Effects of Pine Wilt Disease on Rhizosphere Microbiota and Fine Root Fungi: Insights into Enzyme Activity, Ectomycorrhizal Infection and Microbial Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling
2.2. Rhizosphere Soil Enzyme Activity Analysis
2.3. Determination of Mycorrhizal Fungal Infection Rate
2.4. Sample DNA Extraction, PCR Amplification and Illumina MiSeq Sequencing
2.5. Bioinformatics and Statistical Analysis
3. Results
3.1. Enzyme Activity in Rhizosphere Soil
3.2. Microbial Community in Rhizosphere Soil
3.2.1. Microbial α-Diversity in Rhizosphere Soil
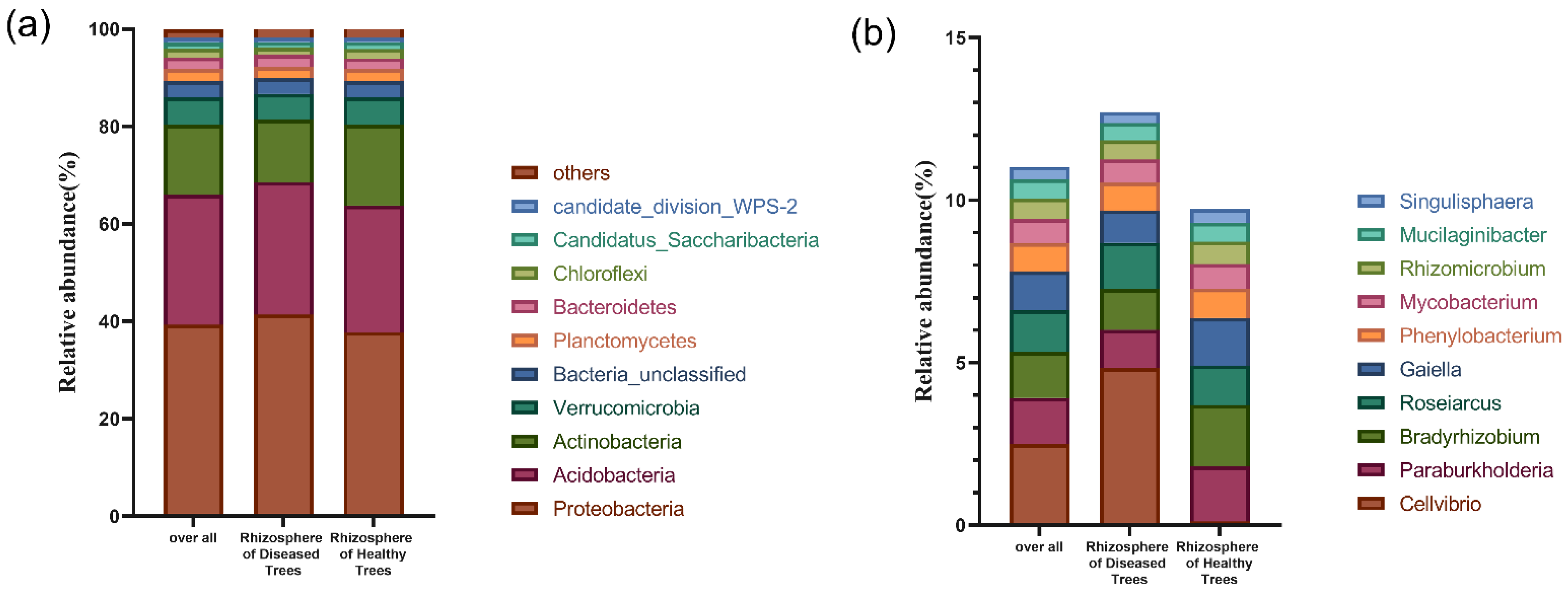
3.2.2. Microbial Community Structure in Rhizosphere Soil
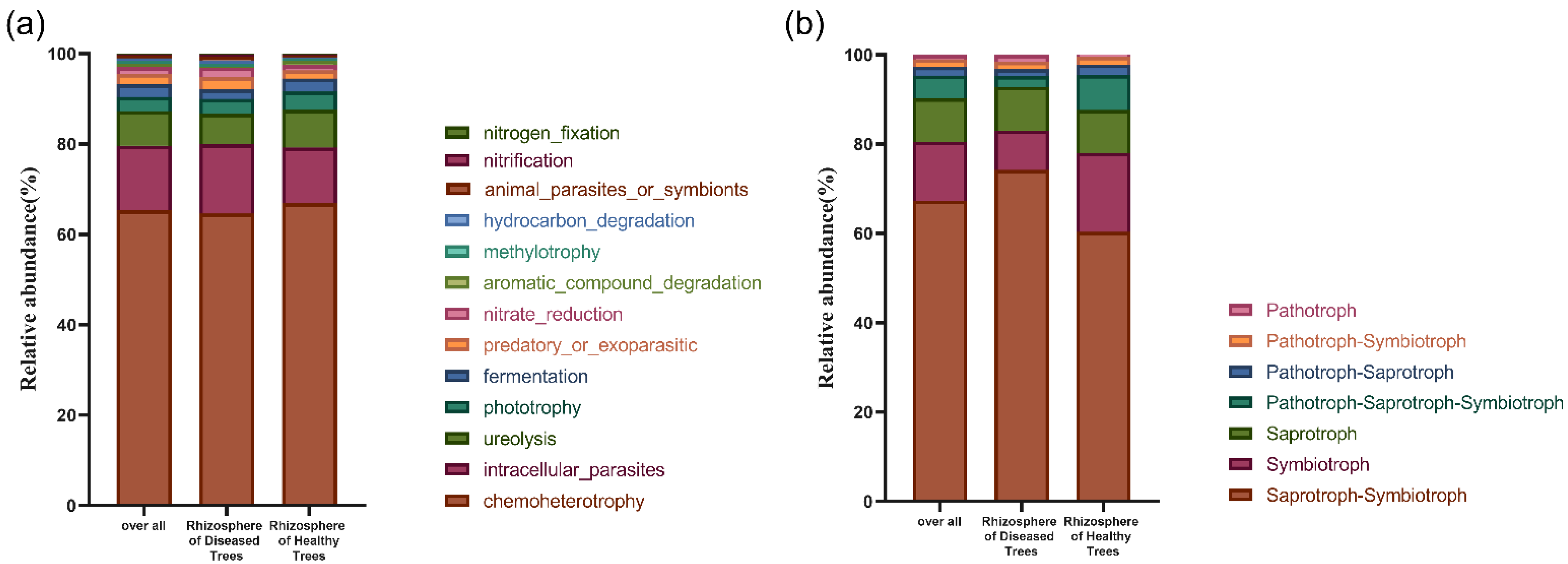
3.2.3. Microbial Community Potential Functional Structures in Rhizosphere Soil
3.3. Mycorrhizal Fungal Infection Rate and Fungal Community in Fine Root
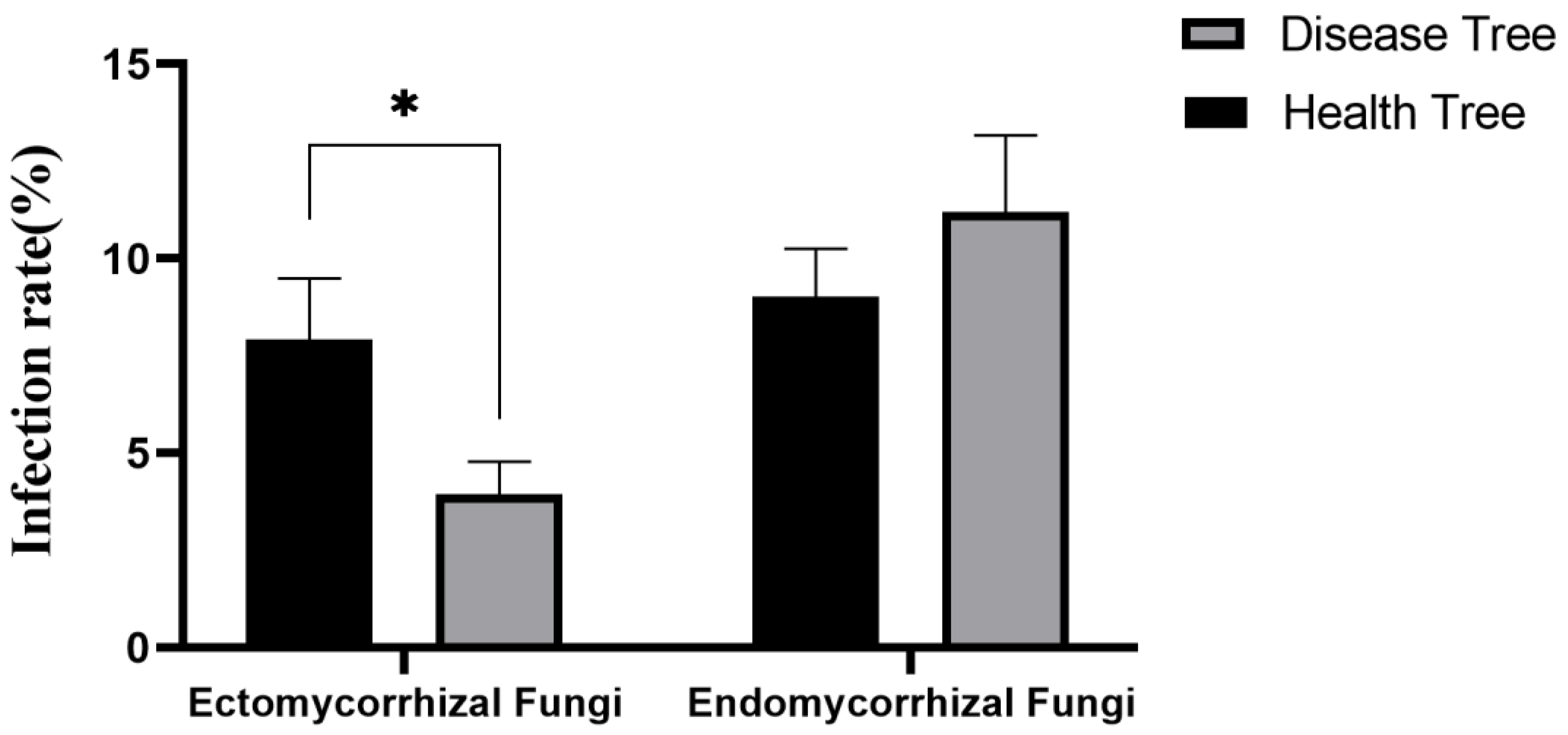
3.3.1. Mycorrhizal Fungal Infection Rate
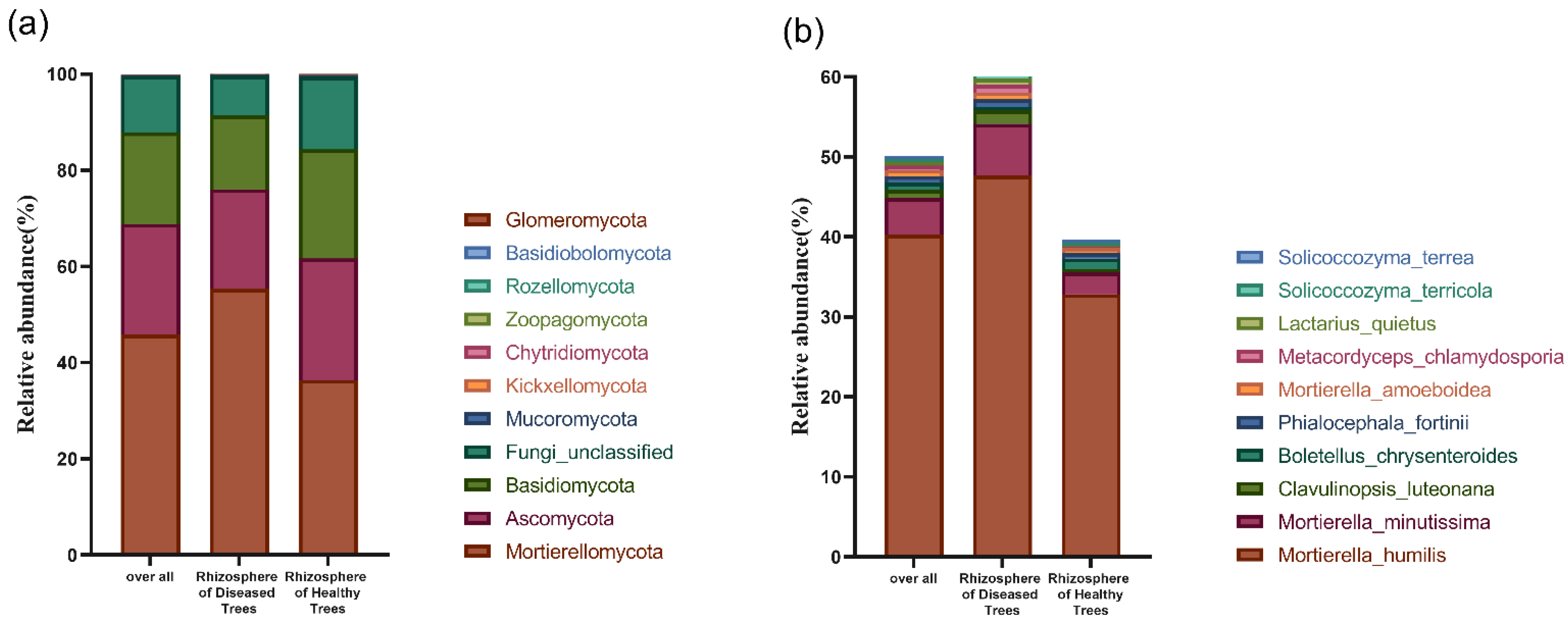
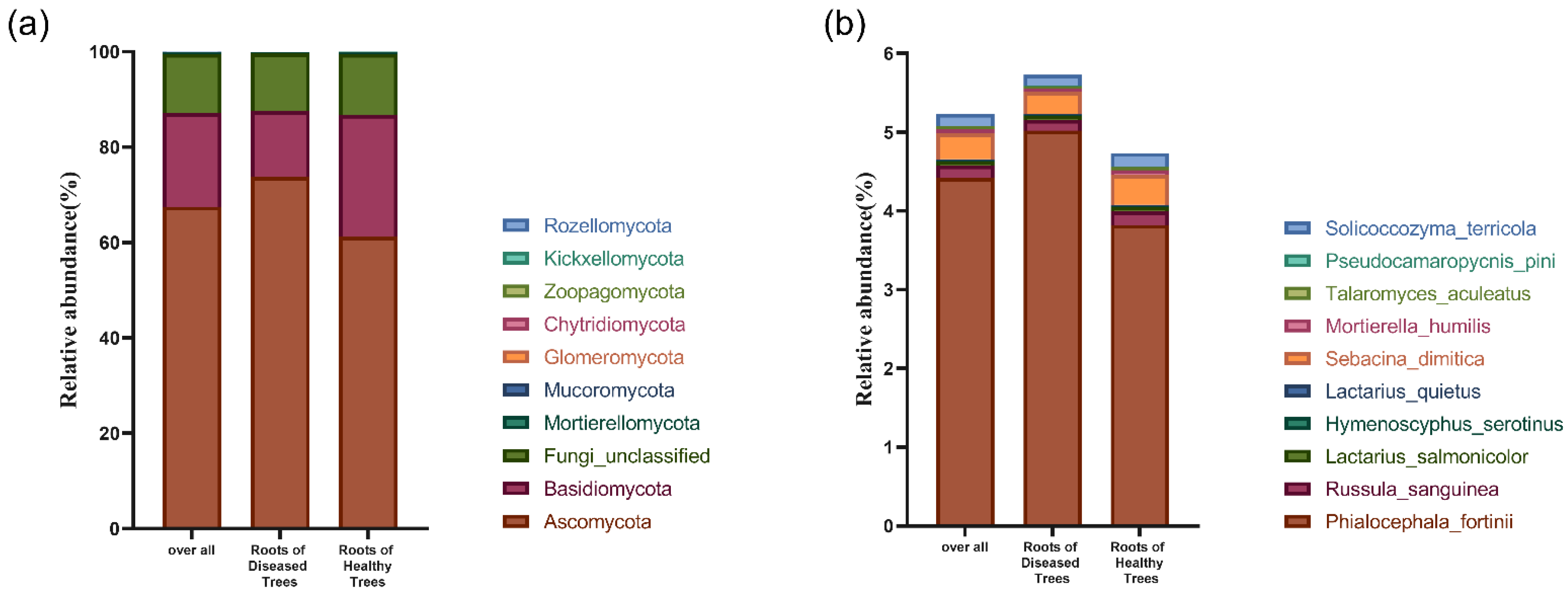
3.3.2. Fungal Community Structure in Fine Root
3.3.3. Fungal Potential Functional Structures in Fine Root of Diseased and Healthy Trees
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fadiji, A.E.; Babalola, O.O. Metagenomics Methods for the Study of Plant-Associated Microbial Communities: A Review. J. Microbiol. Methods 2020, 170, 105860. [Google Scholar] [CrossRef] [PubMed]
- Brader, G.; Compant, S.; Vescio, K.; Mitter, B.; Trognitz, F.; Ma, L.-J.; Sessitsch, A. Ecology and Genomic Insights into Plant-Pathogenic and Plant-Nonpathogenic Endophytes. Annu. Rev. Phytopathol. 2017, 55, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; White, J.F. Indigenous Endophytic Seed Bacteria Promote Seedling Development and Defend against Fungal Disease in Browntop Millet (Urochloa ramosa L.). J. Appl. Microbiol. 2018, 124, 764–778. [Google Scholar] [CrossRef]
- Agler, M.T.; Ruhe, J.; Kroll, S.; Morhenn, C.; Kim, S.-T.; Weigel, D.; Kemen, E.M. Microbial Hub Taxa Link Host and Abiotic Factors to Plant Microbiome Variation. PLoS Biol. 2016, 14, e1002352. [Google Scholar] [CrossRef]
- Omomowo, O.I.; Babalola, O.O. Bacterial and Fungal Endophytes: Tiny Giants with Immense Beneficial Potential for Plant Growth and Sustainable Agricultural Productivity. Microorganisms 2019, 7, 481. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Rothballer, M.; Schmid, M. Lorenz Hiltner, a Pioneer in Rhizosphere Microbial Ecology and Soil Bacteriology Research. Plant Soil 2008, 312, 7–14. [Google Scholar] [CrossRef]
- Jacoby, R.P.; Chen, L.; Schwier, M.; Koprivova, A.; Kopriva, S. Recent Advances in the Role of Plant Metabolites in Shaping the Root Microbiome. F1000Research 2020, 9, F1000 Faculty Rev-151. [Google Scholar] [CrossRef]
- Dennis, P.; Miller, A.; Hirsch, P. Are Root Exudates More Important than Other Sources of Rhizodeposits in Structuring Rhizosphere Bacterial Communities? FEMS Microbiol. Ecol. 2010, 72, 313–327. [Google Scholar] [CrossRef]
- Jacoby, R.; Koprivova, A.; Kopriva, S. Pinpointing Secondary Metabolites That Shape the Composition and Function of the Plant Microbiome. J. Exp. Bot. 2021, 72, 57–69. [Google Scholar] [CrossRef]
- Bailly, A.; Weisskopf, L. The Modulating Effect of Bacterial Volatiles on Plant Growth: Current Knowledge and Future Challenges. Plant Signal. Behav. 2012, 7, 79–85. [Google Scholar] [CrossRef]
- Oldroyd, G. Speak, Friend, and Enter: Signalling Systems That Promote Beneficial Symbiotic Associations in Plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, A.; Nizampatnam, N.; Kondreddy, A.; Pradhan, B.; Chatterjee, S. Xanthomonas Campestris Cell-Cell Signalling Molecule DSF (Diffusible Signal Factor) Elicits Innate Immunity in Plants and Is Suppressed by the Exopolysaccharide Xanthan. J. Exp. Bot. 2015, 66, 6697–6714. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.; Brzostek, E.; Midgley, M. The Mycorrhizal-Associated Nutrient Economy: A New Framework for Predicting Carbon-Nutrient Couplings in Temperate Forests. New Phytol. 2013, 199, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Sulman, B.; Shevliakova, E.; Brzostek, E.; Kivlin, S.; Malyshev, S.; Menge, D.; Zhang, X. Diverse Mycorrhizal Associations Enhance Terrestrial C Storage in a Global Model. Glob. Biogeochem. Cycles 2019, 33, 501–523. [Google Scholar] [CrossRef]
- Kivlin, S.; Emery, S.; Rudgers, J. Fungal symbionts alter plant responses to global change. Am. J. Bot. 2013, 100, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, S.; Wirth, C.; Jucker, T.; van der Plas, F.; Scherer-Lorenzen, M.; Verheyen, K.; Allan, E.; Benavides, R.; Bruelheide, H.; Ohse, B.; et al. Biodiversity and Ecosystem Functioning Relations in European Forests Depend on Environmental Context. Ecol. Lett. 2017, 20, 1414–1426. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.A.; Grace, E.J.; Smith, S.E. More than a Carbon Economy: Nutrient Trade and Ecological Sustainability in Facultative Arbuscular Mycorrhizal Symbioses. New Phytol. 2009, 182, 347–358. [Google Scholar] [CrossRef]
- Dwinell, L.D. The Pinewood Nematode: Regulation and Mitigation. Annu. Rev. Phytopathol. 1997, 35, 153–166. [Google Scholar] [CrossRef]
- Kim, B.-N.; Kim, J.H.; Ahn, J.-Y.; Kim, S.; Cho, B.-K.; Kim, Y.-H.; Min, J. A Short Review of the Pinewood Nematode, Bursaphelenchus Xylophilus. Toxicol. Environ. Health Sci. 2020, 12, 297–304. [Google Scholar] [CrossRef]
- Vicente, C.; Ikuyo, Y.; Mota, M.; Hasegawa, K. Pinewood Nematode-Associated Bacteria Contribute to Oxidative Stress Resistance of Bursaphelenchus Xylophilus. BMC Microbiol. 2013, 13, 299. [Google Scholar] [CrossRef]
- Wu, X.; Yuan, W.; Tian, X.; Fan, B.; Fang, X.; Ye, J.; Ding, X. Specific and Functional Diversity of Endophytic Bacteria from Pine Wood Nematode Bursaphelenchus Xylophilus with Different Virulence. Int. J. Biol. Sci. 2013, 9, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Qu, Z.-L.; Liu, B.; Tan, J.-J.; Asiegbu, F.O.; Sun, H. Bacterial Community Structure of Pinus Thunbergii Naturally Infected by the Nematode Bursaphelenchus Xylophilus. Microorganisms 2020, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qu, Z.-L.; Liu, B.; Ma, Y.; Xu, J.; Shen, W.-X.; Sun, H. The Impact of Pine Wood Nematode Infection on the Host Fungal Community. Microorganisms 2021, 9, 896. [Google Scholar] [CrossRef]
- Deng, S.; Katoh, M.; Guan, Q.; Yin, N.; Li, M. Interpretation of Forest Resources at the Individual Tree Level at Purple Mountain, Nanjing City, China, Using WorldView-2 Imagery by Combining GPS, RS and GIS Technologies. Remote Sens. 2014, 6, 87–110. [Google Scholar] [CrossRef]
- Deng, S.; Katoh, M.; Guan, Q.; Yin, N.; Li, M. Estimating Forest Aboveground Biomass by Combining ALOS PALSAR and WorldView-2 Data: A Case Study at Purple Mountain National Park, Nanjing, China. Remote Sens. 2014, 6, 7878–7910. [Google Scholar] [CrossRef]
- Qu, Z.-L.; Braima, A.; Liu, B.; Ma, Y.; Sun, H. Soil Fungal Community Structure and Function Shift during a Disease-Driven Forest Succession. Microbiol. Spectr. 2022, 10, e0079522. [Google Scholar] [CrossRef] [PubMed]
- Millberg, H.; Boberg, J.; Stenlid, J. Changes in Fungal Community of Scots Pine (Pinus Sylvestris) Needles along a Latitudinal Gradient in Sweden. Fungal Ecol. 2015, 17, 126–139. [Google Scholar] [CrossRef]
- Kikuchi, T.; Aikawa, T.; Oeda, Y.; Karim, N.; Kanzaki, N. A Rapid and Precise Diagnostic Method for Detecting the Pinewood Nematode Bursaphelenchus Xylophilus by Loop-Mediated Isothermal Amplification. Phytopathology 2009, 99, 1365–1369. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The Effects of Long Term Nitrogen Deposition on Extracellular Enzyme Activity in an Acer Saccharum Forest Soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil Enzymes in a Changing Environment: Current Knowledge and Future Directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Br. Mycol. Soc. 1970, 55, 158-IN18. [Google Scholar] [CrossRef]
- Li, Z.; Fu, J.; Zhou, R.; Wang, D. Effects of Phenolic Acids from Ginseng Rhizosphere on Soil Fungi Structure, Richness and Diversity in Consecutive Monoculturing of Ginseng. Saudi J. Biol. Sci. 2018, 25, 1788–1794. [Google Scholar] [CrossRef]
- Hui, N.; Jumpponen, A.; Francini, G.; Kotze, D.J.; Liu, X.; Romantschuk, M.; Strömmer, R.; Setälä, H. Soil Microbial Communities Are Shaped by Vegetation Type and Park Age in Cities under Cold Climate. Environ. Microbiol. 2017, 19, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Needleman, S.B.; Wunsch, C.D. A General Method Applicable to the Search for Similarities in the Amino Acid Sequence of Two Proteins. J. Mol. Biol. 1970, 48, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Olesen, S.W.; Duvallet, C.; Alm, E.J. dbOTU3: A New Implementation of Distribution-Based OTU Calling. PLoS ONE 2017, 12, e0176335. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Sánchez-Ramírez, S.; Kõljalg, U.; Bahram, M.; Döring, M.; Schigel, D.; May, T.; Ryberg, M.; Abarenkov, K. High-Level Classification of the Fungi and a Tool for Evolutionary Ecological Analyses. Fungal Divers. 2018, 90, 135–159. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling Function and Taxonomy in the Global Ocean Microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An Open Annotation Tool for Parsing Fungal Community Datasets by Ecological Guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A Web-Based Tool for the Analysis of Sets through Venn Diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.S.; Anderson, M.; Gorley, R.N.; Clarke, K.; Andersom, M. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; Primer-E Limited.: Auckland, New Zealand, 2008. [Google Scholar]
- Zhang, W.; Wang, X.; Li, Y.; Liu, Z.; Li, D.; Wen, X.; Feng, Y.; Zhang, X. Pinewood Nematode Alters the Endophytic and Rhizospheric Microbial Communities of Pinus Massoniana. Microb. Ecol. 2021, 81, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yu, D.; Zhou, W.; Zhou, L.; Zhu, W. Variations of Phyllosphere and Rhizosphere Microbial Communities of Pinus Koraiensis Infected by Bursaphelenchus Xylophilus. Microb. Ecol. 2022, 84, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Sahib, M.R.; Pervaiz, Z.H.; Williams, M.A.; Saleem, M.; DeBolt, S. Rhizobacterial Species Richness Improves Sorghum Growth and Soil Nutrient Synergism in a Nutrient-Poor Greenhouse Soil. Sci. Rep. 2020, 10, 15454. [Google Scholar] [CrossRef]
- Taha, M.; Foda, M.; Shahsavari, E.; Aburto-Medina, A.; Adetutu, E.; Ball, A. Commercial Feasibility of Lignocellulose Biodegradation: Possibilities and Challenges. Curr. Opin. Biotechnol. 2016, 38, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, Q.; Wei, Y.; Cui, H.; Zhang, X.; Wang, X.; Shan, S.; Wei, Z. Effect of Actinobacteria Agent Inoculation Methods on Cellulose Degradation during Composting Based on Redundancy Analysis. Bioresour. Technol. 2016, 219, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, L.; da Costa, M.S. The Family Gaiellaceae. In The Prokaryotes: Actinobacteria; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 357–360. ISBN 978-3-642-30138-4. [Google Scholar]
- Shi, S.; Richardson, A.E.; O’Callaghan, M.; DeAngelis, K.M.; Jones, E.E.; Stewart, A.; Firestone, M.K.; Condron, L.M. Effects of Selected Root Exudate Components on Soil Bacterial Communities. FEMS Microbiol. Ecol. 2011, 77, 600–610. [Google Scholar] [CrossRef]
- Badri, D.V.; Chaparro, J.M.; Zhang, R.; Shen, Q.; Vivanco, J.M. Application of Natural Blends of Phytochemicals Derived from the Root Exudates of Arabidopsis to the Soil Reveal That Phenolic-Related Compounds Predominantly Modulate the Soil Microbiome. J. Biol. Chem. 2013, 288, 4502–4512. [Google Scholar] [CrossRef]
- Zhang, C.; Tayyab, M.; Abubakar, A.Y.; Yang, Z.; Pang, Z.; Islam, W.; Lin, Z.; Li, S.; Luo, J.; Fan, X.; et al. Bacteria with Different Assemblages in the Soil Profile Drive the Diverse Nutrient Cycles in the Sugarcane Straw Retention Ecosystem. Diversity 2019, 11, 194. [Google Scholar] [CrossRef]
- Caravaca, F.; Torres, P.; Díaz, G.; Roldán, A. Elevated CO2 Affects the Rhizosphere Microbial Community and the Growth of Two Invader Plant Species Differently in Semiarid Mediterranean Soils. Land Degrad. Dev. 2022, 33, 117–132. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, G.; Wu, Z.; Wen, X.; Zhong, H.; Zhong, Z.; Bian, F.; Gai, X. Agroforestry Alters the Rhizosphere Soil Bacterial and Fungal Communities of Moso Bamboo Plantations in Subtropical China. Appl. Soil Ecol. 2019, 143, 192–200. [Google Scholar] [CrossRef]
- Shi, Y.; Qiu, L.; Guo, L.; Man, J.; Shang, B.; Pu, R.; Ou, X.; Dai, C.; Liu, P.; Yang, Y.; et al. K Fertilizers Reduce the Accumulation of Cd in Panax Notoginseng (Burk.) F.H. by Improving the Quality of the Microbial Community. Front. Plant Sci. 2020, 11, 888. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, X.; Tang, Y.; Yan, C.; Hu, X.; Huang, X.; Lin, M.; Liu, Z. Integrated Analysis Reveals an Association between the Rhizosphere Microbiome and Root Rot of Arecanut Palm. Pedosphere 2021, 31, 725–735. [Google Scholar] [CrossRef]
- Solís-García, I.A.; Ceballos-Luna, O.; Cortazar-Murillo, E.M.; Desgarennes, D.; Garay-Serrano, E.; Patiño-Conde, V.; Guevara-Avendaño, E.; Méndez-Bravo, A.; Reverchon, F. Phytophthora Root Rot Modifies the Composition of the Avocado Rhizosphere Microbiome and Increases the Abundance of Opportunistic Fungal Pathogens. Front. Microbiol. 2021, 11, 3484. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, M.; Tamaki, H.; Manome, A.; Koyama, O.; Kamagata, Y. Isolation and Characterization of Antagonistic Fungi against Potato Scab Pathogens from Potato Field Soils. FEMS Microbiol. Lett. 2010, 305, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.G.; El-Sayed, E.-S.R.; Younis, N.A.; Hamdy, A.E.H.A.; Easa, S.M. Harnessing Endophytic Fungi for Biosynthesis of Selenium Nanoparticles and Exploring Their Bioactivities. AMB Express 2022, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, R.; Peng, W.; Yang, Y.; Ma, X.; Zhang, W.; Ji, A.; Liu, L.; Liu, P.; Yan, L.; et al. Tea Plants With Gray Blight Have Altered Root Exudates That Recruit a Beneficial Rhizosphere Microbiome to Prime Immunity Against Aboveground Pathogen Infection. Front. Microbiol. 2021, 12, 774438. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Venice, F. Mucoromycota: Going to the Roots of Plant-Interacting Fungi. Fungal Biol. Rev. 2020, 34, 100–113. [Google Scholar] [CrossRef]
- Reva, V.; Fonseca, L.; Lousada, J.L.; Abrantes, I.; Viegas, D.X. Impact of the Pinewood Nematode, Bursaphelenchus Xylophilus, on Gross Calorific Value and Chemical Composition of Pinus Pinaster Woody Biomass. Eur. J. For. Res. 2012, 131, 1025–1033. [Google Scholar] [CrossRef]
- Kowal, J.; Arrigoni, E.; Jarvis, S.; Zappala, S.; Forbes, E.; Bidartondo, M.I.; Suz, L.M. Atmospheric Pollution, Soil Nutrients and Climate Effects on Mucoromycota Arbuscular Mycorrhizal Fungi. Environ. Microbiol. 2022, 24, 3390–3404. [Google Scholar] [CrossRef]
- Yu, L.; Nicolaisen, M.; Larsen, J.; Ravnskov, S. Succession of Root-Associated Fungi in Pisum Sativum during a Plant Growth Cycle as Examined by 454 Pyrosequencing. Plant Soil 2012, 358, 225–233. [Google Scholar] [CrossRef]
- Dhyani, A.; Jain, R.; Pandey, A. Contribution of Root-Associated Microbial Communities on Soil Quality of Oak and Pine Forests in the Himalayan Ecosystem. Trop. Ecol. 2019, 60, 271–280. [Google Scholar] [CrossRef]
- Han, G.; Mannaa, M.; Kim, N.; Jeon, H.; Jung, H.; Lee, H.; Kim, J.; Park, J.; Park, A.; Kim, J.; et al. Response of Pine Rhizosphere Microbiota to Foliar Treatment with Resistance-Inducing Bacteria against Pine Wilt Disease. Microorganisms 2021, 9, 688. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Wang, H.; Zhang, Y.; Li, Z.; Wang, C.; Dai, D.; Tang, M. Inoculation With Ectomycorrhizal Fungi and Dark Septate Endophytes Contributes to the Resistance of Pinus Spp. to Pine Wilt Disease. Front. Microbiol. 2021, 12, 687304. [Google Scholar] [CrossRef] [PubMed]
- Redecker, D.; Schüßler, A.; Stockinger, H.; Stürmer, S.L.; Morton, J.B.; Walker, C. An Evidence-Based Consensus for the Classification of Arbuscular Mycorrhizal Fungi (Glomeromycota). Mycorrhiza 2013, 23, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Rodríguez, V.M.; Abilleira, R.; Velasco, P. Trichoderma Hamatum Can Act as an Inter-Plant Communicator of Foliar Pathogen Infections by Colonizing the Roots of Nearby Plants: A New Inter-Plant “Wired Communication”. Plant Sci. 2023, 330, 111664. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Wang, C.; Wang, H.; Chen, H.; Tang, M. Pine Wilt Disease Alters Soil Properties and Root-Associated Fungal Communities in Pinus Tabulaeformis Forest. Plant Soil 2016, 404, 237–249. [Google Scholar] [CrossRef]
- el Zahar Haichar, F.; Heulin, T.; Guyonnet, J.P.; Achouak, W. Stable Isotope Probing of Carbon Flow in the Plant Holobiont. Curr. Opin. Biotechnol. 2016, 41, 9–13. [Google Scholar] [CrossRef]
- Sugiyama, A. Flavonoids and Saponins in Plant Rhizospheres: Roles, Dynamics, and the Potential for Agriculture. Biosci. Biotechnol. Biochem. 2021, 85, 1919–1931. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.; George, T.S.; Limpens, E.; Feng, G. Arbuscular Mycorrhizal Fungi Conducting the Hyphosphere Bacterial Orchestra. Trends Plant Sci. 2022, 27, 402–411. [Google Scholar] [CrossRef]
- Dong, H.; Ge, J.; Sun, K.; Wang, B.; Xue, J.; Wakelin, S.A.; Wu, J.; Sheng, W.; Liang, C.; Xu, Q.; et al. Change in Root-Associated Fungal Communities Affects Soil Enzymatic Activities during Pinus Massoniana Forest Development in Subtropical China. For. Ecol. Manag. 2021, 482, 118817. [Google Scholar] [CrossRef]
- Lang, A.K.; Jevon, F.V.; Vietorisz, C.R.; Ayres, M.P.; Hatala Matthes, J. Fine Roots and Mycorrhizal Fungi Accelerate Leaf Litter Decomposition in a Northern Hardwood Forest Regardless of Dominant Tree Mycorrhizal Associations. New Phytol. 2021, 230, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, C.; Marín, M.; Baixeras, J.; Latorre, A.; Porcar, M. Selecting Microbial Strains from Pine Tree Resin: Biotechnological Applications from a Terpene World. PLoS ONE 2014, 9, e100740. [Google Scholar] [CrossRef] [PubMed]
- Pommerening-Röser, A.; Koops, H.-P. Environmental pH as an Important Factor for the Distribution of Urease Positive Ammonia-Oxidizing Bacteria. Microbiol. Res. 2005, 160, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Meng, W.; Qu, Z.; Zhang, Y.; Liu, B.; Ma, Y.; Chang, L.; Sun, H. Changes in Bacterial Communities and Functions Associated with Litter Degradation during Forest Succession Caused by Forest Disease. Phytobiomes J. 2023. [Google Scholar] [CrossRef]
- Proença, D.N.; Francisco, R.; Kublik, S.; Schöler, A.; Vestergaard, G.; Schloter, M.; Morais, P.V. The Microbiome of Endophytic, Wood Colonizing Bacteria from Pine Trees as Affected by Pine Wilt Disease. Sci. Rep. 2017, 7, 4205. [Google Scholar] [CrossRef]

| RhizospHere, Soil | C Cycle | N Cycle | P Cycle | S Cycle | |||
|---|---|---|---|---|---|---|---|
| XYL | GLR | CEL | GLS | NAG | PHO | SUL | |
| Healthy tree | 98.1 ± 17.77 | 13.82 ± 5.35 | 77.32 ± 17.07 * | 278.07 ± 20.43 * | 312.35 ± 37.76 | 2104.92 ± 343.06 | 7.52 ± 0.19 |
| Diseased tree | 71.02 ± 4.83 | 5.53 ± 2.61 | 33.73 ± 12.25 | 139 ± 40.4 | 313.28 ± 57.87 | 1822.14 ± 261.87 | 8.27 ± 0.62 |
| Diversity Indices | ||||
|---|---|---|---|---|
| Samples | Host Status | Sobs | Shannon | Shannoneven |
| Rhizosphere bacteria | Healthy | 2433.67 ± 220.6 | 5.83 ± 0.22 | 0.75 ± 0.02 |
| Diseased | 2367 ± 465.3 | 5.63 ± 0.76 | 0.72 ± 0.08 | |
| Rhizosphere fungi | Healthy | 553 ± 23.42 * | 3.02 ± 0.46 | 0.46 ± 0.09 |
| Diseased | 509 ± 40.75 | 2.54 ± 0.4 | 0.4 ± 0.06 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Z.; Gao, Z.; Liao, Y.; Liu, Y.; Dong, L.; Sun, H. Effects of Pine Wilt Disease on Rhizosphere Microbiota and Fine Root Fungi: Insights into Enzyme Activity, Ectomycorrhizal Infection and Microbial Composition. Forests 2023, 14, 1884. https://doi.org/10.3390/f14091884
Jiao Z, Gao Z, Liao Y, Liu Y, Dong L, Sun H. Effects of Pine Wilt Disease on Rhizosphere Microbiota and Fine Root Fungi: Insights into Enzyme Activity, Ectomycorrhizal Infection and Microbial Composition. Forests. 2023; 14(9):1884. https://doi.org/10.3390/f14091884
Chicago/Turabian StyleJiao, Ziwen, Ziwen Gao, Yangchunzi Liao, Yi Liu, Lina Dong, and Hui Sun. 2023. "Effects of Pine Wilt Disease on Rhizosphere Microbiota and Fine Root Fungi: Insights into Enzyme Activity, Ectomycorrhizal Infection and Microbial Composition" Forests 14, no. 9: 1884. https://doi.org/10.3390/f14091884
APA StyleJiao, Z., Gao, Z., Liao, Y., Liu, Y., Dong, L., & Sun, H. (2023). Effects of Pine Wilt Disease on Rhizosphere Microbiota and Fine Root Fungi: Insights into Enzyme Activity, Ectomycorrhizal Infection and Microbial Composition. Forests, 14(9), 1884. https://doi.org/10.3390/f14091884






