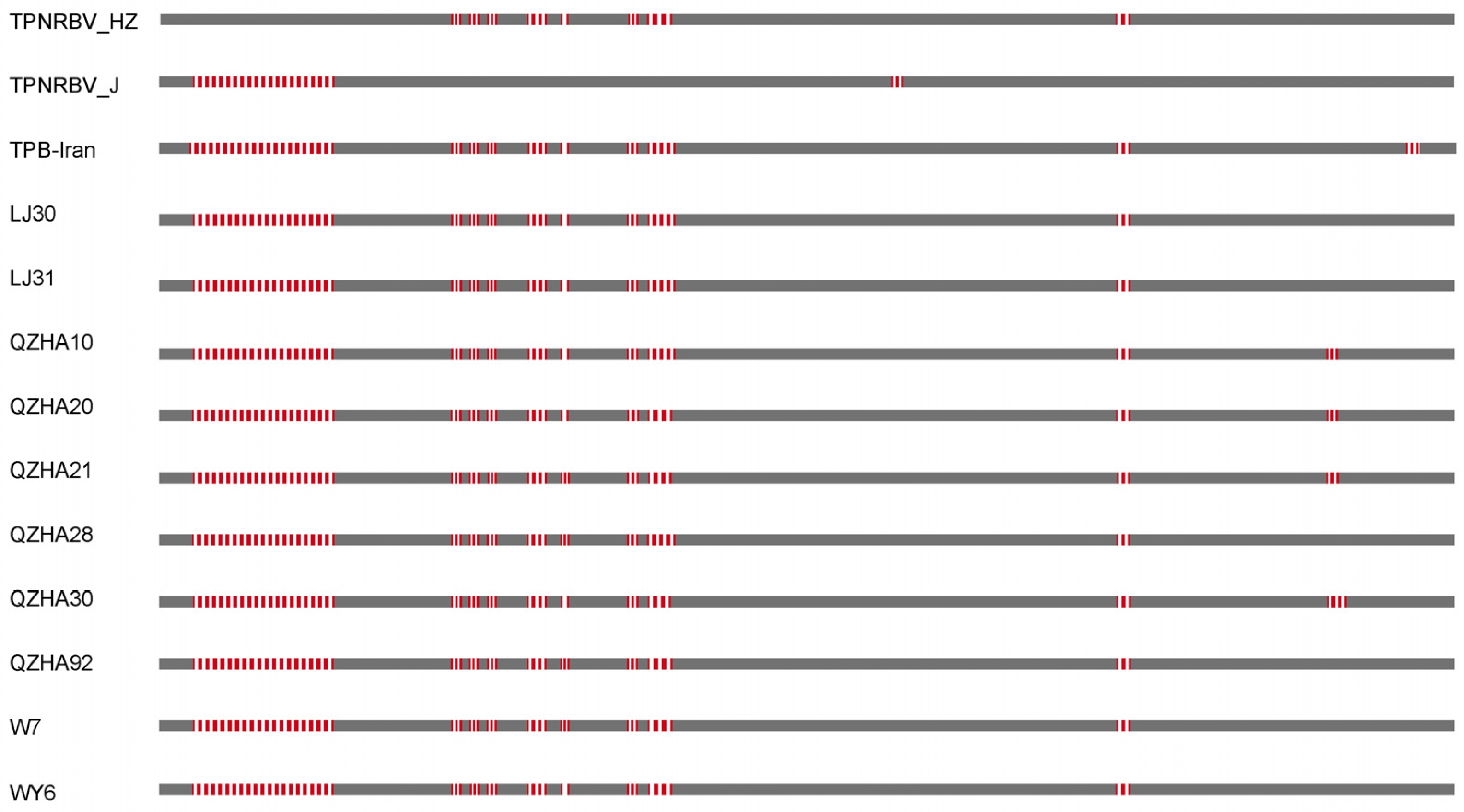
3.2. Genetic Variability of TPNRBV
3.3. A Conserved Sequence Motif at the 5′ Termini of TPNRBV Genomic Segments
3.4. Extensive Deletion/Insertion Mutations in the 3′-Noncoding Regions of TPNRBV RNA4
3.5. The Role of Natural Selection and Recombination in Shaping the Genetic Structure of TPNRBV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liang, L.; Ridoutt, B.; Wang, L.; Xie, B.; Li, M.; Li, Z. China’s Tea Industry: Net Greenhouse Gas Emissions and Mitigation Potential. Agriculture 2021, 11, 363. [Google Scholar] [CrossRef]
- Quito-Avila, D.; Freitas-Astúa, J.; Melzer, M. Bluner-, cile-, and higreviruses (Kitaviridae). In Encyclopedia of Virology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 247–251. [Google Scholar]
- Ramos-González, P.; Arena, G.; Tassi, A.; Chabi-Jesus, C.; Kitajima, E.; Freitas-Astúa, J. Kitaviruses: A window to atypical plant viruses causing nonsystemic diseases. Annu. Rev. Phytopathol. 2023, 61. [Google Scholar] [CrossRef]
- Hao, X.; Zhang, W.; Zhao, F.; Liu, Y.; Qian, W.; Wang, Y.; Wang, L.; Zeng, J.; Yang, Y.; Wang, X. Discovery of plant viruses from tea plant (Camellia sinensis (L.) O. Kuntze) by metagenomic sequencing. Front. Microbiol. 2018, 9, 2175. [Google Scholar] [CrossRef]
- Ramos-González, P.L.; Kondo, H.; Morozov, S.; Vasilakis, N.; Varsani, A.; Cao, M.; Freitas-Astúa, J. The border between kitavirids and nege-like viruses: Tracking the evolutionary pace of plant- and arthropod-infecting viruses. Front. Plant Sci. 2022, 13, 932523. [Google Scholar] [CrossRef]
- Nazerian, E.; Bayat, H. Occurrence of tea plant necrotic ring blotch virus in Iran. J. Plant Prot. Res. 2021, 61, 200–202. [Google Scholar]
- Maruyama, N.; Nozomu, I.; Nishikawa, M.; Nijo, T.; Yoshida, T.; Kitazawa, Y.; Maejima, K.; Namba, S.; Yamaji, Y. Complete genome sequence of tea plant necrotic ring blotch virus detected from a tea plant in Japan. Microbiol. Resour. Announc. 2022, 11, e00323-22. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Mock, R.; Huang, Q.; Abad, J.; Hartung, J.; Kinard, G. A reliable and inexpensive method of nucleic acid extraction for the PCR-based detection of diverse plant pathogens. J. Virol. Methods 2008, 154, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Muhire, B.M.; Varsani, A.; Martin, D.P. SDT: A virus classification tool based on pairwise sequence alignment and identity calculation. PLoS ONE 2014, 9, e108277. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2014, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.-Y.; Gao, F.; Jakovlić, I.; Lei, H.-P.; Hu, Y.; Zhang, H.; Zou, H.; Wang, G.-T.; Zhang, D. Using PhyloSuite for molecular phylogeny and tree-based analyses. iMeta 2023, 2, e87. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015, 1, vev003. [Google Scholar] [CrossRef] [PubMed]
- Kosakovsky Pond, S.L.; Posada, D.; Gravenor, M.B.; Woelk, C.H.; Frost, S.D. GARD: A genetic algorithm for recombination detection. Bioinformatics 2006, 22, 3096–3098. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chen, C.; Arab, D.A.; Du, Z.; He, Y.; Ho, S.Y.W. EasyCodeML: A visual tool for analysis of selection using CodeML. Ecol. Evol. 2019, 9, 3891–3898. [Google Scholar] [CrossRef]
- Haralampiev, I.; Prisner, S.; Nitzan, M.; Schade, M.; Jolmes, F.; Schreiber, M.; Loidolt-Krüger, M.; Jongen, K.; Chamiolo, J.; Nilson, N.; et al. Selective flexible packaging pathways of the segmented genome of influenza A virus. Nat. Commun. 2020, 11, 4355. [Google Scholar] [CrossRef]
- Ren, H.; Chen, Y.; Zhao, F.; Ding, C.; Zhang, K.; Wang, L.; Yang, Y.; Hao, X.; Wang, X. Quantitative distribution and transmission of tea plant necrotic ring blotch virus in Camellia sinensis. Forests 2022, 13, 1306. [Google Scholar] [CrossRef]
- Chabi-Jesus, C.; Ramos-González, P.L.; Postclam-Barro, M.; Fontenele, R.S.; Harakava, R.; Bassanezi, R.B.; Moreira, A.S.; Kitajima, E.W.; Varsani, A.; Freitas-Astúa, J. Molecular epidemiology of citrus leprosis virus C: A new viral lineage and phylodynamic of the main viral subpopulations in the Americas. Front. Microbiol. 2021, 12, 641252. [Google Scholar] [CrossRef]
- Ramos-González, P.L.; Chabi-Jesus, C.; Guerra-Peraza, O.; Breton, M.C.; Arena, G.D.; Nunes, M.A.; Kitajima, E.W.; Machado, M.A.; Freitas-Astúa, J. Phylogenetic and molecular variability studies reveal a new genetic clade of citrus leprosis virus C. Viruses 2016, 8, 153. [Google Scholar] [CrossRef]

| City | Location | Tested Samples | Positive Sample | Positive Rate (%) |
|---|---|---|---|---|
| Fuzhou | Lianjiang | 75 | 2 | 2.67 |
| Quanzhou | Hui’an | 20 | 16 | 80 |
| Nanping | Wuyishan | 101 | 4 | 3.96 |
| Total | 196 | 22 | 11.22 |
| Segment | Length | TVs | SVs | PIs | Hd | Pi |
|---|---|---|---|---|---|---|
| RNA1 | 5586 | 569 | 278 | 291 | 1.000 ± 0.030 | 0.027 ± 0.006 |
| RNA2 | 3636 | 226 | 160 | 66 | 1.000 ± 0.002 | 0.016 ± 0.006 |
| RNA3 | 2455 | 218 | 68 | 150 | 0.987 ± 0.035 | 0.027 ± 0.003 |
| RNA4 | 948 | 39 | 18 | 21 | 0.933 ± 0.077 | 0.014 ± 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Shen, J.; Li, M.; Gao, Y.; Du, Z.; Gao, F. The Occurrence and Genetic Variability of Tea Plant Necrotic Ring Blotch Virus in Fujian Province, China. Forests 2023, 14, 1755. https://doi.org/10.3390/f14091755
Chen X, Shen J, Li M, Gao Y, Du Z, Gao F. The Occurrence and Genetic Variability of Tea Plant Necrotic Ring Blotch Virus in Fujian Province, China. Forests. 2023; 14(9):1755. https://doi.org/10.3390/f14091755
Chicago/Turabian StyleChen, Xihong, Jianguo Shen, Min Li, Yujie Gao, Zhenguo Du, and Fangluan Gao. 2023. "The Occurrence and Genetic Variability of Tea Plant Necrotic Ring Blotch Virus in Fujian Province, China" Forests 14, no. 9: 1755. https://doi.org/10.3390/f14091755
APA StyleChen, X., Shen, J., Li, M., Gao, Y., Du, Z., & Gao, F. (2023). The Occurrence and Genetic Variability of Tea Plant Necrotic Ring Blotch Virus in Fujian Province, China. Forests, 14(9), 1755. https://doi.org/10.3390/f14091755







