Spatial Heterogeneity of Total and Labile Soil Organic Carbon Pools in Poplar Agroforestry Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design and Soil and Root Sampling
2.3. Soil Analyses
2.4. Vegetation Analyses
2.5. Statistical Analyses
3. Results
3.1. Biomass of Poplar and Understory Vegetation in Different Planting Systems
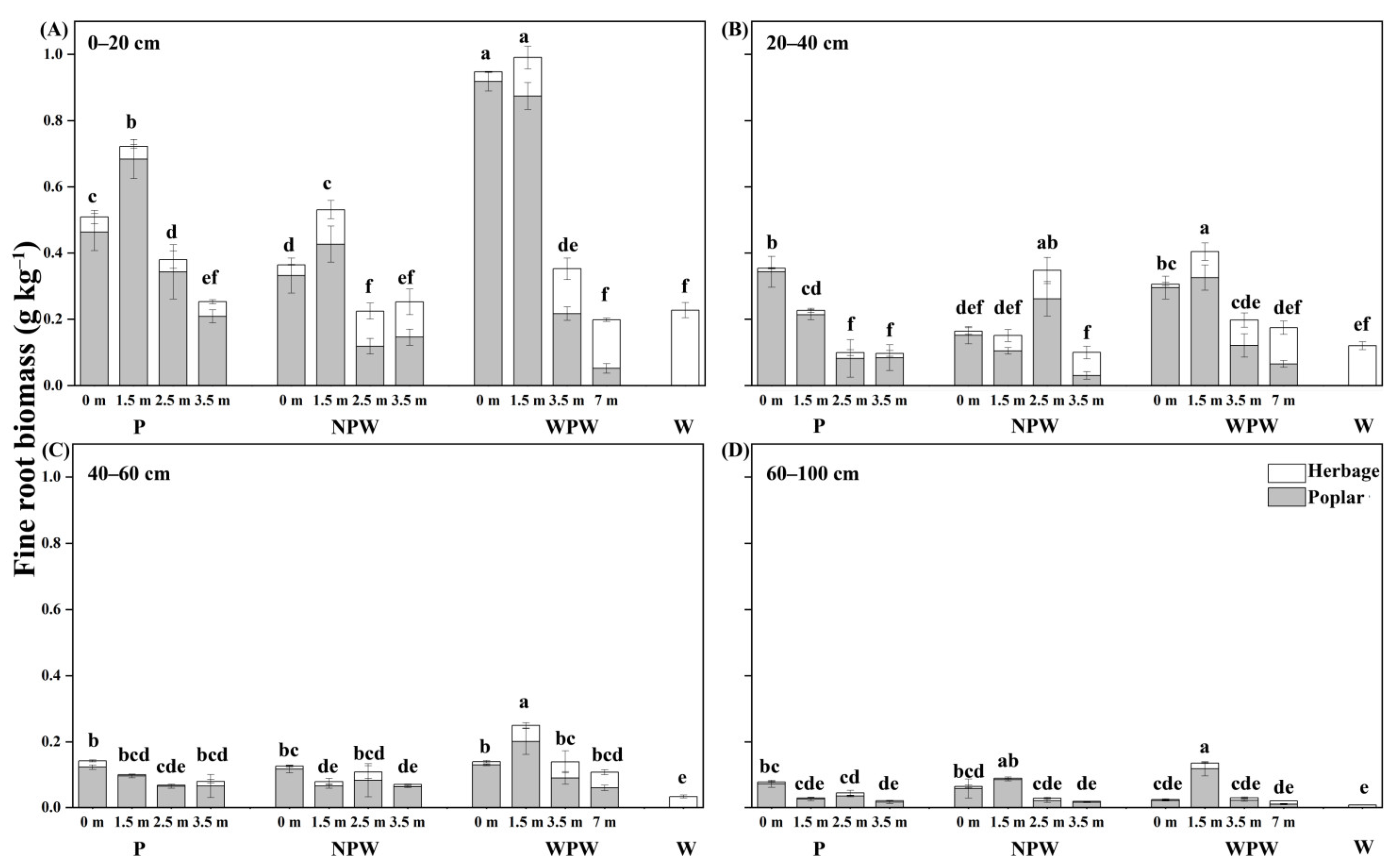
3.2. Vertical and Horizontal Distribution of FRB
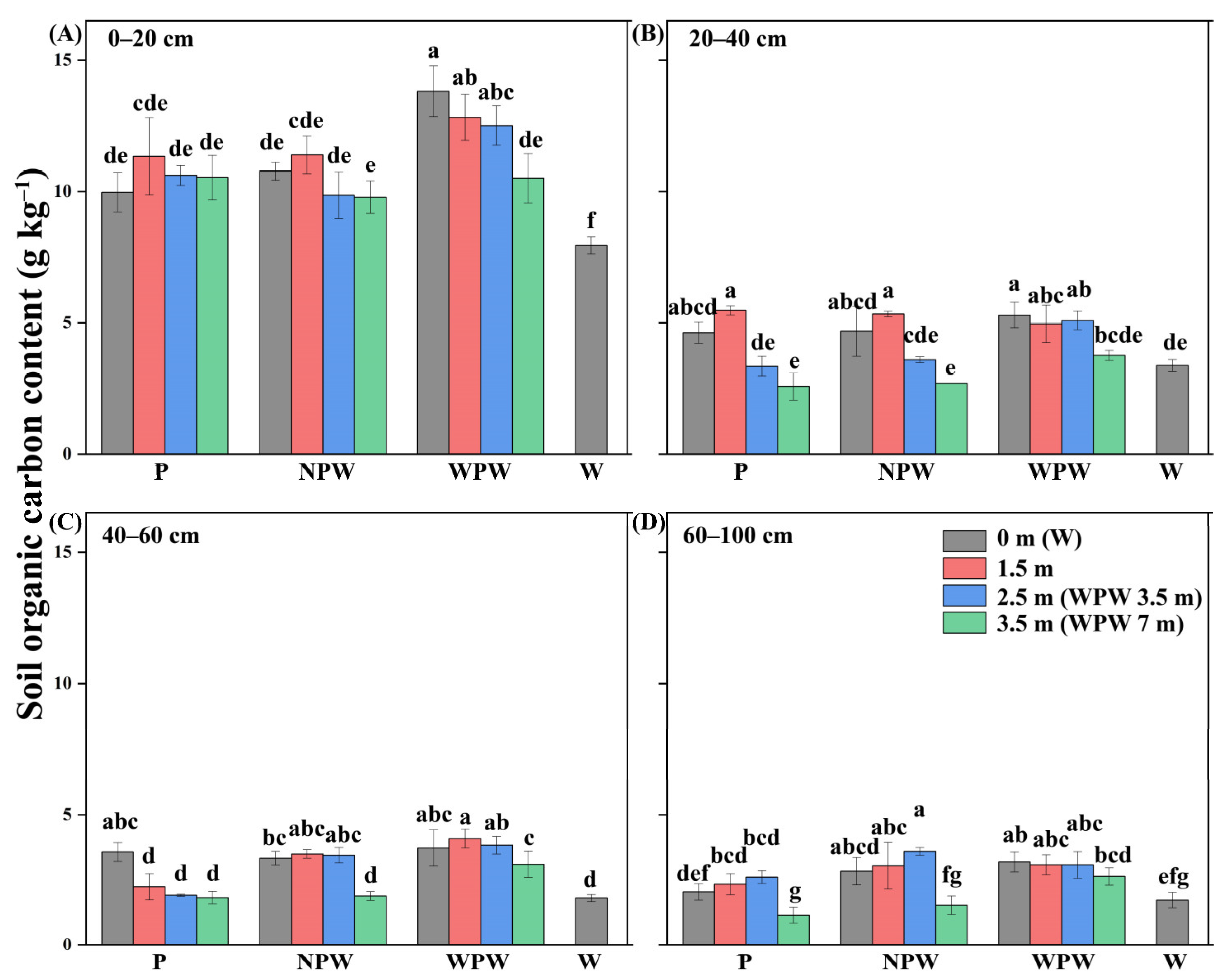
3.3. Vertical and Horizontal Distributions of the SOC Content
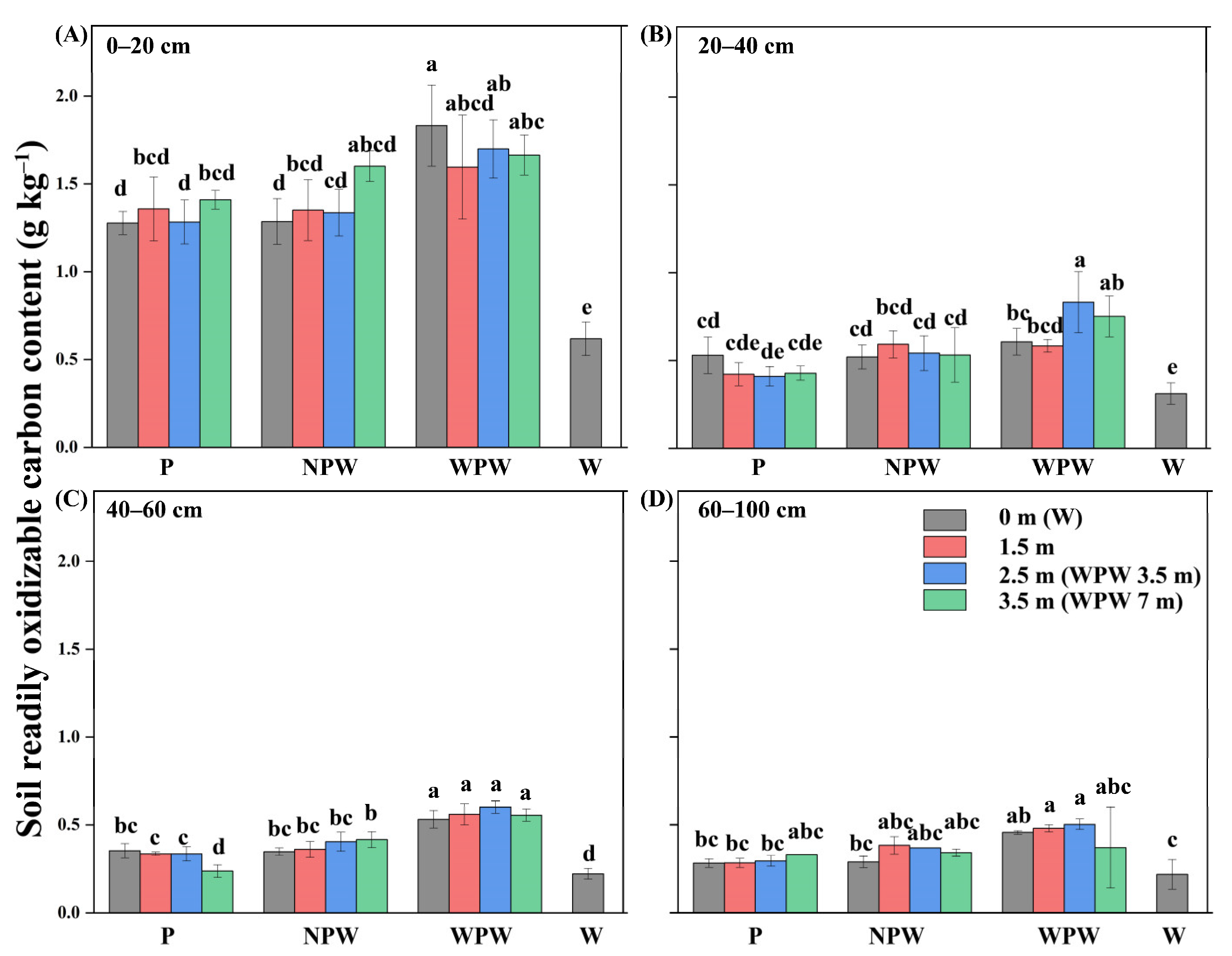
3.4. Vertical and Horizontal Distributions of the Soil ROC Content
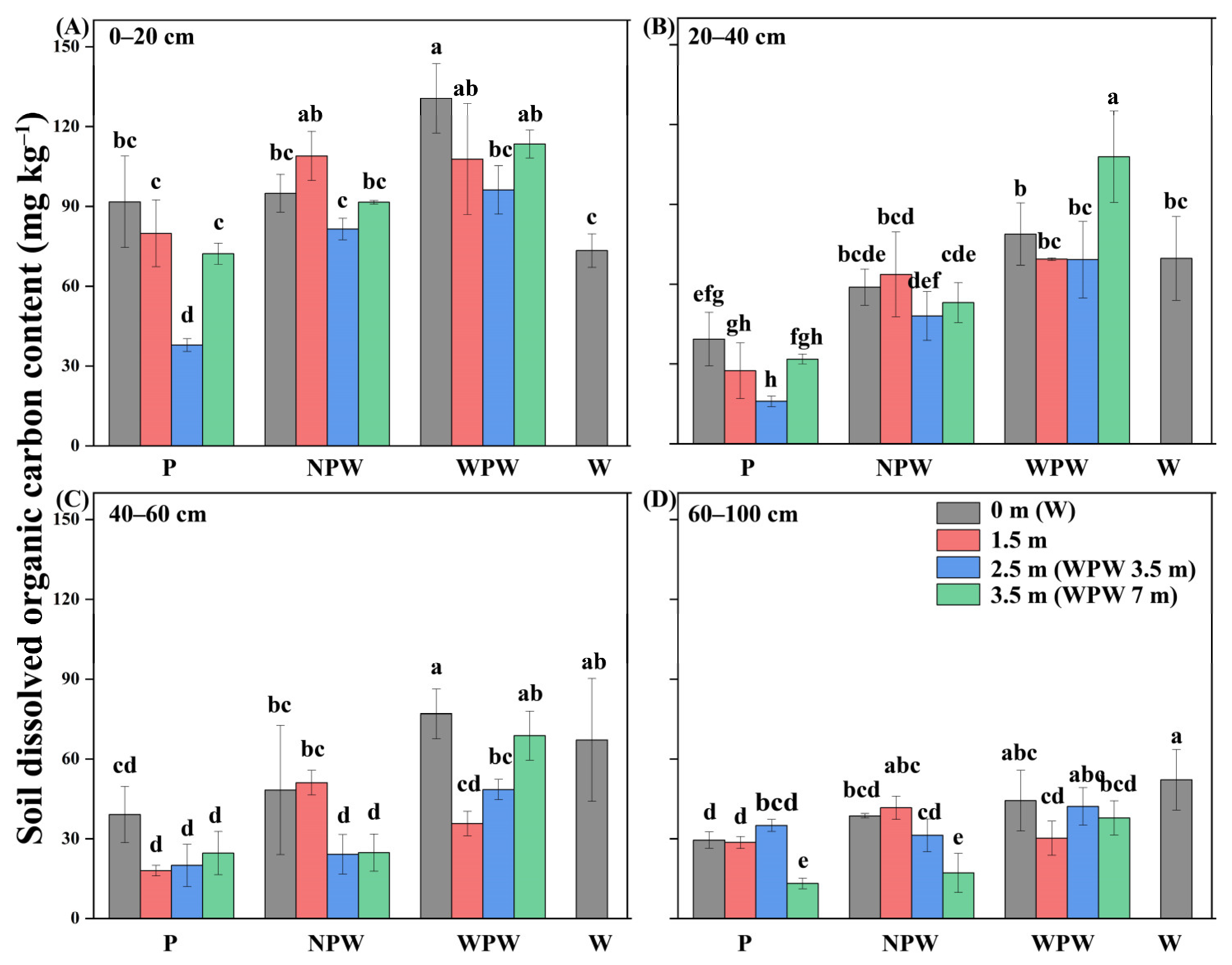
3.5. Vertical and Horizontal Distributions of the Soil DOC Content
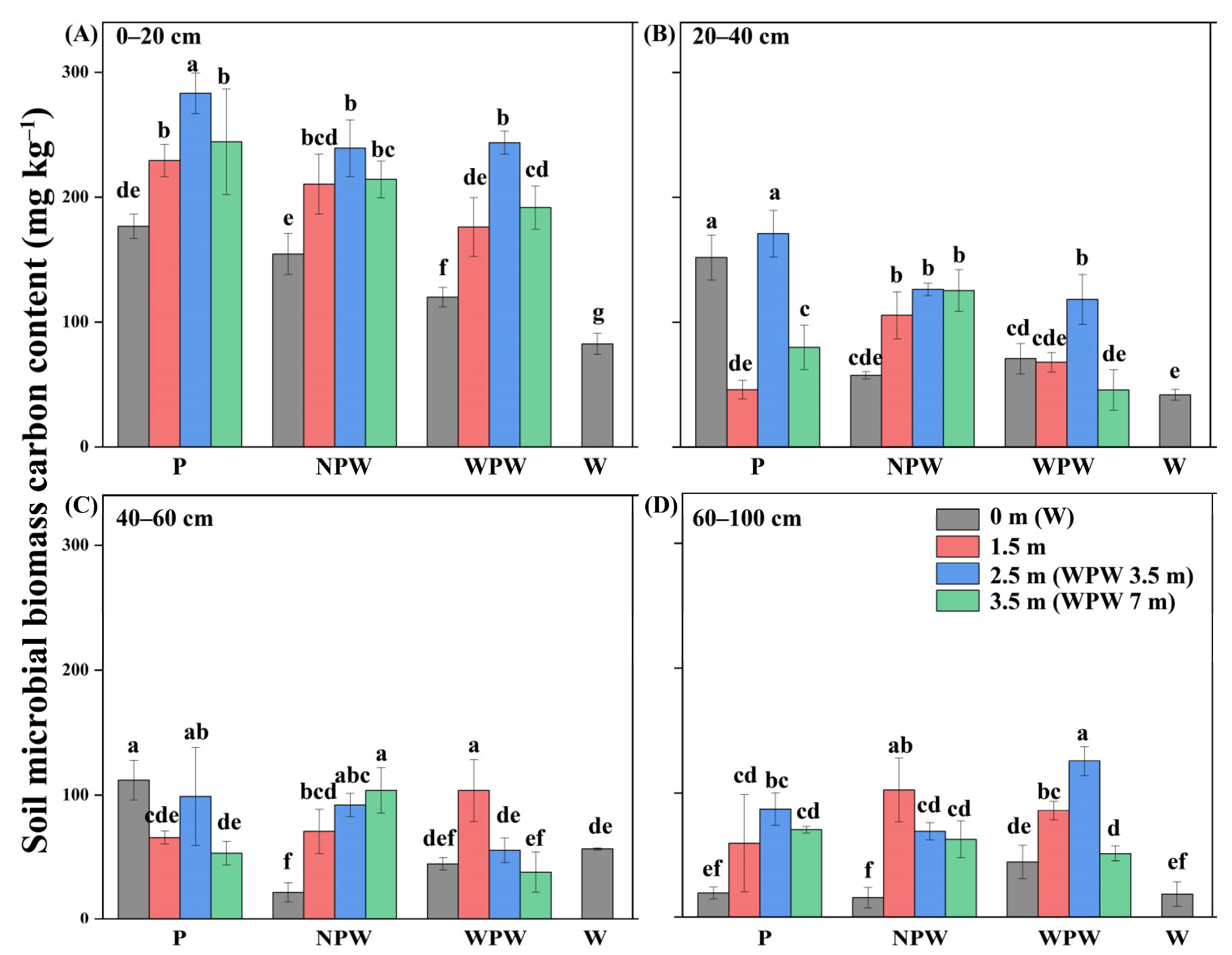
3.6. Vertical and Horizontal Distributions of the Soil MBC Content
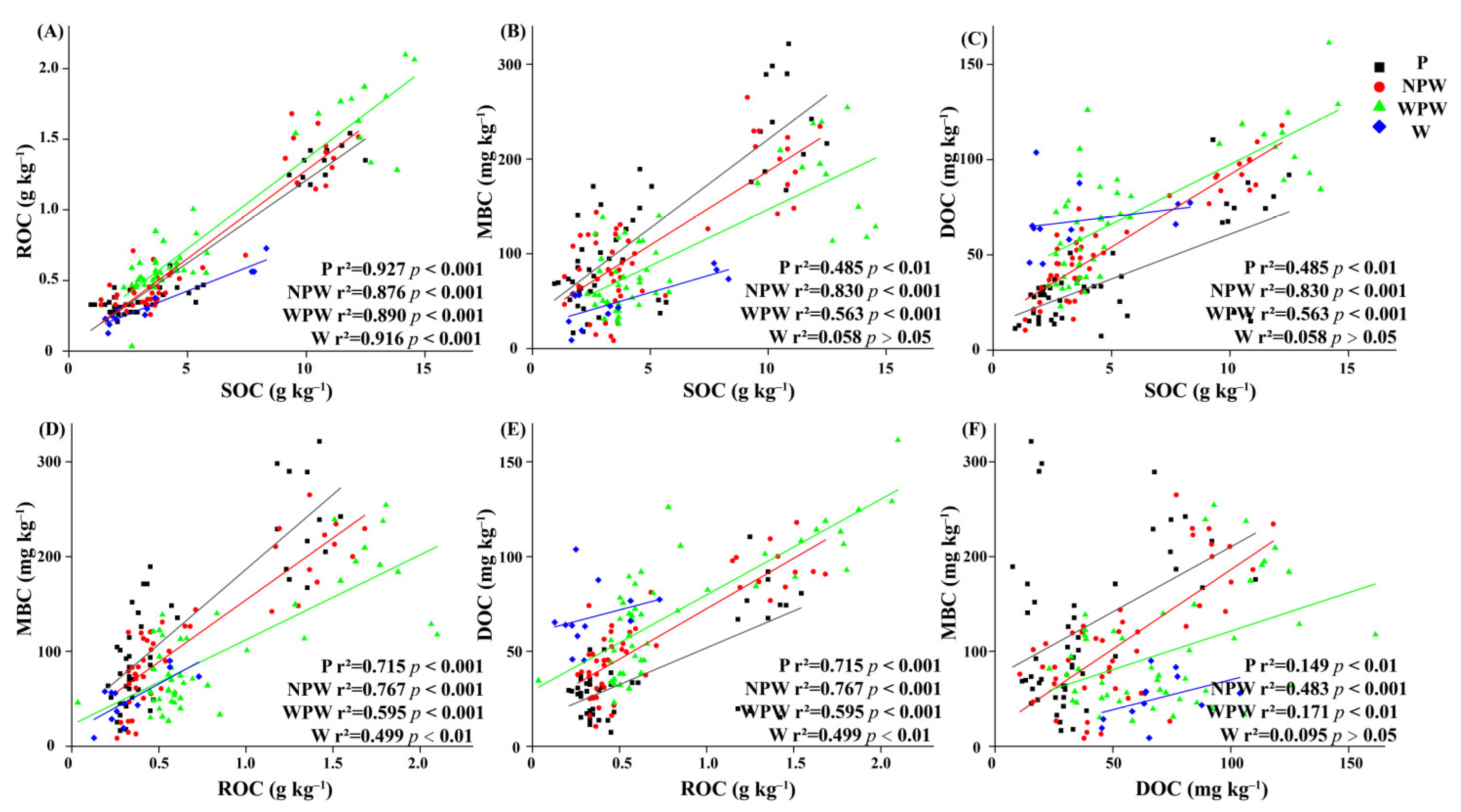
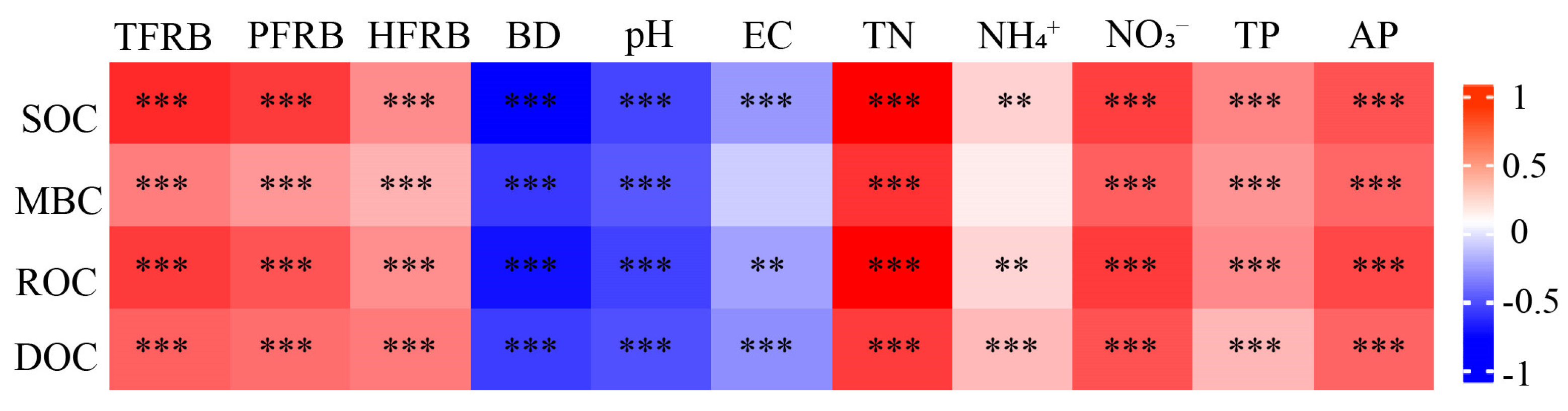
3.7. Relationship between FRB, Soil Properties and Soil Organic Fractions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Caesar, L.; McCarthy, G.D.; Thornalley, D.J.R.; Cahill, N.; Rahmstorf, S. Current atlantic meridional overturning circulation weakest in last millennium. Nat. Geosci. 2021, 14, 118–120. [Google Scholar] [CrossRef]
- Dlugokencky, E.; Tans, P. Trends in Atmospheric Carbon Dioxide, National Oceanic & Atmospheric Administration, Earth System Research Laboratory (NOAA/ESRL). 2022. Available online: https://gml.noaa.gov/ccgg/trends/global.html (accessed on 18 September 2022).
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Albrecht, A.; Kandji, S.T. Carbon sequestration in tropical agroforestry systems. Agric. Ecosyst. Environ. 2003, 99, 15–27. [Google Scholar] [CrossRef]
- Verchot, L.V.; Van Noordwijk, M.; Kandji, S.; Tomich, T.; Ong, C.; Albrecht, A.; Mackensen, J.; Bantilan, C.; Anupama, K.V.; Palm, C. Climate change: Linking adaptation and mitigation through agroforestry. Mitig. Adapt. Strat. Glob. Chang. 2007, 12, 901–918. [Google Scholar] [CrossRef]
- Pardon, P.; Reubens, B.; Reheul, D.; Mertens, J.; De Frenne, P.; Coussement, T.; Janssens, P.; Verheyen, K. Trees increase soil organic carbon and nutrient availability in temperate agroforestry systems. Agric. Ecosyst. Environ. 2017, 247, 98–111. [Google Scholar] [CrossRef]
- Rhoades, C.C. Single-tree influences on soil properties in agroforestry: Lessons from natural forest and savanna ecosystems. Agrofor. Syst. 1996, 35, 71–94. [Google Scholar] [CrossRef]
- Upson, M.A.; Burgess, P.J. Soil organic carbon and root distribution in a temperate arable agroforestry system. Plant Soil 2013, 373, 43–58. [Google Scholar] [CrossRef]
- Howlett, D.S.; Moreno, G.; Mosquera Losada, M.R.; Nair, P.K.R.; Nair, V.D. Soil carbon storage as influenced by tree cover in the Dehesa cork oak silvopasture of central-western Spain. J. Environ. Monit. 2011, 13, 1897–1904. [Google Scholar] [CrossRef]
- Ramachandran Nair, P.K.; Nair, V.D.; Mohan Kumar, B.; Showalter, J.M. Carbon sequestration in agroforestry systems. Adv. Agron. 2010, 108, 237–307. [Google Scholar] [CrossRef]
- Bambrick, A.D.; Whalen, J.K.; Bradley, R.L.; Cogliastro, A.; Gordon, A.M.; Olivier, A.; Thevathasan, N.V. Spatial heterogeneity of soil organic carbon in tree-based intercropping systems in Quebec and Ontario, Canada. Agrofor. Syst. 2010, 79, 343–353. [Google Scholar] [CrossRef]
- Peichl, M.; Thevathasan, N.V.; Gordon, A.M.; Huss, J.; Abohassan, R.A. Carbon sequestration potentials in temperate tree-based intercropping systems, Southern Ontario, Canada. Agrofor. Syst. 2006, 66, 243–257. [Google Scholar] [CrossRef]
- Jackson, R.B.; Mooney, H.A.; Schulze, E.D. A global budget for fine root biomass, surface area, and nutrient contents. Proc. Natl. Acad. Sci. USA 1997, 94, 7362–7366. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The microbial efficiency-matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Monroe, P.H.M.; Barreto-Garcia, P.A.B.; Lima, M.C.D.; Santos, R.K.A.; Oliveira, E.P.; Silva, S.R.; Gama, D.C. Fine root contribution to the soil carbon stock of an agroforestry system in a Caatinga-Atlantic Forest transition zone. Rev. Bras. Ciênc. Ambient. 2020, 56, 128–136. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Soil organic carbon sequestration in agroforestry systems. A review. Agron. Sustain. Dev. 2014, 34, 443–454. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, X.; Wei, X.; Shao, M.; Li, T.; Yu, Q. Total soil organic carbon increases but becomes more labile after afforestation in China’s Loess Plateau. For. Ecol. Manag. 2020, 461, 117911. [Google Scholar] [CrossRef]
- Lan, Z.; Zhang, S.; Sial, T.A.; Wu, L.; Chang, W.; Li, X.; Zhang, J.; Fan, J.; Feng, X. Fine-scale spatial distribution of soil organic carbon and its fractions after afforestation with Pinus sylvestris and Salix psammophila in a semiarid desert of China. J. Plant Ecol. 2022, 15, 141–154. [Google Scholar] [CrossRef]
- Fang, C.; Moncrieff, J.B. The variation of soil microbial respiration with depth in relation to soil carbon composition. Plant Soil 2005, 268, 243–253. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.K.; Liang, W.J.; Jiang, Y.; Dai, G.H.; Wang, X.G.; Han, S.J. Distribution of soil organic carbon fractions along the altitudinal gradient in Changbai mountain, China. Pedosphere 2011, 21, 615–620. [Google Scholar] [CrossRef]
- Benbi, D.K.; Brar, K.; Toor, A.S.; Singh, P. Total and labile pools of soil organic carbon in cultivated and undisturbed soils in northern India. Geoderma 2015, 237, 149–158. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Ouyang, Z. Effects of land use, climate, topography and soil properties on regional soil organic carbon and total nitrogen in the upstream watershed of Miyun Reservoir, North China. J. Environ. Sci. 2012, 24, 387–395. [Google Scholar] [CrossRef]
- Cardinael, R.; Chevallier, T.; Barthès, B.G.; Saby, N.P.A.; Parent, T.; Dupraz, C.; Bernoux, M.; Chenu, C. Impact of alley cropping agroforestry on stocks, forms and spatial distribution of soil organic carbon—A case study in a Mediterranean context. Geoderma 2015, 259–260, 288–299. [Google Scholar] [CrossRef]
- Dad, J.M.; Shafiq, M. Spatial distribution of soil organic carbon in apple orchard soils of Kashmir Himalaya, India. Carbon Manag. 2021, 12, 485–498. [Google Scholar] [CrossRef]
- Yao, X.; Yu, K.; Deng, Y.; Zeng, Q.; Lai, Z.; Liu, J. Spatial distribution of soil organic carbon stocks in Masson pine (Pinus massoniana) forests in subtropical China. Catena 2019, 178, 189–198. [Google Scholar] [CrossRef]
- Böhm, W. Methods of Studying Root Systems; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1979. [Google Scholar]
- Gao, X.; Meng, T.; Zhao, X. Variations of soil organic carbon following land use change on deep-loess hillsopes in China. Land Degrad. Dev. 2017, 28, 1902–1912. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, P.; Chang, S.X.; Wu, J.; Lin, L. Organic mulch and fertilization affect soil carbon pools and forms under intensively managed bamboo (Phyllostachys praecox) forests in southeast China. J. Soils Sediments 2010, 10, 739–747. [Google Scholar] [CrossRef]
- Laganière, J.R.M.; Angers, D.A.; Paré, D. Carbon accumulation in agricultural soils after afforestation: A meta-analysis. Glob. Chang. Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Shi, L.; Feng, W.; Xu, J.; Kuzyakov, Y. Agroforestry systems: Meta-analysis of soil carbon stocks, sequestration processes, and future potentials. Land Degrad. Dev. 2018, 29, 3886–3897. [Google Scholar] [CrossRef]
- Baah-Acheamfour, M.; Carlyle, C.N.; Bork, E.W.; Chang, S.X. Trees increase soil carbon and its stability in three agroforestry systems in central Alberta, Canada. For. Ecol. Manag. 2014, 328, 131–139. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Gillespie, A.; Peak, D.; Van Rees, K.C.J. Spectroscopic investigation of soil organic matter composition for shelterbelt agroforestry systems. Geoderma 2017, 298, 1–13. [Google Scholar] [CrossRef]
- Eddy, W.C.; Yang, W.H. Improvements in soil health and soil carbon sequestration by an agroforestry for food production system. Agric. Ecosyst. Environ. 2022, 333, 107945. [Google Scholar] [CrossRef]
- Mayer, S.; Wiesmeier, M.; Sakamoto, E.; Hübner, R.; Cardinael, R.; Kühnel, A.; Kögel-Knabner, I. Soil organic carbon sequestration in temperate agroforestry systems—A meta-analysis. Agric. Ecosyst. Environ. 2022, 323, 107689. [Google Scholar] [CrossRef]
- Morales Ruiz, D.E.; Aryal, D.R.; Pinto Ruiz, R.; Hernández, F.; Lugo, F.; Villanueva, G. Carbon contents and fine root production in tropical silvopastoral systems. Land Degrad. Dev. 2020, 32, 738–756. [Google Scholar] [CrossRef]
- Hertel, D.; Harteveld, M.A.; Leuschner, C. Conversion of a tropical forest into agroforest alters the fine root-related carbon flux to the soil. Soil Biol. Biochem. 2009, 41, 481–490. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B.D. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef]
- Defrenet, E.; Roupsard, O.; Meersche, K.; Charbonnier, F.; Perez-Molina, J.; Khac, E.; Prieto, I.; Stokes, A.; Roumet, C.; Rapidel, B.; et al. Root biomass, turnover and net primary productivity of a coffee agroforestry system in Costa Rica: Effects of soil depth, shade trees, distance to row and coffee age. Ann. Bot. 2016, 118, 833–851. [Google Scholar] [CrossRef]
- Liao, Y.; McCormack, M.L.; Fan, H.; Wang, H.; Wu, J.; Tu, J.; Liu, W.; Guo, D. Relation of fine root distribution to soil C in a Cunninghamia lanceolata plantation in subtropical China. Plant Soil 2014, 381, 225–234. [Google Scholar] [CrossRef]
- Morais, V.A.; Santos, C.A.; Mello, J.M.; Dadid, H.C.; Araújo, E.J.G.; Scolforo, J.R.S. Spatial and vertical distribution of litter and belowground carbon in a Brazilian Cerrado vegetation. Cerne 2017, 23, 43–52. [Google Scholar] [CrossRef]
- Panchal, P.; Preece, C.; Penuelas, J.; Giri, J. Soil carbon sequestration by root exudates. Trends Plant Sci. 2022, 27, 749–757. [Google Scholar] [CrossRef]
- Guillot, E.; Hinsinger, P.; Dufour, L.; Roy, J.; Bertrand, I. With or without trees: Resistance and resilience of soil microbial communities to drought and heat stress in a Mediterranean agroforestry system. Soil Biol. Biochem. 2019, 129, 122–135. [Google Scholar] [CrossRef]
- Muñoz, C.; Zagal, E.; Ovalle, C. Influence of trees on soil organic matter in Mediterranean agroforestry systems: An example from the ‘Espinal’ of central Chile. Eur. J. Soil Sci. 2007, 58, 728–735. [Google Scholar] [CrossRef]
- Bojko, O.; Kabala, C.; Mendyk, Ł.; Markiewicz, M.; Pagacz-Kostrzewa, M.; Glina, B. Labile and stabile soil organic carbon fractions in surface horizons of mountain soils—Relationships with vegetation and altitude. J. Mt. Sci.-Eng. 2017, 14, 2391–2405. [Google Scholar] [CrossRef]
- Islam, M.; Dey, A.; Rahman, M. Effect of tree diversity on soil organic carbon content in the homegarden agroforestry system of north-eastern bangladesh. Small-Scale For. 2015, 14, 91–101. [Google Scholar] [CrossRef]
- Nair, P.K.R.; Nair, V.D.; Kumar, B.M.; Haile, S.G. Soil carbon sequestration in tropical agroforestry systems: A feasibility appraisal. Environ. Sci. Policy 2009, 12, 1099–1111. [Google Scholar] [CrossRef]
- Jing, Y.; Zhao, X.; Liu, S.; Tian, P.; Sun, Z.; Chen, L.; Wang, Q. Microbial residue distribution in microaggregates decreases with stand age in subtropical plantations. Forests 2022, 13, 1145. [Google Scholar] [CrossRef]
- Le Goff, N.L.; Ottorini, J.M. Root biomass and biomass increment in a beech (Fagus sylvatica L.) stand in North-East France. Ann. For. Sci. 2001, 58, 1–13. [Google Scholar] [CrossRef]
- Yu, P.; Liu, S.; Han, K.; Guan, S.; Zhou, D. Conversion of cropland to forage land and grassland increases soil labile carbon and enzyme activities in northeastern China. Agric. Ecosyst. Environ. 2017, 245, 83–91. [Google Scholar] [CrossRef]
- Bissett, A.; Abell, G.C.J.; Brown, M.; Thrall, P.H.; Bodrossy, L.; Smith, M.C.; Baker, G.H.; Richardsson, A.E. Land-use and management practices affect soil ammonia oxidiser community structure, activity and connectedness. Soil Biol. Biochem. 2014, 78, 138–148. [Google Scholar] [CrossRef]
- Wang, X.Y.; Ge, Y.; Wang, J. Positive effects of plant diversity on soil microbial biomass and activity are associated with more root biomass production. J. Plant Interact. 2017, 12, 533–541. [Google Scholar] [CrossRef]
- Sokol, N.W.; Kuebbing, S.E.; Karlsen-Ayala, E.; Bradford, M.A. Evidence for the primacy of living root inputs, not root or shoot litter, in forming soil organic carbon. New Phytol. 2019, 221, 233–246. [Google Scholar] [CrossRef]
- Navarro-Cano, J.A.; Goberna, M.; Valiente-Banuet, A.; Montesinos-Navarro, A.; Garcia, C.; Verdu, M. Plant phylodiversity enhances soil microbial productivity in facilitation-driven communities. Oecologia 2014, 174, 909–920. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, G.; Wu, Z.; Wen, X.; Zhong, H.; Zhong, Z.; Bian, F.; Gai, X. Agroforestry alters the rhizosphere soil bacterial and fungal communities of moso bamboo plantations in subtropical China. Appl. Soil Ecol. 2019, 143, 192–200. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Ji, X.; Zhang, Z.; Zhang, H.; Zha, T.; Jiang, L. Elevation and total nitrogen are the critical factors that control the spatial distribution of soil organic carbon content in the shrubland on the Bashang Plateau, China. Catena 2021, 204, 105415. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Chang. Biol. 2012, 18, 1918–1927. [Google Scholar] [CrossRef]
- Gärdenäs, A.I.; Ågren, G.I.; Bird, J.A.; Clarholm, M.; Hallin, S.; Ineson, P.; Kätterer, T.; Knicker, H.; Nilsson, S.I.; Näsholm, T.; et al. Knowledge gaps in soil carbon and nitrogen interactions—From molecular to global scale. Soil Biol. Biochem. 2011, 43, 702–717. [Google Scholar] [CrossRef]
- Huang, W.J.; Zhou, G.Y.; Liu, J.X. Nitrogen and phosphorus status and their influence on aboveground production under increasing nitrogen deposition in three successional forests. Acta Oecol. 2012, 44, 20–27. [Google Scholar] [CrossRef]
- Merino, A.; Jimenez, E.; Fernandez, C.; Fonturbel, M.T.; Campo, J.; Vega, J.A. Soil organic matter and phosphorus dynamics after low intensity prescribed burning in forests and shrubland. J. Environ. Manag. 2019, 234, 214–225. [Google Scholar] [CrossRef]
- Nawaz, M.F.; Bourrié, G.; Trolard, F. Soil compaction impact and modelling. A review. Agron. Sustain. Dev. 2013, 33, 291–309. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, L.; Yang, T.; Qian, Z.; Xu, C.; Tian, D.; Tang, L. Poplar agroforestry systems in eastern China enhance the spatiotemporal stability of soil microbial community structure and metabolism. Land Degrad. Dev. 2022, 33, 916–930. [Google Scholar] [CrossRef]
- Farley, K.A.; Kelly, E.F.; Hofstede, R.G.M. Soil organic carbon and water retention after conversion of grasslands to pine plantations in the Ecuadorian Andes. Ecosystems 2004, 7, 729–739. [Google Scholar] [CrossRef]
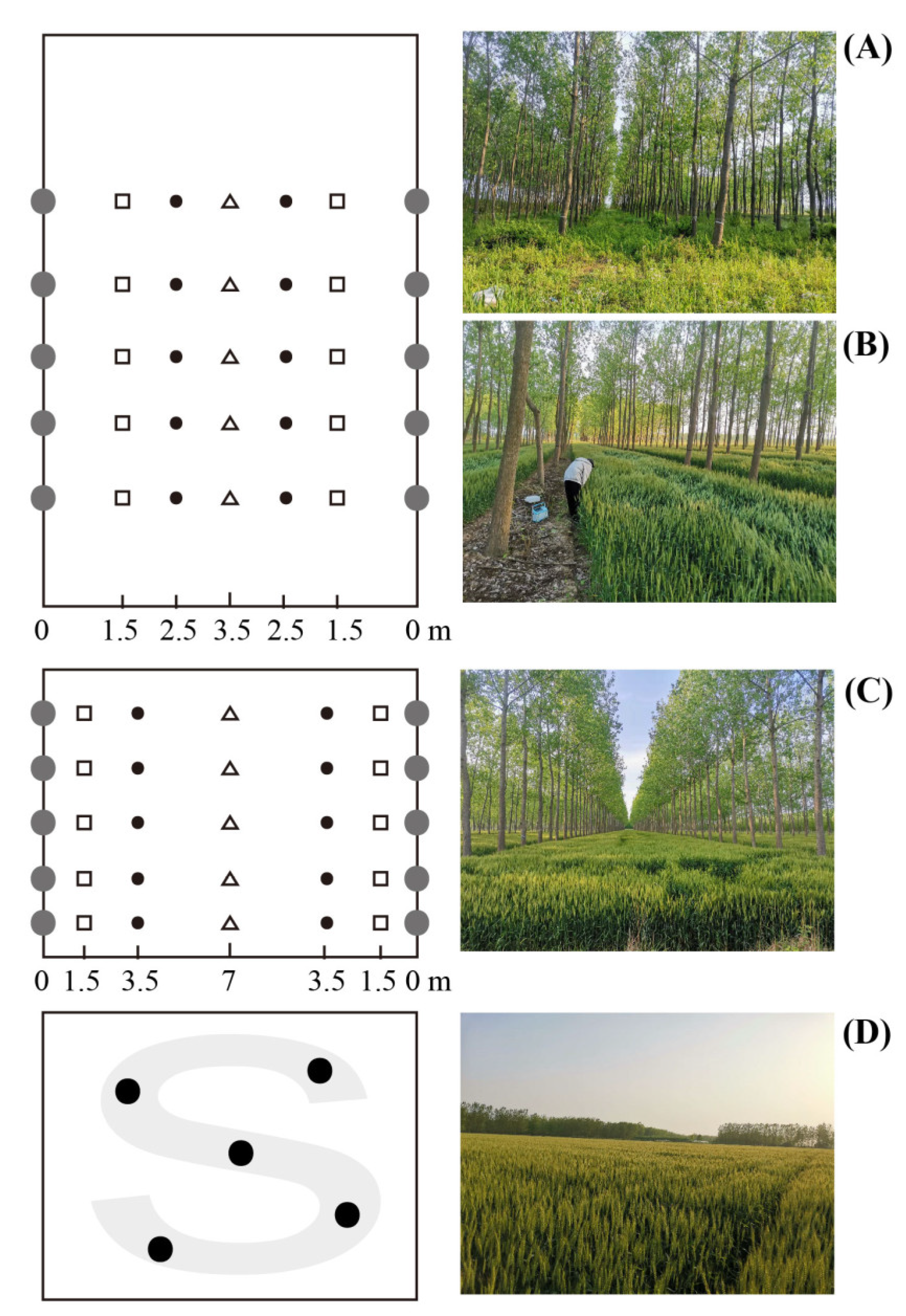

| Treatments | Age (yrs) | Planting Density (m × m) | Area (km2) | Mean DBH (cm) | Mean Height (m) |
|---|---|---|---|---|---|
| P | 12 | 3 × 7 | 0.5 | 25.16 ± 2.98 | 24.41 ± 1.92 |
| NPW | 12 | 3 × 7 | 0.5 | 23.71 ± 2.64 | 23.27 ± 1.38 |
| WPW | 12 | 3 × 14 | 2 | 28.51 ± 3.86 | 24.48 ± 2.02 |
| W | – | – | 2 | – | – |
| Planting Systems | Source of Variation | TFRB | SOC | MBC | ROC | DOC | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F | df | F | df | F | df | F | df | F | ||
| P | D | 3 | 9.544 *** | 3 | 12.587 *** | 3 | 22.627 *** | 3 | 0.36 | 3 | 27.026 *** |
| S | 3 | 151.309 *** | 3 | 603.586 *** | 3 | 175.571 *** | 3 | 567.787 *** | 3 | 74.071 *** | |
| D×S | 9 | 8.125 *** | 9 | 5.416 *** | 9 | 10.27 *** | 9 | 1.898 | 9 | 13.456 *** | |
| NPW | D | 3 | 6.809 ** | 3 | 28.745 *** | 3 | 49.896 *** | 3 | 3.146 | 3 | 13.568 *** |
| S | 3 | 190.931 *** | 3 | 605.156 *** | 3 | 200.754 *** | 3 | 361.723 *** | 3 | 107.116 *** | |
| D×S | 9 | 9.053 *** | 9 | 3.159 ** | 9 | 2.726 * | 9 | 1.904 | 9 | 1.189 | |
| WPW | D | 3 | 41.396 *** | 3 | 14.838 *** | 3 | 50.354 *** | 3 | 0.792 | 3 | 10.069 *** |
| S | 3 | 262.239 *** | 3 | 636.584 *** | 3 | 186.378 *** | 3 | 135.184 *** | 3 | 75.269 *** | |
| D×S | 9 | 28.631 *** | 9 | 2.468 * | 9 | 11.434 *** | 9 | 0.703 | 9 | 2.465 * | |
| Total system | PS | 3 | 5.546 ** | 3 | 26.888 *** | 3 | 13.207 *** | 3 | 74.074 *** | 3 | 41.783 *** |
| S | 3 | 62.406 *** | 3 | 475.441 *** | 3 | 60.73 *** | 3 | 374.202 *** | 3 | 44.785 *** | |
| PS×S | 9 | 4.134 *** | 9 | 3.052 ** | 9 | 2.944 ** | 9 | 8.698 *** | 9 | 4.487 *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Su, X.; Wang, T.; Yang, T.; Xu, C.; Lin, Z.; Tian, D.; Tang, L. Spatial Heterogeneity of Total and Labile Soil Organic Carbon Pools in Poplar Agroforestry Systems. Forests 2023, 14, 1869. https://doi.org/10.3390/f14091869
Wang B, Su X, Wang T, Yang T, Xu C, Lin Z, Tian D, Tang L. Spatial Heterogeneity of Total and Labile Soil Organic Carbon Pools in Poplar Agroforestry Systems. Forests. 2023; 14(9):1869. https://doi.org/10.3390/f14091869
Chicago/Turabian StyleWang, Bo, Xiaolong Su, Tongli Wang, Tao Yang, Cheng Xu, Zeyang Lin, Di Tian, and Luozhong Tang. 2023. "Spatial Heterogeneity of Total and Labile Soil Organic Carbon Pools in Poplar Agroforestry Systems" Forests 14, no. 9: 1869. https://doi.org/10.3390/f14091869
APA StyleWang, B., Su, X., Wang, T., Yang, T., Xu, C., Lin, Z., Tian, D., & Tang, L. (2023). Spatial Heterogeneity of Total and Labile Soil Organic Carbon Pools in Poplar Agroforestry Systems. Forests, 14(9), 1869. https://doi.org/10.3390/f14091869







