Establishment of a Transient Transformation Protocol in Cinnamomum camphora
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
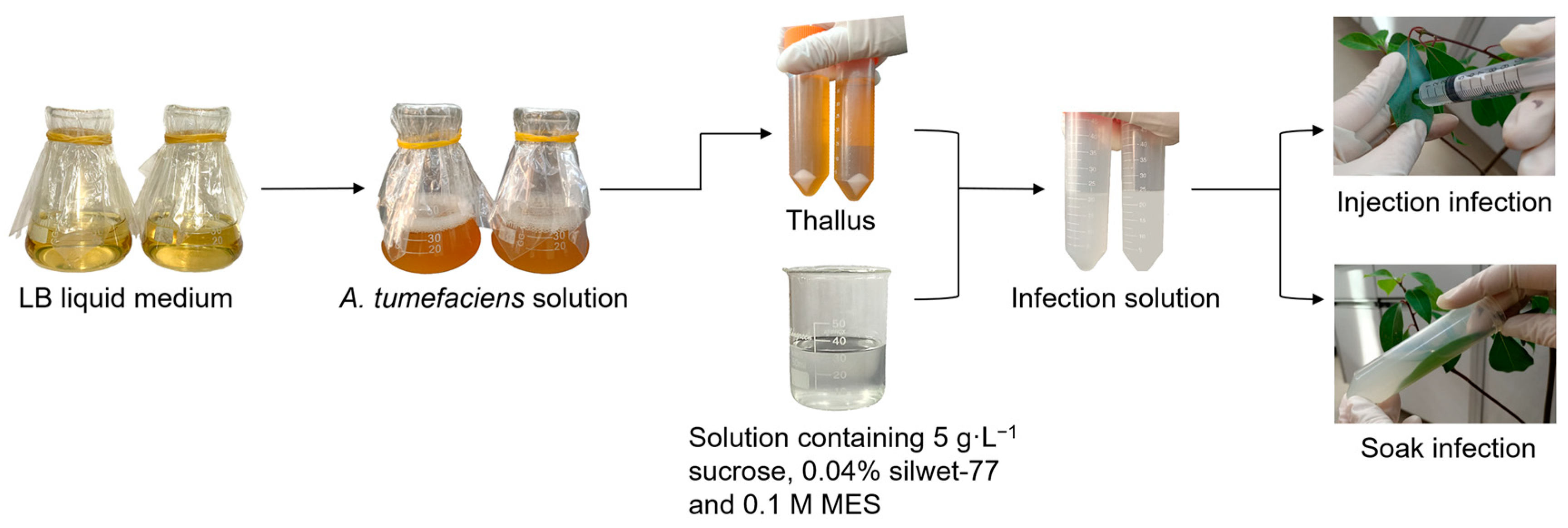
2.2. Preparation for Infection Solution
2.3. Infection Methods
2.3.1. Injection Infection
2.3.2. Soak Infection
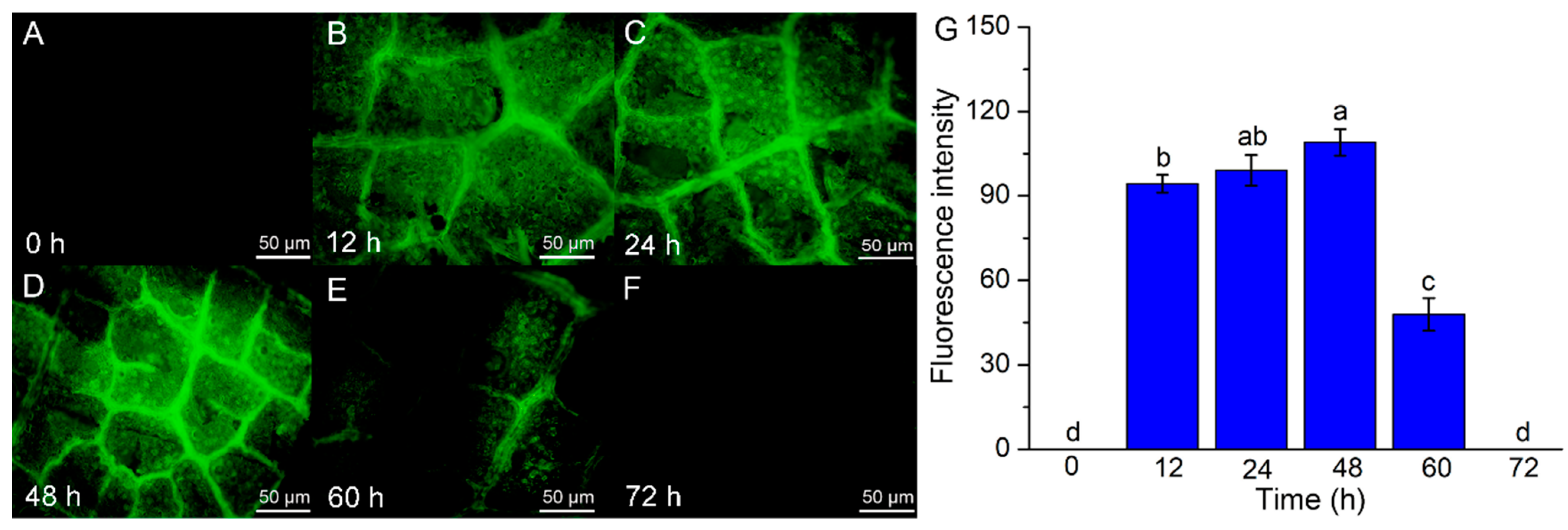
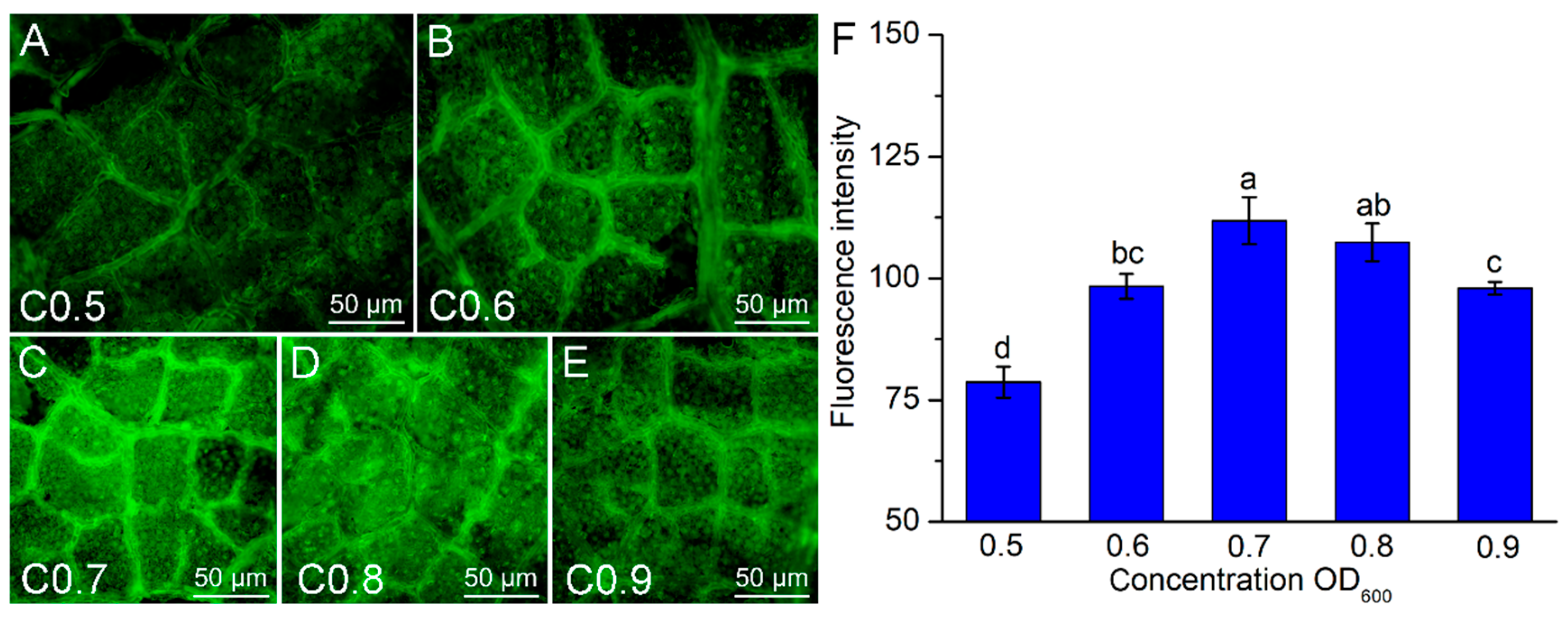
2.4. Determination of Optimum Co-Cultivation Time, Infection Solution Concentration, and A. tumefaciens Growth Density
2.5. Assay of Cytoplasmic Ca2+ Concentration under High Temperature
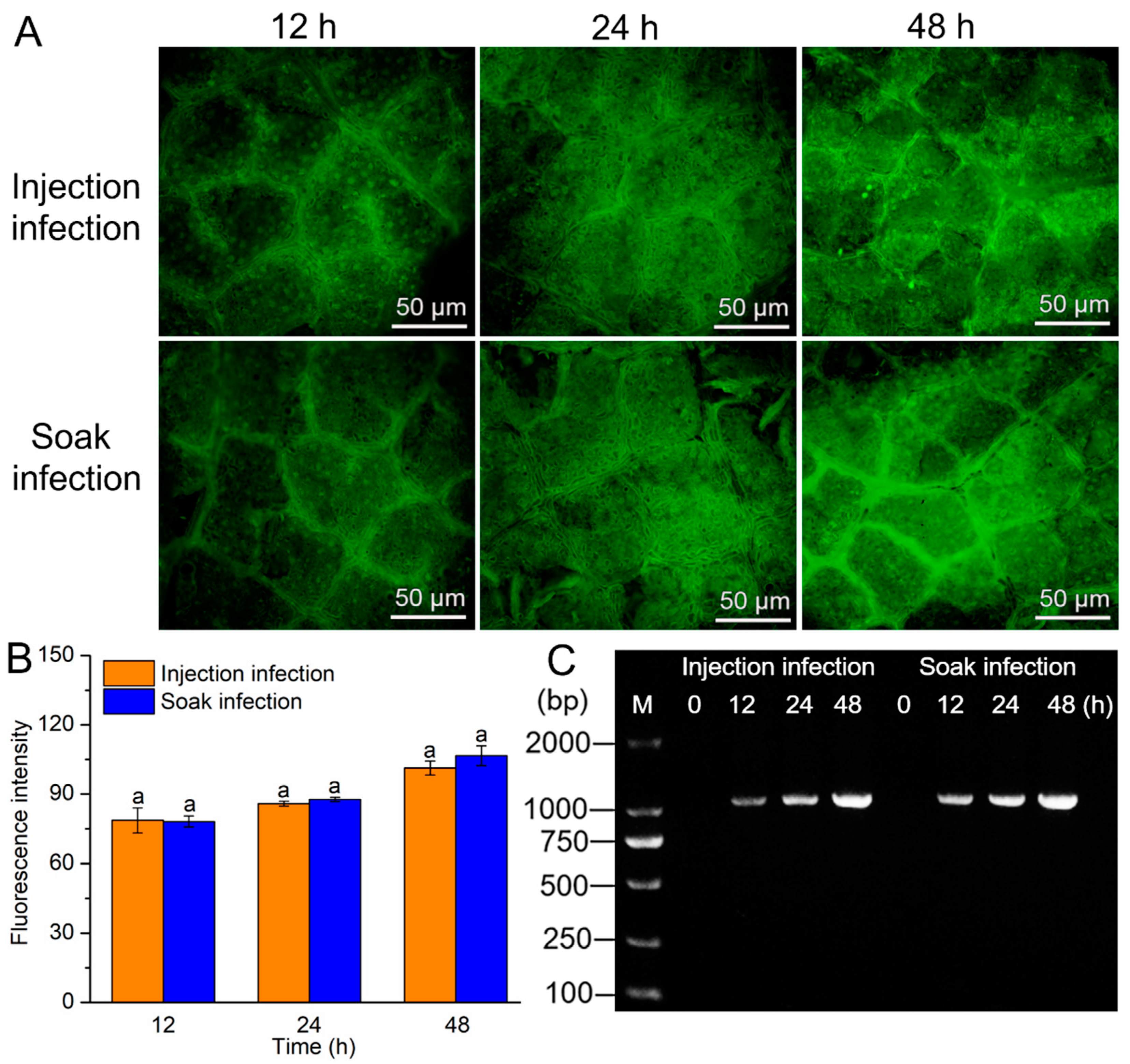
2.6. PCR Analysis
2.7. Fluorescence Intensity Measurement
2.8. Statistical Analyses
3. Results and Discussion
3.1. Effects of Infection Methods on the Transformation Efficiency
3.2. Effects of Co-Cultivation Time on the Transformation Efficiency
3.3. Effects of Infection Solution Concentration on the Transformation Efficiency
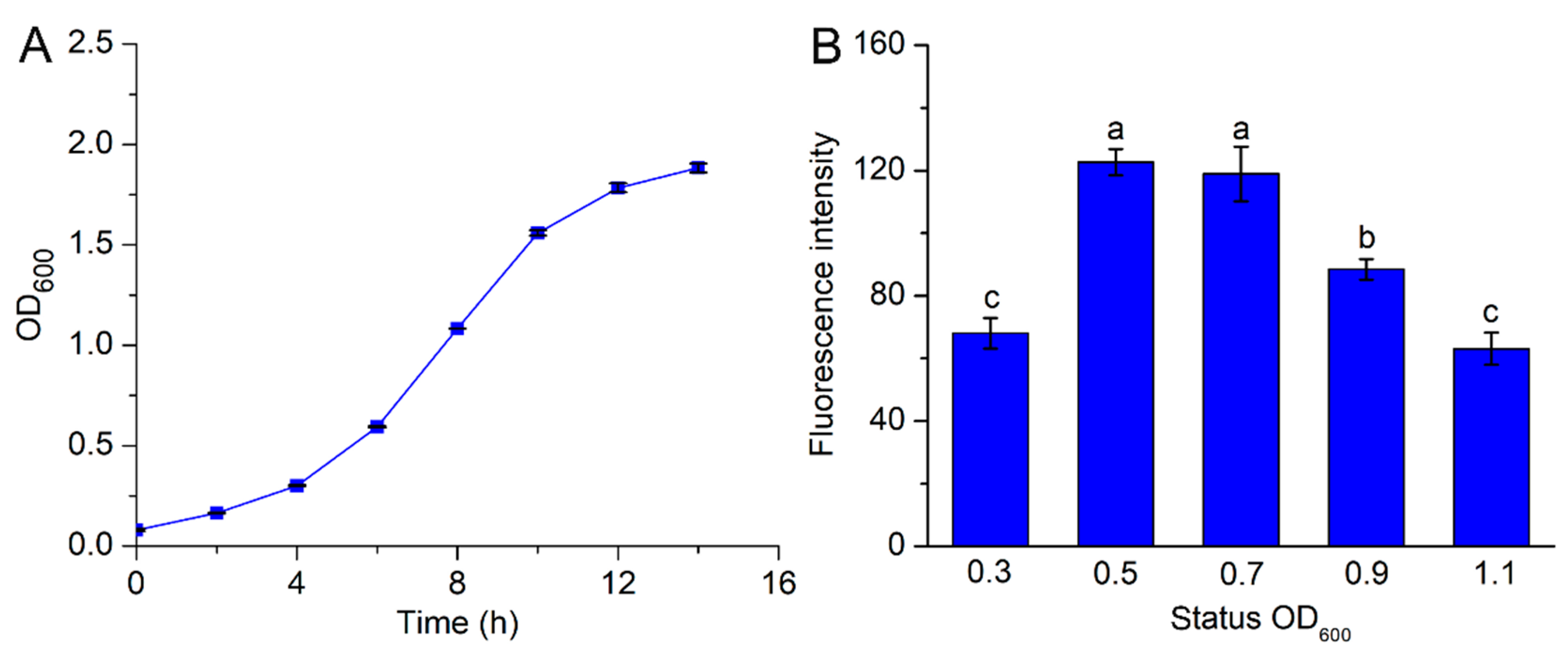
3.4. Effects of A. tumefaciens Growth Density on the Transformation Efficiency
3.5. Effects of High Temperature on the Fluorescence Intensity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lian, Z.; Nguyen, C.D.; Liu, L.; Wang, G.; Chen, J.; Wang, S.; Yi, G.; Wilson, S.; Ozias-Akins, P.; Gong, H.; et al. Application of developmental regulators to improve in-planta or in vitro transformation in plants. Plant Biotechnol. J. 2022, 20, 1622. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Zhou, N.; Ni, X.N.; Okoye, C.; Wang, Y.L.; Li, X.; Gao, L.; Zhou, G.K.; Jiang, J.X. Developing a long-term and powerful in vitro culture and Agrobacterium-mediated transformation system for Miscanthus sinensis (Poaceae). Ind. Crop Prod. 2021, 161, 113190. [Google Scholar] [CrossRef]
- Janani, C.; Sundararajan, B.; Kumari, B.D.R. Construction and transformation of peroxisome proliferator activated receptor gamma (RnPPARγ) gene using Agrobacterium tumefaciens into Glycine max L. Merr. Gene Rep. 2019, 16, 100427. [Google Scholar] [CrossRef]
- Li, X.N.; Li, H.Y.; Zhao, Y.Z.; Zong, P.X.; Zhan, Z.X.; Piao, Z.Y. Establishment of a simple and efficient Agrobacterium-mediated genetic transformation system to Chinese cabbage (Brassica rapa L. ssp. Pekinensis). Hortic. Plant J. 2021, 7, 117–128. [Google Scholar] [CrossRef]
- Giorgio, G.; Ivana, G. Genetic transformation of fruit trees: Current status and remaining challenges. Transgenic Res. 2012, 21, 1163–1181. [Google Scholar]
- Van Nocker, S.; Gardiner, S.E. Breeding better cultivars, faster: Applications of new technologies for the rapid deployment of superior horticultural tree crops. Hortic. Res. 2014, 1, 14022. [Google Scholar] [CrossRef]
- Pushpa, K.; Rinku, C.; Narendra, T.; Prashant, K. A genotype-independent, simple, effective and efficient in planta Agrobacterium-mediated genetic transformation protocol. Methods Psrotoc. 2022, 5, 69. [Google Scholar]
- Franz, L.; Anja, R.; Hannah, T.; Ralph, P. Gene gun-mediated transient gene expression for functional studies in plant immunity. Methods Mol. Biol. 2022, 2523, 63–77. [Google Scholar]
- Wang, Y.Y.; Zhang, Y.A.; Dong, Y.X.; Li, D.L.; Shi, S.L.; Li, S.H.; Li, L.Z.; He, Y.J.; Li, J.Y.; Chen, H.Y.; et al. A highly efficient mesophyll protoplast isolation and PEG-mediated transient expression system in eggplant. Sci. Hortic. 2022, 304, 111303. [Google Scholar] [CrossRef]
- Yang, Y.; Li, R.; Qi, M. In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J. 2000, 22, 543–551. [Google Scholar] [CrossRef]
- Chen, X.J.; He, S.T.; Jiang, L.; Li, X.Z.; Guo, W.L.; Chen, B.H.; Zhou, J.G.; Skliar, V. An efficient transient transformation system for gene function studies in pumpkin (Cucurbita moschata D.). Sci. Hortic. 2021, 282, 110028. [Google Scholar] [CrossRef]
- Zottini, M.; Barizza, E.; Costa, A.; Formentin, E.; Ruberti, C.; Carimi, F.; Schiavo, F.L. Agroinfiltration of grapevine leaves for fast transient assays of gene expression and for long-term production of stable transformed cells. Plant Cell Rep. 2008, 27, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.W.; Dong, B.; Zhou, J.; Miao, Y.F.; Yang, L.Y.; Wang, Y.G.; Xiao, Z.; Fang, Q.; Wan, Q.Q.; Zhao, H.B. Highly efficient transient gene expression of three tissues in Osmanthus fragrans mediated by Agrobacterium tumefaciens. Sci. Hortic. 2023, 310, 111725. [Google Scholar] [CrossRef]
- Fraley, R.T.; Rogers, S.G.; Horsch, R.B.; Sanders, P.R.; Flick, J.S.; Adams, S.P.; Bittner, M.L.; Brand, L.A.; Fink, C.L.; Fry, J.S.; et al. Expression of bacterial genes in plant cells. Proc. Natl. Acad. Sci. USA 1983, 80, 4803–4807. [Google Scholar] [CrossRef]
- Nam, J.; Mysore, K.S.; Zheng, C.; Knue, M.K.; Matthysse, A.G.; Gelvin, S.B. Identification of T-DNA tagged Arabidopsis mutants that are resistant to transformation by Agrobacterium. Mol. Gen. Genet. 1999, 261, 429–438. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, J.Y.; Kim, Y.W.; Lee, M.H.; Choi, S.H. Improvement of transformation efficiencies using Agrobacterium-mediated transformation of Korean rice. Korean J. Crop Sci. 2004, 49, 61–68. [Google Scholar]
- Vinoth, S.; Gurusaravanan, P.; Jayabalan, N. Optimization of factors influencing microinjection method for Agrobacterium tumefaciens-mediated transformation of tomato. Appl. Biochem. Biotechnol. 2013, 169, 1173–1187. [Google Scholar] [CrossRef]
- Lv, Q.R.; Chen, C.S.; Xu, Y.J.; Hu, S.K.; Wang, L.; Sun, K.; Chen, X.; Li, X.H. Optimization of Agrobacterium tumefaciens-mediated transformation systems in tea plant (Camellia sinensis). Hortic. Plant J. 2017, 3, 105–109. [Google Scholar] [CrossRef]
- Lv, Y.D.; Zhang, M.L.; Wu, T.; Wu, T.L.; Zhong, Y. The infiltration efficiency of Agrobacterium-mediated transient transformation in four apple cultivars. Sci. Hortic. 2019, 256, 108597. [Google Scholar] [CrossRef]
- Wen, S.S.; Ge, X.L.; Wang, R.; Yang, H.F.; Bai, Y.; Guo, Y.H.; Zhang, J.; Lu, M.Z.; Zhao, S.T.; Wang, L.Q. An efficient Agrobacterium-mediated transformation method for Hybrid Poplar 84K (Populus alba × P. glandulosa) using calli as explants. Int. J. Mol. Sci. 2022, 23, 2216. [Google Scholar] [CrossRef]
- Yang, W.H.; Ren, J.Q.; Liu, W.R.; Liu, D.; Xie, K.D.; Zhang, F.; Wang, P.W.; Guo, W.W.; Wu, X.M. An efficient transient gene expression system for protein subcellular localization assay and genome editing in citrus protoplasts. Hortic. Plant J. 2022, 9, 425–436. [Google Scholar] [CrossRef]
- Chen, C.H.; Zhong, Y.D.; Yu, F.X.; Xu, M. Deep sequencing identifies miRNAs and their target genes involved in the biosynthesis of terpenoids in Cinnamomum camphora. Ind. Crop. Prod. 2020, 145, 111853. [Google Scholar] [CrossRef]
- Ma, R.; Su, P.; Jin, B.; Guo, J.; Tian, M.; Mao, L.Y.; Tang, J.F.; Chen, T.; Lai, C.J.S.; Zeng, W.; et al. Molecular cloning and functional identification of a high-efficiency (+)-borneol dehydrogenase from Cinnamomum camphora (L.) Presl. Plant Physiol. Biochem. 2021, 158, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhong, Y.; Yu, F.; Xu, M. Novel SSR marker development and genetic diversity analysis of Cinnamomum camphora based on transcriptome sequencing. Plant Genet. Resour. 2018, 16, 568–571. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, A.; Li, Z.; Zhang, H.; Liu, L.; Wu, Z.; Li, Y.; Liu, T.; Xu, M.; Yu, F. Genetic diversity and population genetic structure of Cinnamomum camphora in South China revealed by EST-SSR markers. Forests 2019, 10, 1019. [Google Scholar] [CrossRef]
- Gong, X.; Yang, A.; Wu, Z.; Chen, C.; Li, H.; Liu, Q.; Yu, F.; Zhong, Y. Employing genome-wide SNP discovery to characterize the genetic diversity in Cinnamomum camphora using genotyping by sequencing. Forests 2021, 12, 1511. [Google Scholar] [CrossRef]
- Shi, X.P.; Dai, X.G.; Liu, G.F.; Zhang, J.W.; Ning, G.G.; Bao, M.Z. Cyclic secondary somatic embryogenesis and efficient plant regeneration in camphor tree (Cinnamomum camphora L.). In Vitro Cell. Dev. Biol.-Plant 2010, 46, 117–125. [Google Scholar] [CrossRef]
- El-Kader, E.M.A.; Serag, A.; Aref, M.S.; Ewais, E.E.A.; Farag, M.A. Metabolomics reveals ionones upregulation in MeJA elicited Cinnamomum camphora (camphor tree) cell culture. Plant Cell Tissue Organ Cult. 2019, 137, 309–318. [Google Scholar] [CrossRef]
- Shen, T.F.; Qi, H.R.; Luan, X.Y.; Xu, W.L.; Yu, F.X.; Zhong, Y.D.; Xu, M. The chromosome-level genome sequence of the camphor tree provides insights into Lauraceae evolution and terpene biosynthesis. Plant Biotechnol. J. 2022, 20, 244–246. [Google Scholar] [CrossRef]
- Krogman, W.; Sparks, J.A.; Blancaflor, E.B. Cell type-specific imaging of calcium signaling in Arabidopsis thaliana seedling roots using GCaMP3. Int. J. Mol. Sci. 2020, 21, 6385. [Google Scholar] [CrossRef]
- Ren, Q.; Xu, Z.; Xue, Y.; Yang, R.; Ma, X.; Sun, J.; Wang, J.; Lin, S.; Wang, W.; Yang, L.; et al. Mechanism of calcium signal response to cadmium stress in duckweed. Plant Signal. Behav. 2022, 17, 2119340. [Google Scholar] [CrossRef] [PubMed]
- Toyota, M.; Spencer, D.; Sawai-Toyota, S.; Wang, J.; Zhang, T.; Koo, A.J.; Howe, G.A.; Gilroy, S. Glutamate triggers long-distance, calcium-based plant defense signaling. Science 2018, 361, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Salehi, Z.; Amirahmadi, A.; Rezaei, A.; Farrokh, P.; Ghasemian, J. Comparison of five DNA extraction methods in three medicinal plants: Peganum harmala L., Tamarix ramosissima Ledeb., and Potentilla reptans L. Mol. Biol. Res. Commun. 2023, 12, 1–61. [Google Scholar]
- Chincinska, I.A. Leaf infiltration in plant science: Old method, new possibilities. Plant Methods 2021, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Vonasek, E.; Nitin, N. Influence of vacuum cooling on Escherichia coli O157:H7 infiltration in fresh leafy greens via a multiphoton-imaging approach. Appl. Environ. Microb. 2016, 82, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Ranjbaran, M.; Solhtalab, M.; Datta, A.K. Mechanistic modeling of light induced chemotactic infiltration of bacteria into leaf stomata. PLoS Comput. Biol. 2020, 16, e1007841. [Google Scholar] [CrossRef]
- Suleiman, W.B. In vitro estimation of superfluid critical extracts of some plants for their antimicrobial potential, phytochemistry, and GC–MS analyses. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 29. [Google Scholar] [CrossRef]
- Yan, X.; Liang, S.; Peng, T.; Zhang, G.; Zeng, Z.; Yu, P.; Gong, D.; Deng, S. Influence of phenolic compounds on physicochemical and functional properties of protein isolate from Cinnamomum camphora seed kernel. Food Hydrocolloid 2020, 102, 105612. [Google Scholar] [CrossRef]
- Zhao, H.M.; Jia, Y.Q.; Cao, Y.T.; Wang, Y.C. Improving T-DNA transfer to Tamarix hispida by adding chemical compounds during Agrobacterium tumefaciens culture. Front. Plant Sci. 2020, 11, 501358. [Google Scholar] [CrossRef]
- Li, S.J.; Xu, B.L.; Niu, X.L.; Lu, X.; Cheng, J.P.; Zhou, M.L.; Hooykaas, P.J.J. JAZ8 interacts with VirE3 attenuating Agrobacterium mediated root tumorigenesis. Front. Plant Sci. 2021, 12, 685533. [Google Scholar] [CrossRef]
- Firoozabady, E.; Heckert, M.; Gutterson, N. Transformation and regeneration of pineapple. Plant Cell Tissue Organ 2006, 84, 1–16. [Google Scholar] [CrossRef]
- Mo, R.L.; Huang, Y.M.; Yang, S.C.; Zhang, Q.L.; Luo, Z.R. Development of Agrobacterium -mediated transient transformation in persimmon (Diospyros kaki Thunb.). Sci. Hortic. 2015, 192, 29–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.C.; Wang, C. Gene overexpression and gene silencing in birch using an Agrobacterium-mediated transient expression system. Mol. Biol. Rep. 2012, 39, 5537–5541. [Google Scholar] [CrossRef]
- Priyadarshani, S.V.G.N.; Cai, H.Y.; Zhou, Q.; Liu, Y.H.; Cheng, Y.; Xiong, J.J.; Patson, D.L.; Cao, S.; Zhao, H.; Qin, Y. An efficient Agrobacterium mediated transformation of pineapple with GFP-tagged protein allows easy, non-destructive screening of transgenic pineapple plants. Biomolecules 2019, 9, 617. [Google Scholar] [CrossRef] [PubMed]
- Petri, C.; Alburquerque, A.; Garcéa-Castillo, S.; Egea, J.; Burgos, L. Factors affecting gene transfer efficiency to apricot leaves during early Agrobacterium-mediated transformation steps. J. Hortic. Sci. Biotechnol. 2004, 79, 704–712. [Google Scholar] [CrossRef]
- Chaparro-Pulido, C.A.; Montiel, M.M.; Palomo-Ríos, E.; Mercado, J.A.; Pliego-Alfaro, F. Development of an efficient transient transformation protocol for avocado (Persea americana Mill.) embryogenic callus. In Vitro Cell. Dev. Biol.-Plant 2014, 50, 292–298. [Google Scholar] [CrossRef]
- Mallón, R.; Vidal, A.M.V.N. High-efficiency Agrobacterium-mediated transformation in Quercus robur: Selection by use of a temporary immersion system and assessment by quantitative PCR. Plant Cell Tissue Organ 2013, 114, 171–185. [Google Scholar] [CrossRef]
- Jiang, S.F.; Cui, Y.; Zhao, R.R.; Qi, S.Z.; Kong, L.S.; Zhao, J.; Li, S.S.; Zhang, J.F. Agrobacterium tumefaciens-mediated transformation of hybrid sweetgum embryogenic callus. J. Beijing For. Univ. 2021, 43, 9–17, (In Chinese with English abstract). [Google Scholar]
- Han, X.; Han, B. Plant regeneration and Agrobacterium-mediated transformation of vacuolar H+-ATPase c subunit gene in hybrid poplar Populus davidiana Dode × P. bollena Lauche. BIO Web Conf. 2017, 8, 03004. [Google Scholar] [CrossRef]
- Miao, M.M.; Xu, R.; Zheng, L.J.; Zhou, H.L.; Zhang, Z.D.; Cheng, H. High-efficiency Agrobacterium tumefaciens-mediated transformation of cucumber (Cucumis sativus L.) using stem nodes as explants. J. Hortic. Sci. Biotechnol. 2009, 84, 199–203. [Google Scholar] [CrossRef]
- An, D.D.; Danhorn, T.; Fuqua, C.; Parsek, M.R. Quorum sensing and motility mediate interactions between Pseudomonas aeruginosa and Agrobacterium tumefaciens in biofilm cocultures. Proc. Natl. Acad. Sci. USA 2006, 103, 3828–3833. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Mandal, N. Reasons and riddance of Agrobacterium tumefaciens overgrowth in plant transformation. Transgenic Res. 2023, 32, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Kasia, P.; Tanja, S.; Jürgen, T.; Karel, Š.; Karel, K.; Silke, K.; Dietmar, H.P.; Michal, K. Determining lineage-specific bacterial growth curves with a novel approach based on amplicon reads normalization using internal standard (ARNIS). ISME J. 2018, 12, 2640–2654. [Google Scholar]
- Benny, A.; Alex, S.; Soni, K.B.; Anith, K.N.; Kiran, A.G.; Viji, M.M. Improved transformation of Agrobacterium assisted by silver nanoparticles. BioTechnologia 2022, 103, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Thilip, C.; Raju, C.S.; Varutharaju, K.; Aslam, A.; Shajahan, A. Improved Agrobacterium rhizogenes-mediated hairy root culture system of Withania somnifera (L.) Dunal using sonication and heat treatment. 3 Biotech 2015, 5, 949–956. [Google Scholar] [CrossRef]
- Iqbal, A.; Dave, N.; Kant, T. Comparative analysis of different nutrient media for growth of Agrobacterium tumefaciens under small volume cultures. J. Appl. Life Sci. Int. 2021, 24, 27–33. [Google Scholar] [CrossRef]
- Irvin, L.; Ortiz, Y.Z.; Rivera, K.R.; Vaidya, B.N.; Sherman, S.H.; Batista, R.A.; Berríos, J.A.N.; Joshee, N.; Arun, A. Micropropagation of rare Scutellaria havanensis Jacq. and preliminary studies on antioxidant capacity and anti-cancer potential. Molecules 2021, 26, 5813. [Google Scholar] [CrossRef]
- Ashraf-Dehkordi, E.; Alemzadeh, A.; Tanaka, N.; Razi, H. Effects of vacuum infiltration, Agrobacterium cell density and acetosyringone concentration on Agrobacterium-mediated transformation of bread wheat. J. Consum. Prot. Food Saf. 2021, 16, 59–69. [Google Scholar] [CrossRef]
- Brown, P.J.B.; Chang, J.H.; Fuqua, C. Agrobacterium tumefaciens: A transformative agent for fundamental insights into host-microbe interactions, genome biology, chemical signaling, and cell biology. J. Bacteriol. 2023, 205, e00005-23. [Google Scholar] [CrossRef]
- Cao, D.V.; Pamplona, R.S.; Kim, J.; Oh, Y.K.; Cho, S.K.; Ahn, J.; Yang, S.W.; Riu, K.Z.; Boo, K.H. Optimization of Agrobacterium-mediated transient expression of heterologous genes in spinach. Plant Biotechnol. Rep. 2017, 11, 397–405. [Google Scholar] [CrossRef]
- Duan, S.Y.; Xin, R.J.; Guan, S.X.; Li, X.T.; Fei, R.W.; Cheng, W.; Pan, Q.; Sun, X.M. Optimization of callus induction and proliferation of Paeonia lactiflora Pall. and Agrobacterium-mediated genetic transformation. Front. Plant Sci. 2022, 13, 996690. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Kang, X.; Ge, J.; Fei, R.; Duan, S.; Sun, X. An efficient Agrobacterium-mediated transient transformation system and its application in gene function elucidation in Paeonia lactiflora Pall. Front. Plant Sci. 2022, 13, 999433. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Harper, J.F.; Weigand, C.; Hua, J. Resting cytosol Ca2+ level maintained by Ca2+ pumps affect environmental responses in Arabidopsis. Plant Physiol. 2023, 191, 2534–2550. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Luo, D.L.; Vignols, F.; Jinn, T.L. Heat shock-induced biphasic Ca2+ signature and OsCaM1-1 nuclear localization mediate downstream signalling in acquisition of thermotolerance in rice (Oryza sativa L.). Plant Cell Environ. 2012, 35, 1543–1557. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.N.; Lu, X.X.; Ren, A.; Shi, L.; Zhu, J.; Jiang, A.L.; Yu, H.S.; Zhao, M.W. Conversion of phosphatidylinositol (PI) to PI4-phosphate (PI4P) and then to PI (4, 5) P2 is essential for the cytosolic Ca2+ concentration under heat stress in Ganoderma lucidum. Environ. Microbiol. 2018, 20, 2456–2468. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Hu, R.; Yang, L.; Zuo, Z. Establishment of a Transient Transformation Protocol in Cinnamomum camphora. Forests 2023, 14, 1872. https://doi.org/10.3390/f14091872
Zhang S, Hu R, Yang L, Zuo Z. Establishment of a Transient Transformation Protocol in Cinnamomum camphora. Forests. 2023; 14(9):1872. https://doi.org/10.3390/f14091872
Chicago/Turabian StyleZhang, Siyi, Rong Hu, Lin Yang, and Zhaojiang Zuo. 2023. "Establishment of a Transient Transformation Protocol in Cinnamomum camphora" Forests 14, no. 9: 1872. https://doi.org/10.3390/f14091872
APA StyleZhang, S., Hu, R., Yang, L., & Zuo, Z. (2023). Establishment of a Transient Transformation Protocol in Cinnamomum camphora. Forests, 14(9), 1872. https://doi.org/10.3390/f14091872





