Seedling Morphological Characteristics on Survival, Uniformity, and Growth during a Full Short Rotation in Eucalyptus grandis x E. urophylla Plantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Evaluations
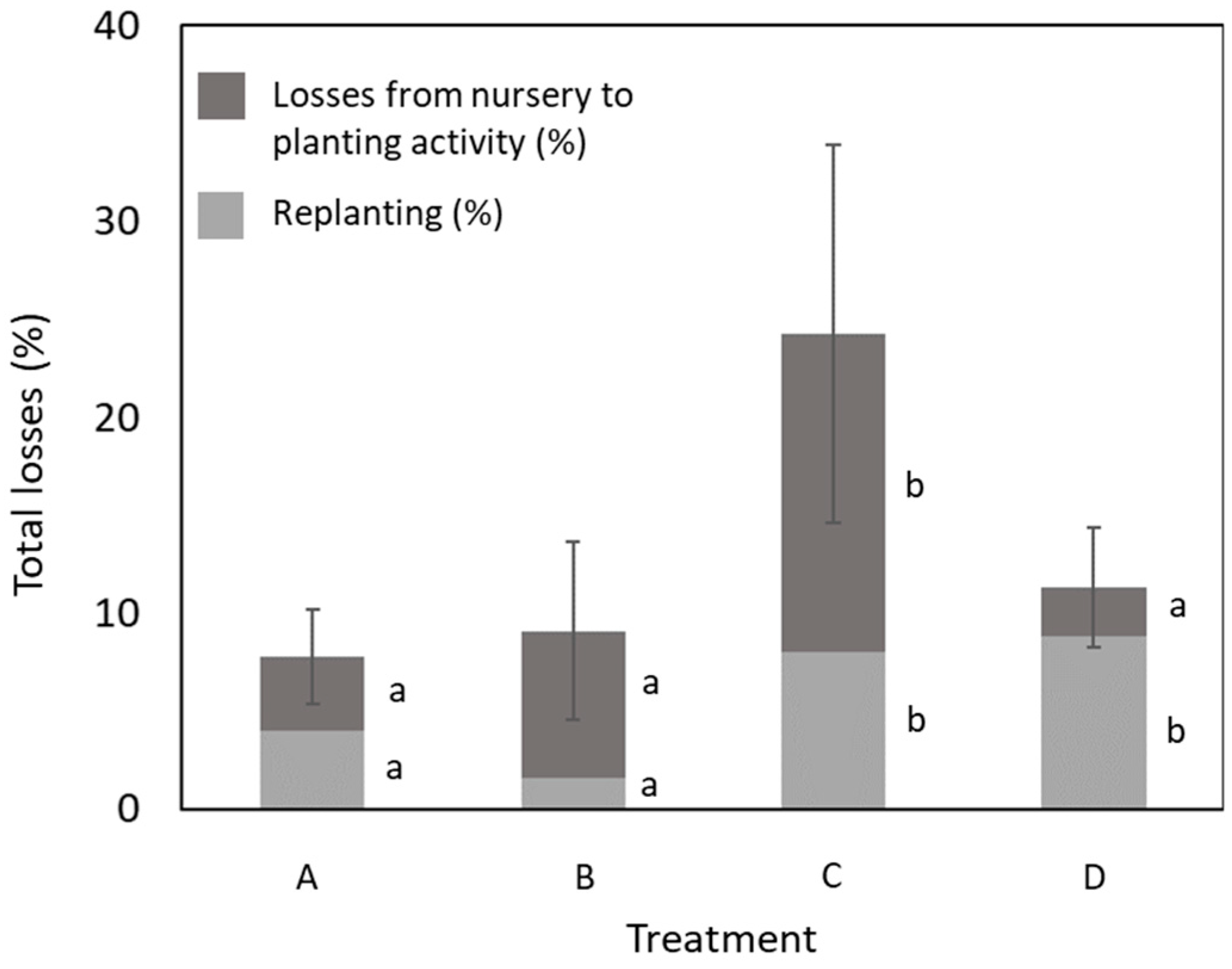
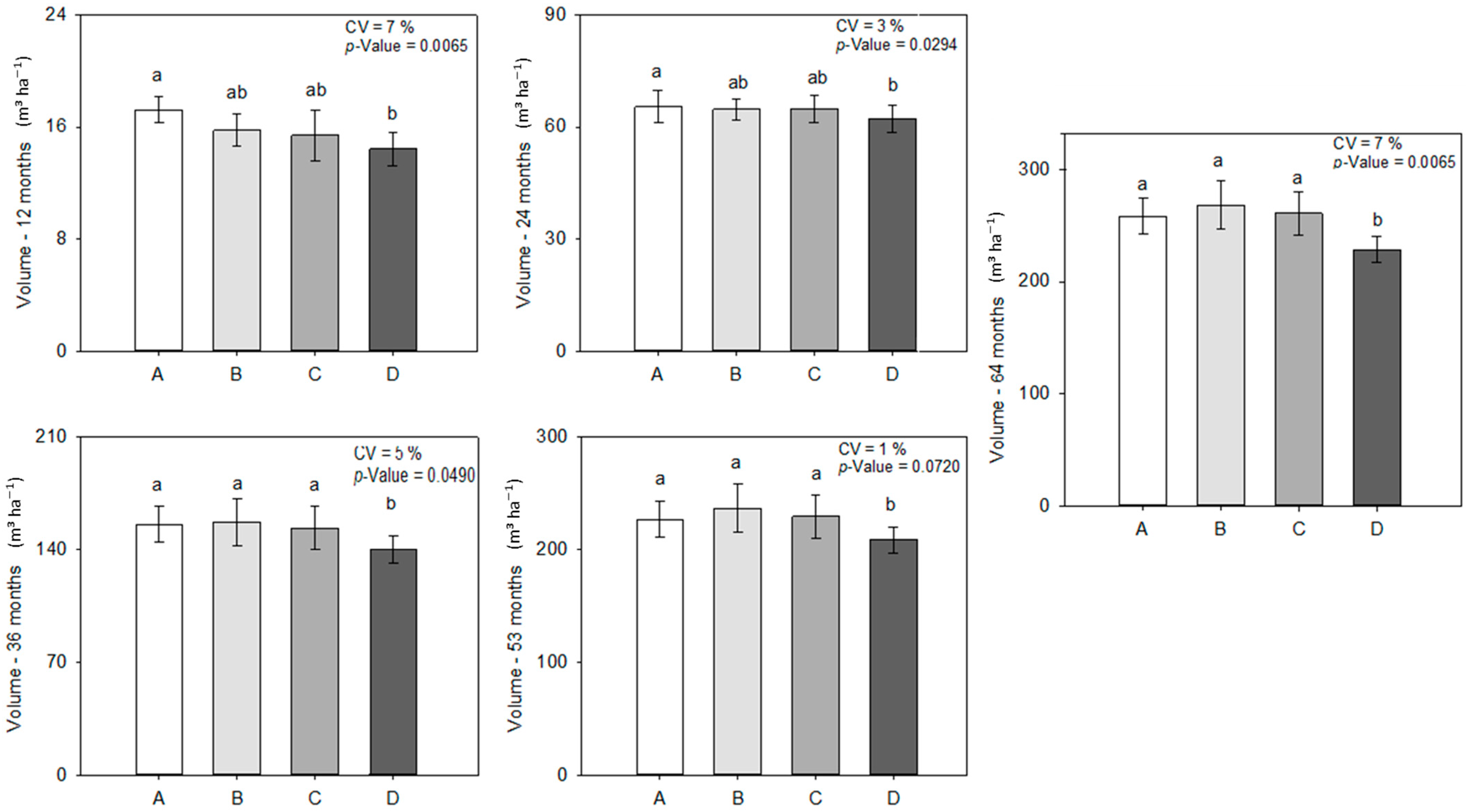
- Mortality, Seedling Losses during the Process and Wood Growth
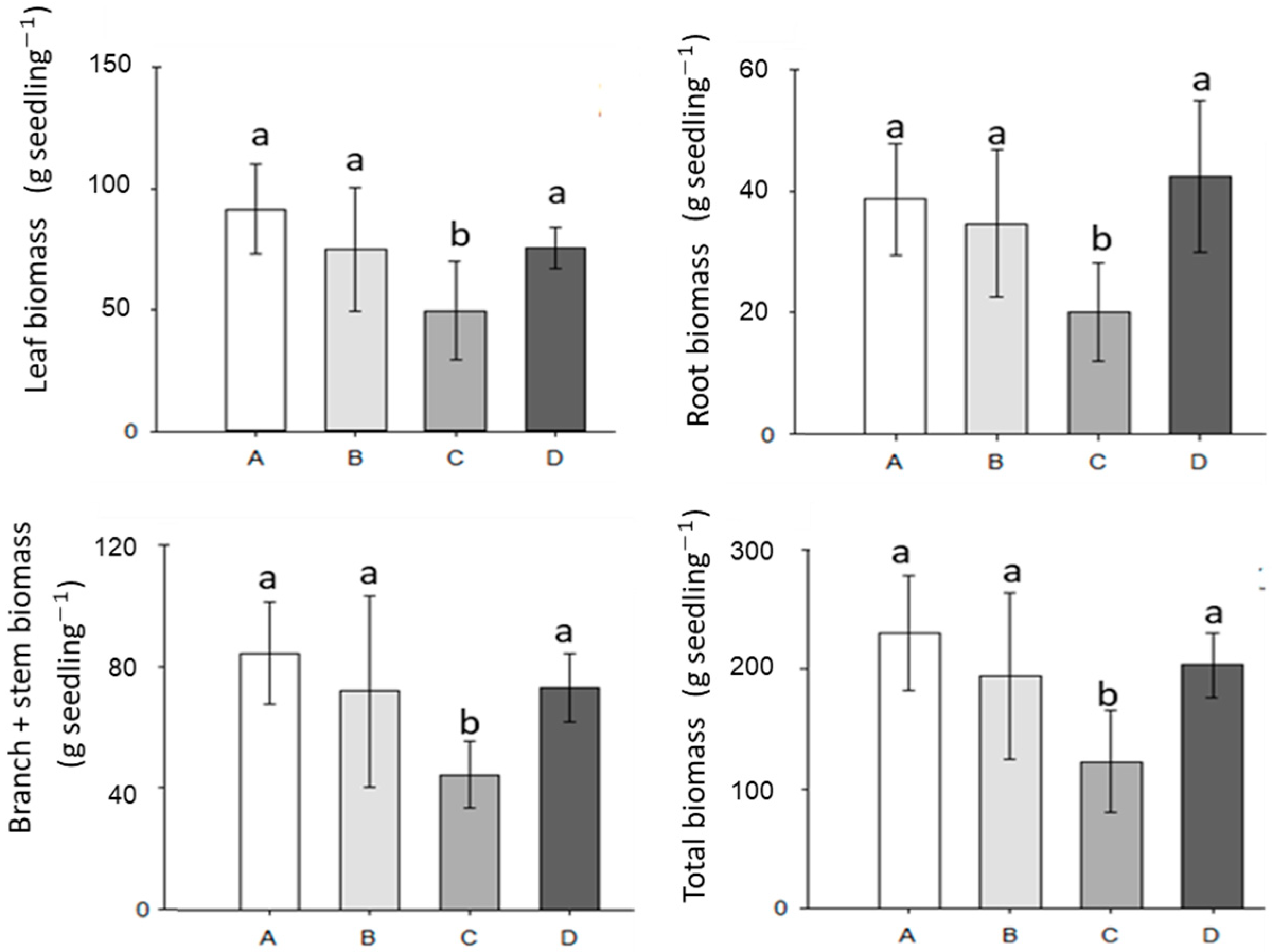
- Leaf Area and Biomass
- Stand Uniformity
2.4. Statistical Analysis
3. Results
3.1. Survival and Establishment of Seedlings in the Field
3.2. Tree Development
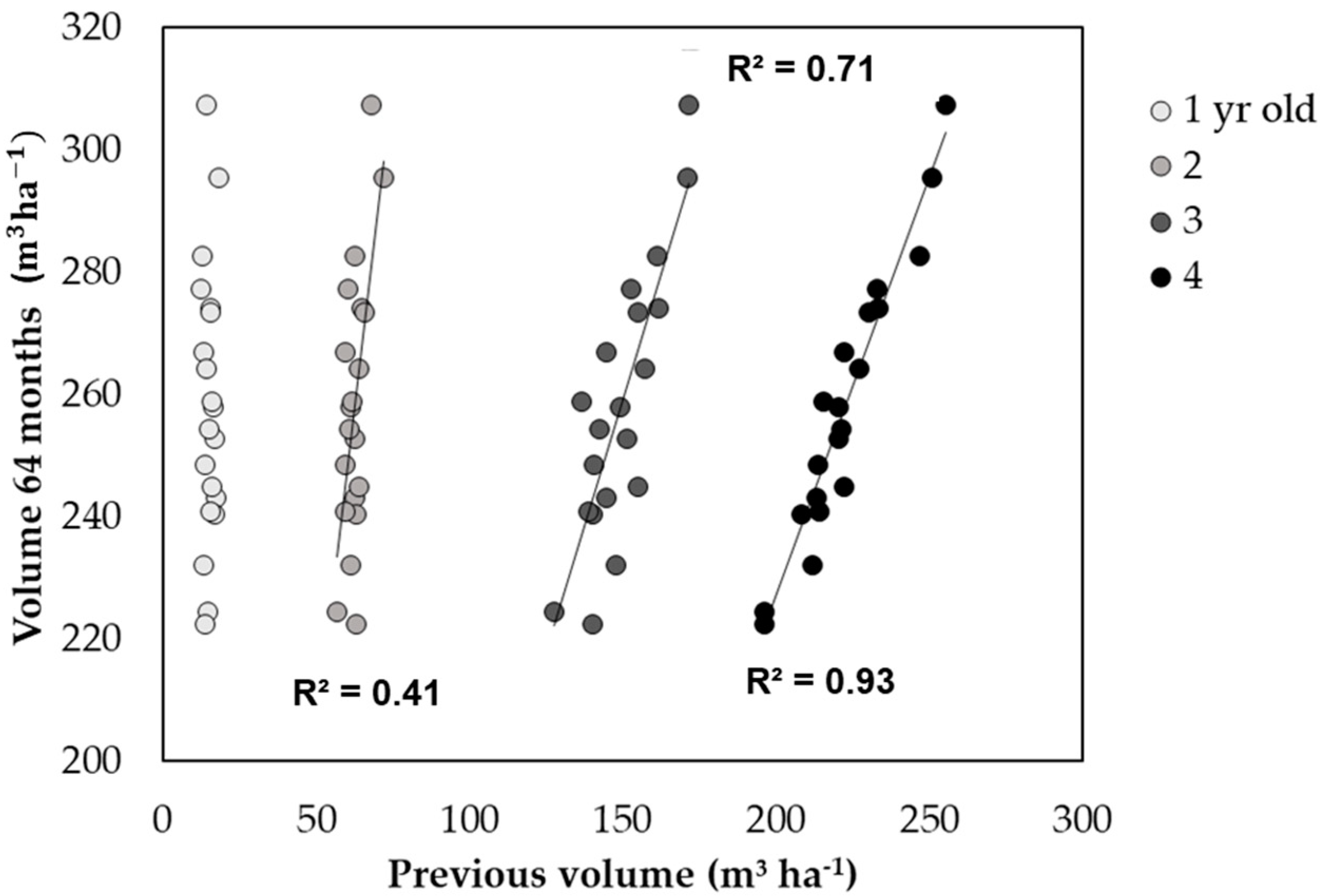
3.3. Relationship between Seedling Quality and Tree Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of the World’s Forests 2022. Forest Pathways for Green Recovery and Building Inclusive, Resilient and Sustainable Economies; FAO: Rome, Italy, 2022. [Google Scholar]
- Árvores, I.B.D. Relatório Anual IBA; IBA: Sao Paulo, Brazil, 2023; p. 96. [Google Scholar]
- Hakamada, R.E.; Stape, J.L.; Zani de Lemos, C.C.; Amaral Almeida, A.E.; Silva, L.F. Using forest inventory and uniformity among trees to monitor silvicultural quality in Eucalyptus clonal plantations. Sci. For. 2015, 43, 27–36. [Google Scholar]
- Gonçalves, J.L.; Alvares, C.A.; Rocha, J.H.; Brandani, C.B.; Hakamada, R. Eucalypt plantation management in regions with water stress. South. For. 2017, 79, 169–183. [Google Scholar] [CrossRef]
- Crous, J.; Sale, G.; Naidoo, T. The influence of species, tree improvement and cultural practices on rotation-end fibre production of Eucalyptus pulpwood plantations in South Africa. South. For. 2019, 81, 307–317. [Google Scholar] [CrossRef]
- Pallett, R.N.; Sale, G. The relative contributions of tree improvement and cultural practice toward productivity gains in Eucalyptus pulpwood stands. For. Ecol. Manag. 2004, 193, 33–43. [Google Scholar] [CrossRef]
- Stape, J.L.; Binkley, D.; Ryan, M.G.; Fonseca, S.; Loos, R.A.; Takahashi, E.N.; Silva, C.R.; Silva, S.R.; Hakamada, R.E.; Ferreira, J.M.d.A.; et al. The Brazil Eucalyptus Potential Productivity Project: Influence of water, nutrients and stand uniformity on wood production. For. Ecol. Manag. 2010, 259, 1684–1694. [Google Scholar] [CrossRef]
- Binkley, D.; Stape, J.L.; Ryan, M.G. Thinking about efficiency of resource use in forests. For. Ecol. Manag. 2004, 193, 5–16. [Google Scholar] [CrossRef]
- Ford, C. Improving Field Survival of Pine Seedlings and Cuttings: The Sappi Plant Quality Index©. Proc. Int. Plant Propagators Soc. 2013, 1055, 11–16. [Google Scholar] [CrossRef]
- Grossnickle, S.; MacDonald, J. Seedling Quality: History, Application, and Plant Attributes. Forests 2018, 9, 283. [Google Scholar] [CrossRef]
- Stape, J.L.; Gonçalves, J.L.M.; Gonçalves, A.N. Relationships between nursery practices and field performance for Eucalyptus plantations in Brazil. New For. 2001, 22, 19–41. [Google Scholar] [CrossRef]
- Grossnickle, S. Importance of root growth in overcoming planting stress. New For. 2005, 30, 273–294. [Google Scholar] [CrossRef]
- dos Leles Santos, P.S.; de Carneiro Araujo, J.G.; Guerra, D.B. Crescimento e arquitetura radicial de plantas de eucalipto oriundas de mudas produzidas em blocos prensados e em tubetes, após o plantio. Cerne 2001, 7, 10–19. [Google Scholar]
- de Freitas, T.A.S.; Fonseca, M.D.S.; de Souza, S.S.M.; Lima, T.M.; Mendonça, A.V.R.; dos Santos, A.P. Crescimento e ciclo de produção de mudas de Eucalyptus em recipientes. Pesqui. Florest. Bras. 2013, 33, 419–428. [Google Scholar] [CrossRef]
- da Silva, A.; Pandolfi, F.; Penchel, R.; dos Reis, E.; Goncalves, E. Relationship between physiological, biochemical and leaf color characteristics in the initial growth of Eucalyptus sp. clones. Cienc. Florest. 2021, 31, 569–589. [Google Scholar] [CrossRef]
- Madrid-Aispuro, R.; Prieto-Ruiz, J.; Hernandez-Diaz, J.; Aldrete, A.; Wehenkel, C.; Chavez-Simental, J. Growth of Pinus cembroides zucc. in nursery and field produced in different type of container. Rev. Fitotec. Mex. 2021, 44, 435–442. [Google Scholar]
- Lopes, J.L.W.; Guerrini, I.A.; Saad, J.C.C. Qualidade de mudas de eucalipto produzidas sob diferentes lâminas de irrigação e dois tipos de substrato. Rev. Árvore 2007, 31, 835–843. [Google Scholar] [CrossRef]
- Oliveira, K.F.; Souza, A.M.d.; Sousa, G.T.d.O.; Costa, A.L.M.d.; Freitas, M.L.M. Estabelecimento de mudas de Eucalyptus spp. e Corymbia citriodora em diferentes substratos. Floresta Ambiente 2014, 21, 30–36. [Google Scholar] [CrossRef]
- Schwegman, K.; Little, K.M.; McEwan, A.; Ackerman, S.A. Harvesting and extraction impacts on Eucalyptus grandis x E-urophylla coppicing potential and rotation-end volume in Zululand, South Africa. South. For. 2018, 80, 51–57. [Google Scholar] [CrossRef]
- Huang, Z.-C.; Zeng, F.-H.; Lu, X.-Y. Efficient regeneration of Eucalyptus urophylla from seedling-derived hypocotyls. Biol. Plant. 2010, 54, 131–134. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; MacDonald, J.E. Why seedlings grow: Influence of plant attributes. New For. 2018, 49, 1–34. [Google Scholar] [CrossRef]
- Dickson, A.; Leaf, A.L.; Hosner, J.F. Quality appraisal of white spruce and white pine seedling stock in nurseries. For. Chron. 1960, 36, 10–13. [Google Scholar] [CrossRef]
- Gallegos-Cedillo, V.; Dianez, F.; Najera, C.; Santos, M. Plant Agronomic Features Can Predict Quality and Field Performance: A Bibliometric Analysis. Agronomy 2021, 11, 2305. [Google Scholar] [CrossRef]
- Carneiro, J.G.A. Produção e Controle de Qualidade de Mudas Florestais; Editora Folha de Viçosa: Viçosa, Brazil, 1995; p. 451. [Google Scholar]
- Alfenas, A.C.; Zauza, E.; Mafia, R.; De Assis, T. Clonagem e Doenças do Eucalipto; Editora UFV: Viçosa, Brazil, 2004. [Google Scholar]
- Figueiredo, F.; Carneiro, J.; Penchel, R.; Thiebaut, J.; Abad, J.; Barroso, D.; Ferraz, T. Correlations between Eucalyptus Clonal Cutting Quality and Performance after Planting. Floresta Ambiente 2019, 26, e20160163. [Google Scholar] [CrossRef]
- Stuepp, C.; Kratz, D.; Gabira, M.; Wendling, I. Survival and initial growth in the field of Eucalyptus seedlings produced in different substrates. Pesqui. Agropecu. Bras. 2020, 55, e01587. [Google Scholar] [CrossRef]
- Hechter, U.; Little, K.; Chan, J.; Crous, J.; da Costa, D. Factors affecting eucalypt survival in South African plantation forestry. South. For. 2022, 84, 253–270. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.d.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Pezzutti, R.V.; Caldato, S.L. Sobrevivência e crescimento inicial de mudas de Pinus taeda L. com diferentes diâmetros do colo. Ciência Florest. 2011, 21, 355–362. [Google Scholar] [CrossRef]
- Trazzi, P.A.; dos Santos, J.A.; Júnior, M.D.; Rioyei, A. A qualidade morfológica de mudas de Pinus taeda afeta o seu crescimento em campo no longo prazo? Sci. For. 2020, 48, e3052. [Google Scholar] [CrossRef]
- Schumacher, F.X.; Hall, F.d.S. Logarithmic Expression of Timber-Tree Volume; USDA Publications: Washington, DC, USA, 1933. [Google Scholar]
- Hakamada, R.E.; Stape, J.L.; Zani de Lemos, C.C.; Amaral Almeida, A.E.; Silva, L.F. Uniformity between trees in a full rotation and its relationship with productivity in clonal Eucalyptus. Cerne 2015, 21, 465–472. [Google Scholar] [CrossRef]
- South, D.B.; Starkey, T.E.; Lyons, A. Why Healthy Pine Seedlings Die after They Leave the Nursery. Forests 2023, 14, 645. [Google Scholar] [CrossRef]
- Schulze, E.D.; Turner, N.C.; Nicolle, D.; Schumacher, J. Species differences in carbon isotope ratios, specific leaf area and nitrogen concentrations in leaves of Eucalyptus growing in a common garden compared with along an aridity gradient. Physiol. Plant. 2006, 127, 434–444. [Google Scholar] [CrossRef]
- Landis, T.D.; Nisley, R.G. The Container Tree Nursery Manual: Seedling Processing, Storage, and Outplanting; US Department of Agriculture, Forest Service: Washington, DC, USA, 2010. [Google Scholar]
- Binkley, D. A hypothesis about the interaction of tree dominance and stand production through stand development. For. Ecol. Manag. 2004, 190, 265–271. [Google Scholar] [CrossRef]
- Fernández-Tschieder, E.; Binkley, D. Linking competition with growth dominance and production ecology. For. Ecol. Manag. 2018, 414, 99–107. [Google Scholar] [CrossRef]
- Júnior, M.D.; Trazzi, P.A.; Higa, A.R.; Seitz, R.A. Effect of container size and planting method on growth of a nine-years-old Pinus taeda stand. Sci. For. 2013, 41, 7–14. [Google Scholar]
- Aphalo, P.; Rikala, R. Field performance of silver-birch planting-stock grown at different spacing and in containers of different volume. New For. 2003, 25, 93–108. [Google Scholar] [CrossRef]
- Barberá, G.G.; Martínez-Fernández, F.; Álvarez-Rogel, J.; Albaladejo, J.; Castillo, V. Short-and intermediate-term effects of site and plant preparation techniques on reforestation of a Mediterranean semiarid ecosystem with Pinus halepensis Mill. New For. 2005, 29, 177–198. [Google Scholar] [CrossRef]
- McKenzie, B.E.; Peterson, C.A. Root browning in Pinus banksiana Lamb. and Eucalyptus pilularis Sm. 1. Anatomy and permeability of the white and tannin zones. Bot. Acta 1995, 108, 127–137. [Google Scholar] [CrossRef]
- da Costa Alpoim, G. The Interaction between Site, Harvest Residue Management and Plant Stock Quality on Eucalyptus Transplant Survival, Growth and Uniformity in Kwazulu-Natal, South Africa; Stellenbosch University: Stellenbosch, South Africa, 2021. [Google Scholar]
- Thomas, D. Survival and growth of drought hardened Eucalyptus pilularis Sm. seedlings and vegetative cuttings. New For. 2009, 38, 245–259. [Google Scholar] [CrossRef]
- de Oliveira Castro, C.A.; dos Santos, G.A.; Takahashi, E.K.; Nunes, A.C.P.; Souza, G.A.; de Resende, M.D.V. Accelerating Eucalyptus breeding strategies through top grafting applied to young seedlings. Ind. Crops Prod. 2021, 171, 113906. [Google Scholar] [CrossRef]
- Correa, T.; Picoli, E.; Pereira, W.; Conde, S.; Resende, R.; de Resende, M.; da Costa, W.; Cruz, C.; Zauza, E. Very Early Biomarkers Screening for Water Deficit Tolerance in Commercial Eucalyptus Clones. Agronomy 2023, 13, 937. [Google Scholar] [CrossRef]
- Davis, A.; Jacobs, D. Quantifying root system quality of nursery seedlings and relationship to outplanting performance. New For. 2005, 30, 295–311. [Google Scholar] [CrossRef]
- Kormanik, P.P. Lateral root morphology as an expression of sweetgum seedling quality. For. Sci. 1986, 32, 595–604. [Google Scholar]
- Saha, R.; Ginwal, H.S.; Chandra, G.; Barthwal, S. Integrated assessment of adventitious rhizogenesis in Eucalyptus: Root quality index and rooting dynamics. J. For. Res. 2020, 31, 2145–2161. [Google Scholar] [CrossRef]

| Parameter | Classification According to Seedling Morphological Characteristics (Treatments) | |||
|---|---|---|---|---|
| A | B | C | D | |
| Age (days) | 100 | 100 | 100 | 180 |
| Diameter (mm) | 2.5–4.5 | 2.0–3.0 | 1.5–2.5 | >3.2 |
| Height (cm) | 25–45 | 17–30 | 13–25 | 35–55 |
| Height of stem lignification (cm) | 6–10 | 3–5 | <3 | >10 |
| Treat | Base Diameter (Days) | Heigth (Days) | ||||
|---|---|---|---|---|---|---|
| 60 | 90 | 120 | 60 | 90 | 120 | |
| mm | cm | |||||
| A | 2.5 ± 0.1 a | 4.4 ± 0.4 a | 8.2 ± 0.3 a | 10.6 ± 1.1 a | 13.0 ± 1.3 a | 39.5 ± 2.1 a |
| B | 2.1 ±0.2 ab | 4.0 ± 0.8 a | 7.8 ± 0.6 ab | 11.1 ± 1.8 a | 12.4 ± 1.5 a | 35.0 ± 3.4 ab |
| C | 1.8 ± 0.2 bc | 3.5 ± 0.7 a | 7.1 ± 0.6 b | 9.5 ± 0.6 a | 11.7 ± 2.6 a | 33.4 ± 4.0 ab |
| D | 2.1 ± 0.1 ab | 4.2 ± 0.5 a | 7.4 ± 0.2 ab | 9.2 ± 0.7 a | 10.7 ± 1.2 a | 32.1 ± 1.3 b |
| CV (%) | 15 | 12 | 5 | 16 | 7 | 1 |
| p-Value | 0.0051 | 0.0342 | 0.0005 | <0.0001 | <0.0001 | 0.0003 |
| Treat | DQI (120 Days) | Specific Leaf Area (Days) | PH50—Heigh3 (Days) | ||||
|---|---|---|---|---|---|---|---|
| 60 | 90 | 120 | 60 | 90 | 120 | ||
| cm2 g−1 | % | ||||||
| A | 0.701 ± 0.109 b | 18 ± 0.55 | 14 ± 0.43 b | 15 ± 2.60 b | 35 ± 2.9 ab | 33 ± 1.57 ab | 33 ± 1.9 ab |
| B | 0.436 ± 0.038 c | 17 ± 1.06 | 15 ± 1.11 b | 14 ± 1.09 b | 33 ± 3.9 ab | 35 ± 3.81 a | 33 ± 5.6 a |
| C | 0.206 ± 0.048 d | 18 ± 0.38 | 18 ± 1.15 a | 16 ± 0.87 a | 30 ± 3.7 b | 27 ± 4.28 b | 28 ± 3.6 b |
| D | 0.941 ± 0.209 b | 17 ± 0.99 | 16 ± 0.58 ab | 13 ± 0.91 b | 36 ± 1.2 a | 35 ± 2.69 a | 32 ± 6.9 a |
| CV (%) | 14 | 5 | 5 | 14 | 9 | 9 | 13 |
| p-Value | 0.0102 | 0.1277 | 0.0019 | 0.0783 | <0.0001 | 0.0032 | 0.0813 |
| Treat | Cumulative Individual Volume of 50% of the Smallest Trees (Months) | ||||
|---|---|---|---|---|---|
| 12 | 24 | 36 | 53 | 64 | |
| % | |||||
| A | 41 ± 1.5 | 40 ± 1.5 | 35 ± 2.3 | 30 ± 2.4 | 28 ± 2.9 |
| B | 39 ± 5.6 | 41 ± 3.2 | 36 ± 4.5 | 32 ± 5.0 | 29 ± 4.1 |
| C | 39 ± 1.0 | 41 ± 0.8 | 36 ± 1.0 | 32 ± 1.7 | 30 ± 1.3 |
| D | 39 ± 3.7 | 41 ± 3.6 | 37 ± 4.1 | 33 ± 4.4 | 27 ± 4.5 |
| CV (%) | 8 | 5 | 7 | 9 | 10 |
| p-Value | 0.8497 | 0.9864 | 0.7749 | 0.4461 | 0.373 |
| Parameters | Pearson’s Correlation Coefficient | |||||
|---|---|---|---|---|---|---|
| Days | ||||||
| 60 | 90 | 120 | - | |||
| DQI versus | SD | 0.5733 * | 0.484 ns | 0.489 ns | - | |
| H | 0.5077 ns | 0.445 ns | 0.201 ns | - | ||
| LB | 0.0356 * | 0.068 ns | 0.193 ns | - | ||
| SBB | 0.2763 ns | 0.120 ns | 0.461 ns | - | ||
| RB | 0.4639 ns | 0.2748 ns | 0.204 ns | - | ||
| TB | 0.2163 ns | 0.150 ns | 0.287 ns | - | ||
| SLA | −0.2898 ns | −0.016 ns | −0.039 ns | - | ||
| PH50 (H3) | 0.6340 * | 0.634 * | 0.625 * | - | ||
| Months | ||||||
| 12 | 24 | 36 | 53 | 64 | ||
| DQI versus | Volume | −0.292 ns | −0.301 ns | −0.134 ns | −0.044 ns | −0.281 ns |
| PV50 | −0.069 ns | −0.312 ns | −0.325 ns | −0.201 ns | −0.246 ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, G.G.; Hakamada, R.; Silva, R.M.L.d.; Lemos, C.C.Z.d.; Florentino, A.L.; Gonçalves, J.L.d.M. Seedling Morphological Characteristics on Survival, Uniformity, and Growth during a Full Short Rotation in Eucalyptus grandis x E. urophylla Plantation. Forests 2023, 14, 1756. https://doi.org/10.3390/f14091756
Moreira GG, Hakamada R, Silva RMLd, Lemos CCZd, Florentino AL, Gonçalves JLdM. Seedling Morphological Characteristics on Survival, Uniformity, and Growth during a Full Short Rotation in Eucalyptus grandis x E. urophylla Plantation. Forests. 2023; 14(9):1756. https://doi.org/10.3390/f14091756
Chicago/Turabian StyleMoreira, Gabriela Gonçalves, Rodrigo Hakamada, Renato Meulman Leite da Silva, Cristiane Camargo Zani de Lemos, Antônio Leite Florentino, and José Leonardo de Moraes Gonçalves. 2023. "Seedling Morphological Characteristics on Survival, Uniformity, and Growth during a Full Short Rotation in Eucalyptus grandis x E. urophylla Plantation" Forests 14, no. 9: 1756. https://doi.org/10.3390/f14091756





