Processing and Properties of Wood-Plastic Composite Containing Alkali-Treated Birch Wood Shavings and Bioadditive Obtained by Biorefinery of Birch Bark
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
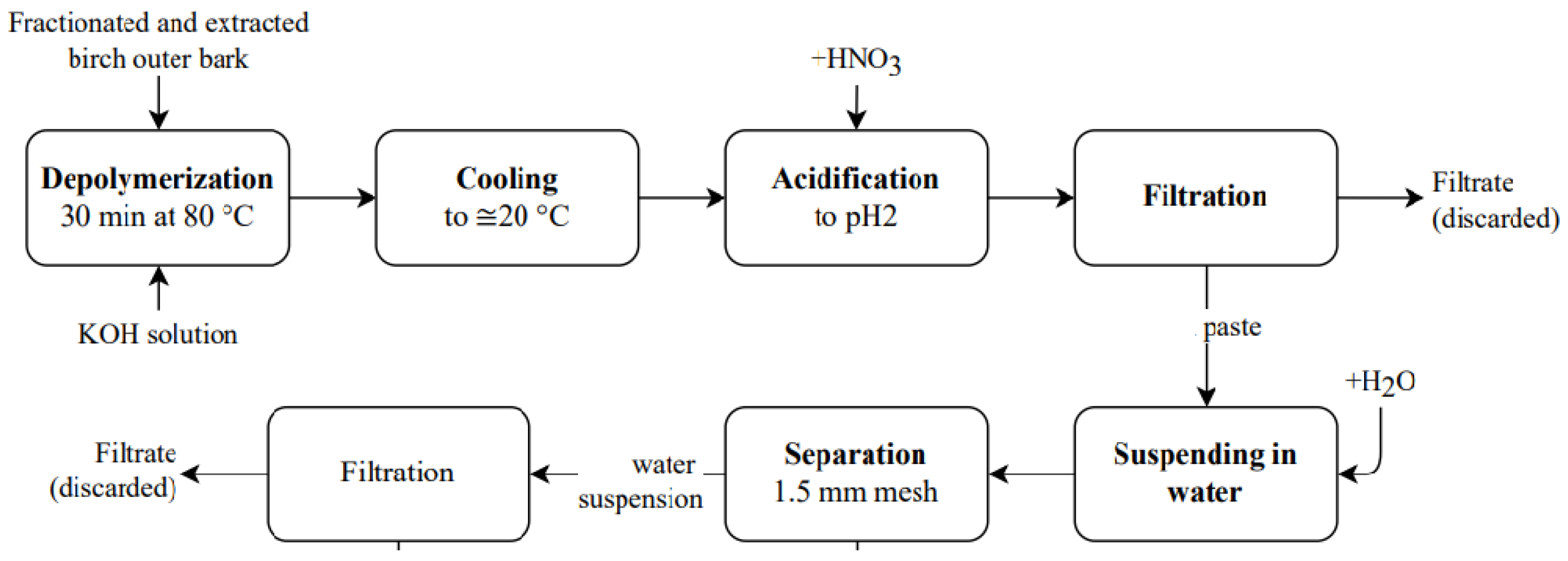
2.1.1. Suberinic Acids and Their Extraction
2.1.2. Alkali Treatment of Milled Birch Wood Shavings
2.1.3. Incorporation of Suberinic Acids in the Composite
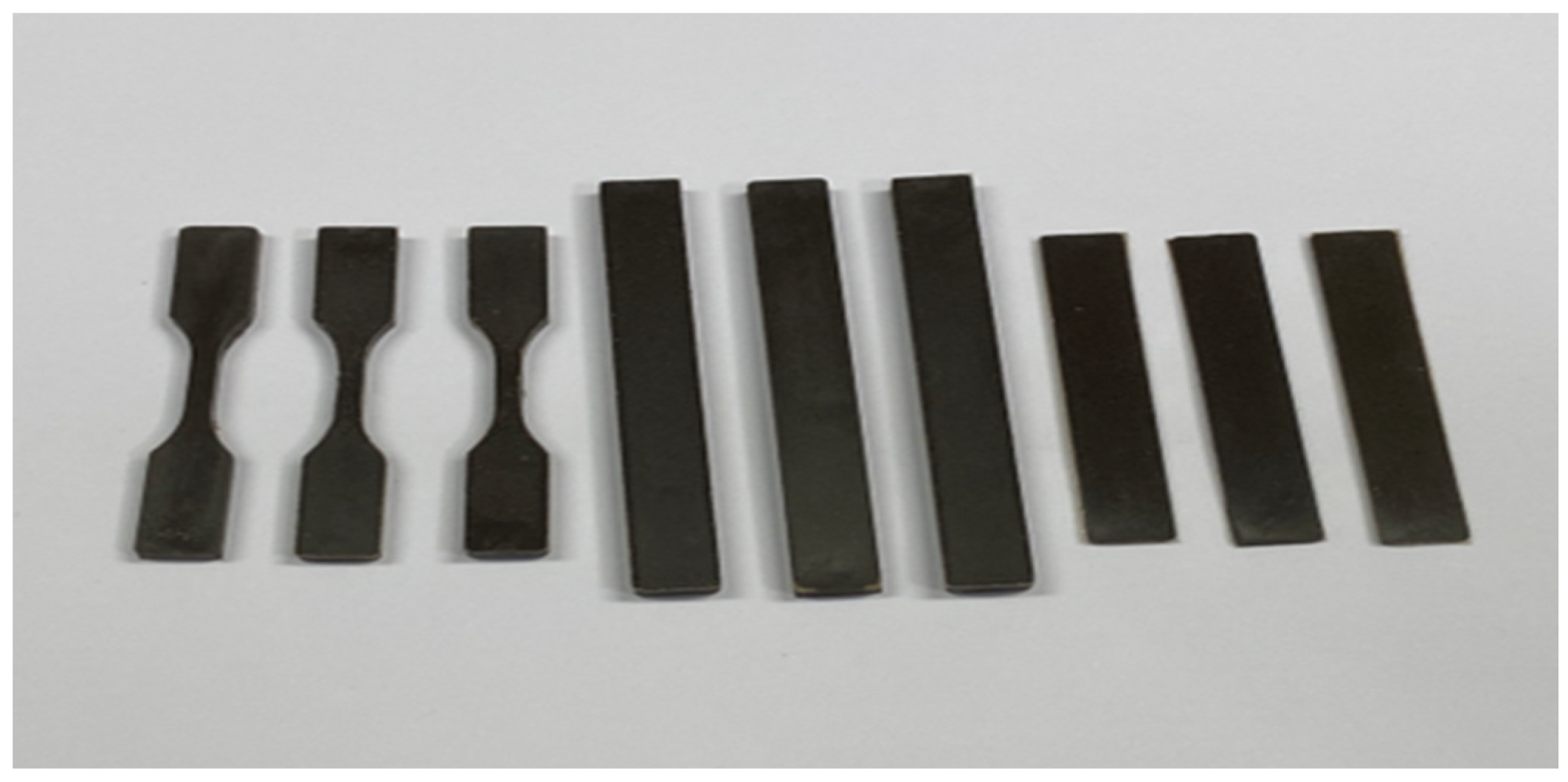
2.1.4. Preparation of Composite Samples
2.2. Methods
2.2.1. Elemental Analysis
2.2.2. Potentiometric and Conductometric Titration
2.2.3. Zeta Potential
2.2.4. Thermogravimetric Analysis (TGA)
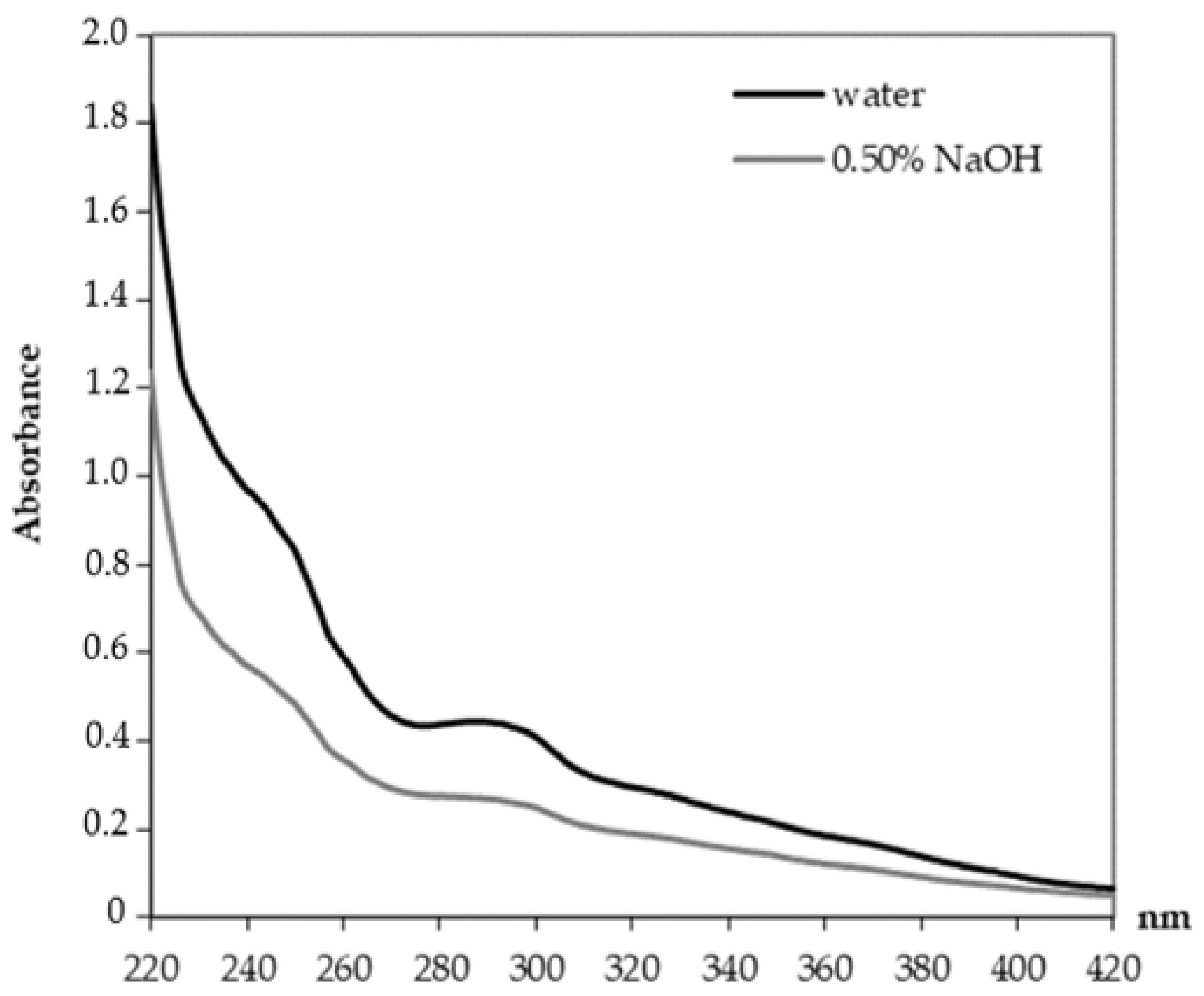
2.2.5. UV Spectroscopy
2.2.6. Fourier Transform Infrared (FT-IR) Spectroscopy
2.2.7. Water Uptake and Swelling
2.2.8. Mechanical Tests
2.2.9. Torque and Injection Molding Pressure
2.2.10. Contact Angles
2.2.11. Milling
3. Results and Discussion
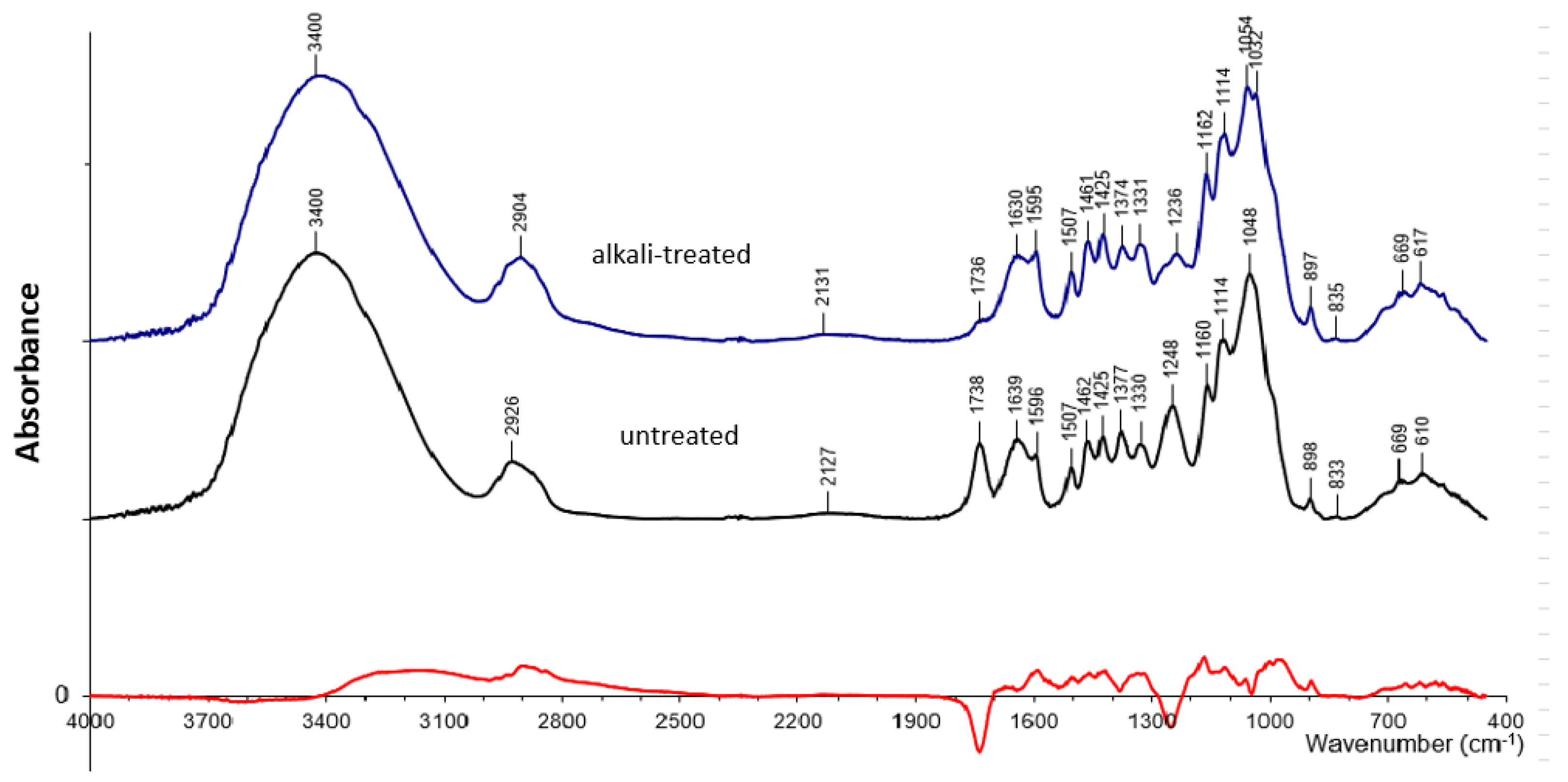
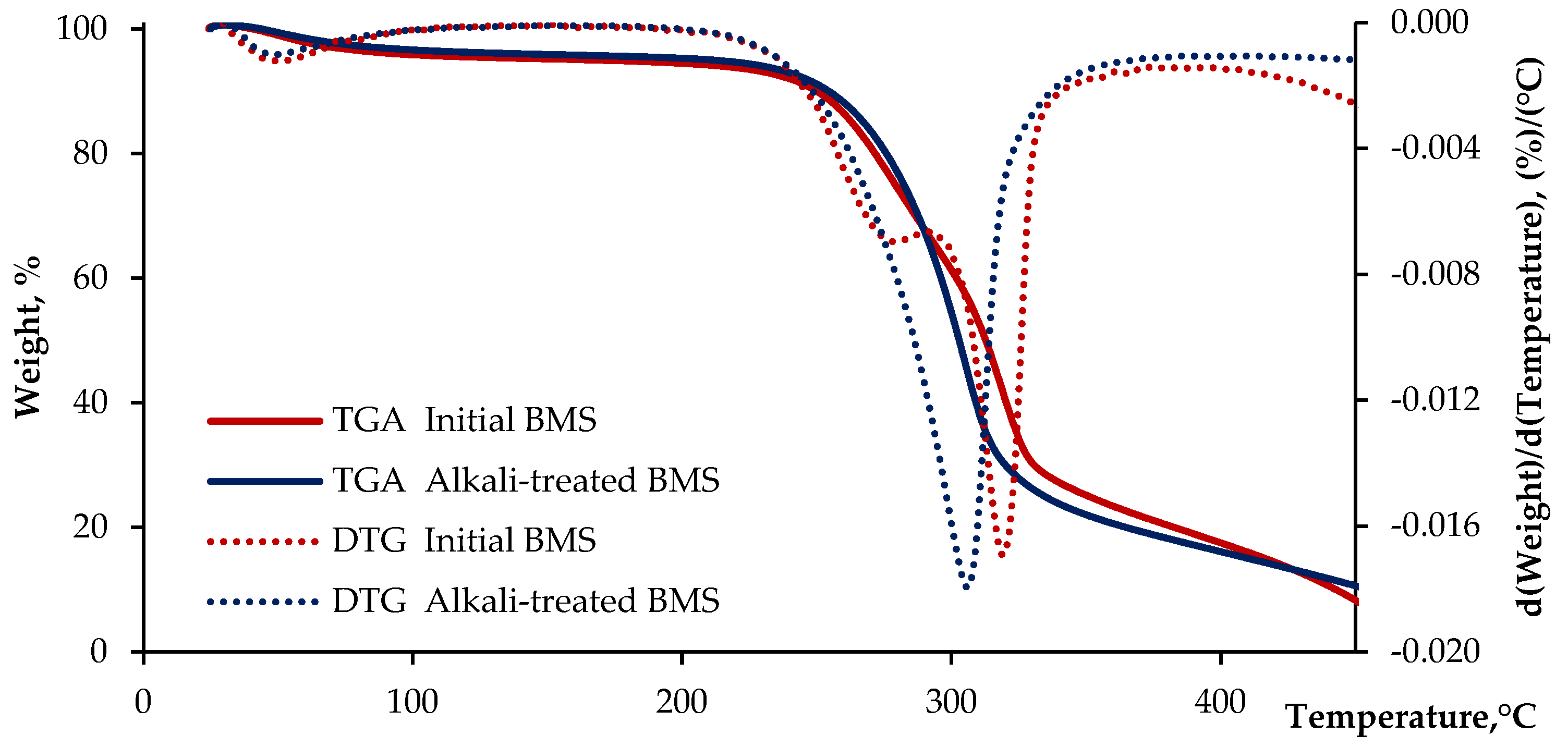
3.1. Characterization of Alkali-Treated Lignocellulosic Filler
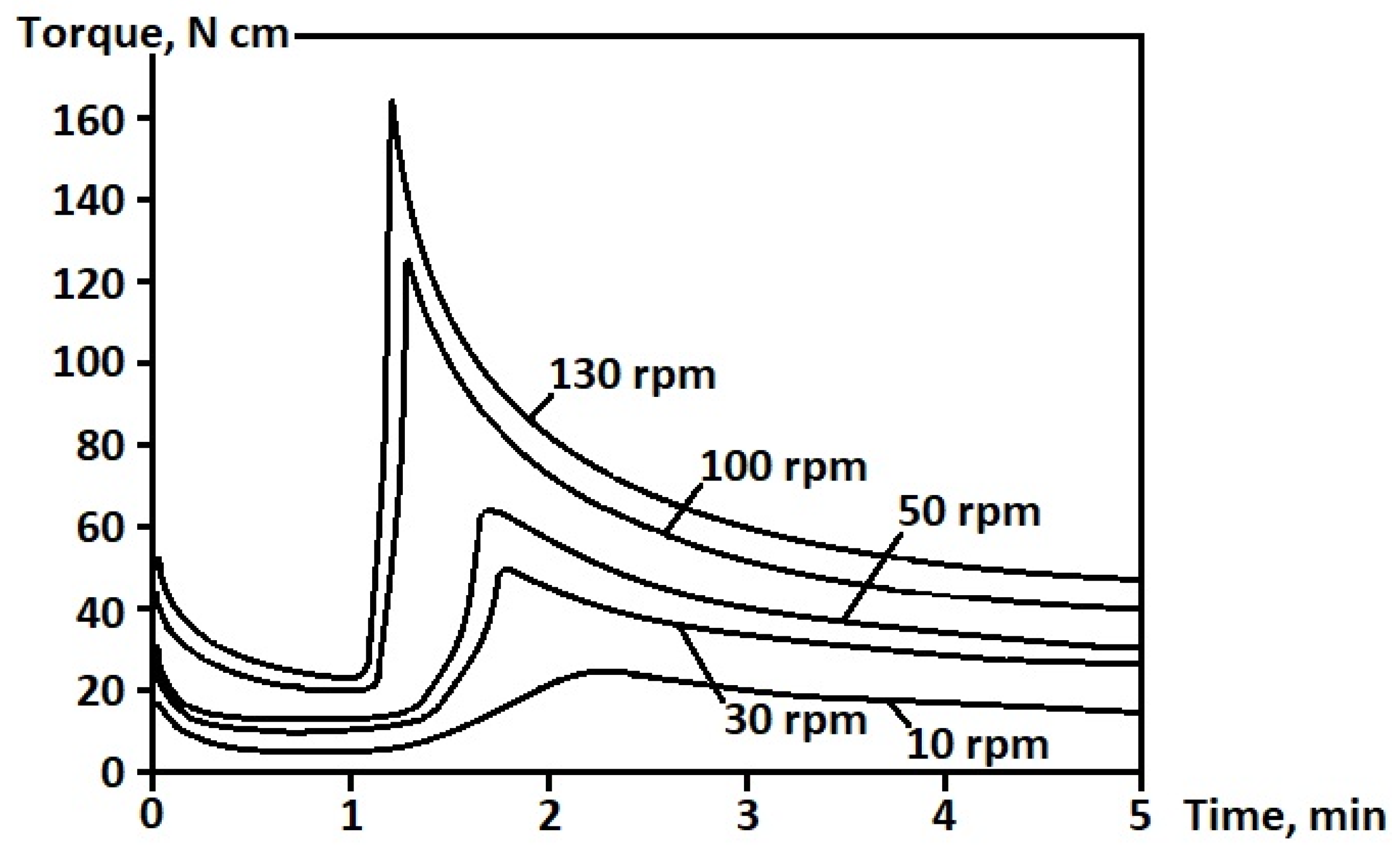
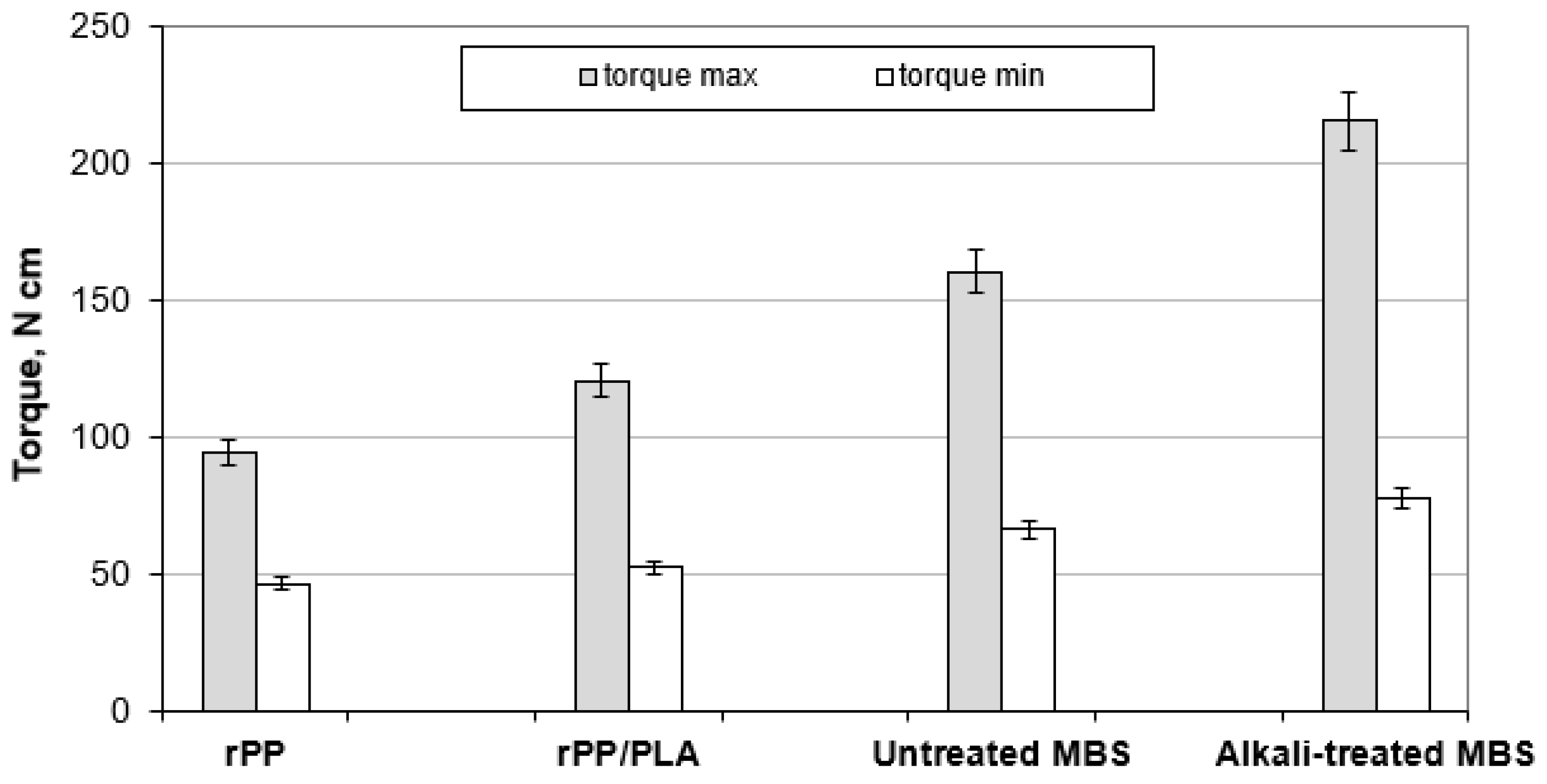
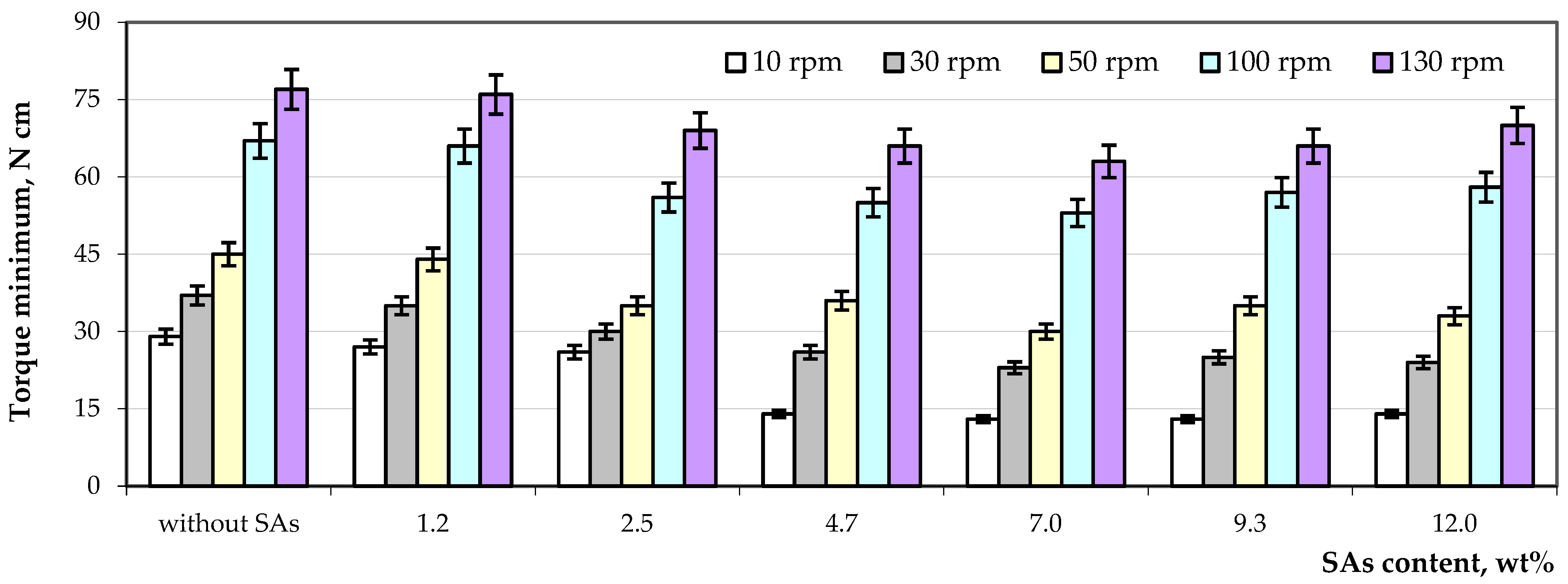
3.2. Processing of Composite Samples
3.3. WPC Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Materials 2030 Roadmap. In Proceedings of the International Conference on Industrial Technologies, Grenoble, France, 27–29 June 2022.
- Cirillo, G.; Kozlowski, M.A.; Spizzirri, U.G. (Eds.) Composites Materials for Food Packaging; Scrivener Publishing: Beverly, MA, USA, 2018. [Google Scholar]
- Zhang, M.; Biesold, G.M.; Choi, W.; Yu, J.; Deng, Y.; Silvestre, C.; Lin, Z. Recent advances in polymers and polymer composites for food packaging. Mater. Today 2022, 53, 134–161. [Google Scholar] [CrossRef]
- Spear, M.J.; Eder, A.; Carus, M. Wood polymer composites. In Wood Composites; Ansell, M.P., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2015; pp. 195–249. [Google Scholar]
- Niska, K.O.; Sain, M. (Eds.) Wood-Polymer Composites; Woodhead Publishing Ltd.: Cambridge, UK, 2008. [Google Scholar]
- Turku, I.; Keskisaari, A.; Køarki, T.; Puurtinen, A.; Marttila, P. Characterization of wood plastic composites manufactured from recycled plastic blends. Compos. Struct. 2017, 161, 469–476. [Google Scholar] [CrossRef]
- Basalp, D.; Tihminlioglu, F.; Sofuoglu, S.C.; Inal, F.; Sofuoglu, A. Utilization of municipal plastic and wood waste in in-dustrial manufacturing of wood plastic composites. Waste Biomass Valorization 2020, 11, 5419–5430. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Harussani, M.M.; Hakimi, M.Y.A.Y.; Haziq, M.Z.M.; Atikah, M.S.N.; Asyraf, M.R.M.; Ishak, M.R.; Razman, M.R.; Nurazzi, N.M.; et al. Polylactic Acid (PLA) Biocomposite: Processing, Additive Manufacturing and Advanced Applications. Polymers 2021, 13, 1326. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; López, J.; Kenny, J.M. Bionanocomposite films based on plasticized PLA–PHB/cellulose nanocrystal blends. Carbohydr. Polym. 2015, 121, 265–275. [Google Scholar] [CrossRef]
- Spiridon, I.; Tanase, C.E. Design, characterization and preliminary biological evaluation of new lignin-PLA biocomposites. Int. J. Biol. Macromol. 2018, 114, 855–863. [Google Scholar] [CrossRef]
- Lee, T.W.; Jeong, Y.G. Enhanced electrical conductivity, mechanical modulus, and thermal stability of immiscible polylactide/polypropylene blends by the selective localization of multi-walled carbon nanotubes. Compos. Sci. Technol. 2014, 103, 78–84. [Google Scholar] [CrossRef]
- Ploypetchara, N.; Suppakul, P.; Atong, D.; Pechyen, C. Blend of polypropylene/poly(lactic acid) for medical packaging application: Physicochemical, thermal, mechanical, and barrier properties. Energy Procedia 2014, 56, 201–210. [Google Scholar] [CrossRef]
- Shulga, G.; Neiberte, B.; Verovkins, A.; Jaunslavietis, J.; Betkers, T. Recycled polypropylene-poly(lactic acid) blend as a polymer matrix for food packaging containers. In Proceedings of the 13th International Conference Biosystems Engineering, Tartu, Estonia, 10–12 May 2023; p. 73. [Google Scholar]
- Matuana, L.M.; Stark, N.M. The use of wood fibers as reinforcements in composites. In Biofiber Reinforcements in Composite Materials; Faruk, O., Sain, M., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2015; pp. 648–688. [Google Scholar]
- Kaewkuk, S.; Sutapun, W.; Jarukumjorn, K. Effects of interfacial modification and fiber content on physical properties of sisal fiber/polypropylene composites. Compos. Part B Eng. 2013, 45, 544–549. [Google Scholar] [CrossRef]
- Li, X.; Tabil, L.G.; Panigrahi, S. Chemical treatments of natural fibers for use in natural fiber-reinforced composites: A review. J. Polym. Environ. 2007, 15, 25–33. [Google Scholar] [CrossRef]
- Taib, R.M.; Ishak, Z.A.M.; Rozman, H.D.; Glasser, W.G. Effect of moisture absorption on the tensile properties of steam-exploded Acacia mangium fiber-polypropylene composites. J. Thermoplast. Compos. Mater. 2006, 19, 475–489. [Google Scholar] [CrossRef]
- Shulga, G.; Neiberte, B.; Verovkins, A.; Vitolina, S.; Jaunslavietis, J.; Livcha, S.; Betkers, T. Aminated wood sanding dust as a filler for recycled polypropylene-based composite. Cellul. Chem. Technol. 2019, 53, 945–953. [Google Scholar] [CrossRef]
- Vitolina, S.; Shulga, G.; Neiberte, B.; Jaunslvietis, J.; Verovkins, A.; Betkers, T. Characteristics of waste wood biomass and its effect on the properties of wood sandling dust/recycled PP composite. Polymers 2022, 14, 468. [Google Scholar] [CrossRef]
- Shulga, G.; Neiberte, B.; Jaunslavietis, J.; Verovkins, A.; Vitolina, S.; Livcha, S. Lignin-containing adhesion enhancer for wood-plastic composites. BioResources 2021, 16, 2804–2823. [Google Scholar] [CrossRef]
- Lin, J.; Yang, Z.; Hu, X.; Hong, G.; Zhang, S.; Song, W. The effect of alkali treatment on properties of dopamine modification of bamboo fiber/polylactic acid composites. Polymers 2018, 10, 403. [Google Scholar] [CrossRef]
- Ferede, E. Evaluation of Mechanical and Water Absorption Properties of Alkaline-Treated Sawdust-Reinforced Polypropylene Composite. J. Eng. 2020, 2020, 3706176. [Google Scholar] [CrossRef]
- Xu, K.; Du, G.; Wang, S. Wood Plastic Composites: Their Properties and Applications. In Engineered Wood Products for Construction; Gong, M., Ed.; IntechOpen: London, UK, 2022; pp. 197–221. [Google Scholar]
- Dai, L.; Wang, X.; Zhang, J.; Wang, F.; Ou, R.; Song, Y. Effects of lubricants on the rheological and mechanical properties of wood flour/polypropylene composites. J. Appl. Polym. Sci. 2019, 136, 47667. [Google Scholar] [CrossRef]
- Li, H.; Law, S.; Sain, M. Process rheology and mechanical property correlation ship of wood flour-polypropylene composites. J. Reinf. Plast. Compos. 2004, 23, 1153–1158. [Google Scholar] [CrossRef]
- Bettini, S.H.P.; Josefovich, M.P.P.d.M.; Muñoz, P.A.R.; Lotti, C.; Mattoso, L.H.C. Effect of lubricant on mechanical and rheological properties of compatibilized PP/sawdust composites. Carbohydr. Polym. 2013, 94, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Q.; Wolcott, M. Rheology of wood plastics melt, part 2: Effects of lubricating systems in HDPE/maple composites. J. Polym. Eng. Sci. 2006, 46, 464–473. [Google Scholar] [CrossRef]
- Rizhikovs, J.; Zandersons, J.; Dobele, G.; Paze, A. Isolation of triterpene-rich extracts from outer birch bark by hot water and alkaline pre-treatment for the appropriate choice of solvents. Ind. Crop. Prod. 2015, 76, 209–214. [Google Scholar] [CrossRef]
- Godina, D.; Makars, R.; Abolins, A.; Kirpluks, M.; Rizikovs, J. Suberinicacid isolation from birch outer bark and their characterization. Mater. Sci. Forum 2022, 1071, 166–173. [Google Scholar] [CrossRef]
- Rizikovs, J.; Godina, D.; Makars, R.; Meile, K.; Kirpluks, M. Suberinic acids as a potential feedstock for polyol synthesis: Separation and characterization. Polymers 2021, 13, 4380. [Google Scholar] [CrossRef]
- Paze, A.; Rizhikovs, J. Study of an appropriate suberinic acids binder for manufacturing of plywood. Key Eng. Mater. 2019, 800, 251–255. [Google Scholar] [CrossRef]
- Paze, A.; Rizhikovs, J.; Godina, D.; Makars, R.; Berzins, R. Development of plywood binder by partial replacement of phenol-formaldehyde resins with birch outer bark components. Key Eng. Mater. 2021, 903, 229–234. [Google Scholar] [CrossRef]
- Jeżo, A.; Wronka, A.; Dębiński, A.; Rizhikovs, J.; Kowaluk, G. Influence of Upcycled Post-Treatment Bark Biomass Addition to the Binder on Produced Plywood Properties. Forests 2023, 14, 110. [Google Scholar] [CrossRef]
- Tupciauskas, R.; Rizhikovs, J.; Grinins, J.; Puke, M.; Plavniece, A. Investigation of suberinic acids bonded particleboard. Eur. Polym. J. 2019, 113, 176–182. [Google Scholar] [CrossRef]
- Rizhikovs, J.; Brazdausks, P.; Paze, A.; Godina, D.; Makars, R. Characterization of suberinic acids from birch outer bark as bio-based adhesive in wood composites. Int. J. Adhes. Adhes. 2022, 112, 102989. [Google Scholar] [CrossRef]
- Makars, R.; Rizikovs, J.; Godina, D.; Paze, A.; Merijs-Meri, R. Utilization of Suberinic Acids Containing Residue as an Adhesive for Particle Boards. Polymers 2022, 14, 2304. [Google Scholar] [CrossRef] [PubMed]
- TAPPI test method T222 om-02. In Acid-Insoluble Lignin in Wood and Pulp; TAPPI Press: Atlanta, GA, USA, 2002.
- TAPPI test method T203 cm-99. In Alpha-, Beta- and Gamma-Cellulose in Pulp; TAPPI Press: Atlanta, GA, USA, 1999.
- TAPPI test method T264 om-97. In Preparation of Wood for Chemical Analysis; TAPPI Press: Atlanta, GA, USA, 1997.
- Godina, D.; Paze, A.; Rizhikovs, J.; Virsis, I.; Nakurte, I. Stability studies of bioactive compounds from birch outer bark ethanolic extracts. Key Eng. Mater. 2018, 762, 152–157. [Google Scholar] [CrossRef]
- Makars, R.; Rizikovs, J.; Paze, A. Study of Catalysts for Suberinic Acid-Based Adhesive Polymerization. Mater. Sci. Forum 2022, 1071, 182–188. [Google Scholar] [CrossRef]
- ASTM D 570-98; Standard Test Method for Water Absorption of Plastics. American Society for Testing and Materials Information Handling Services: West Conshohocken, PA, USA, 2018.
- ASTM D638; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2007.
- ISO 178:2010; Plastics-Determination of Flexural Properties. International Organization for Standardization: Geneva, Switzerland, 2010.
- Buoso, M.C.; de Poli, M.; Matthaes, P.; Silvestrin, L.; Zafiropoulos, D. Nondestructive wood discrimination: FTIR—Fourier Transform Infrared Spectroscopy in the characterization of different wood species used for artistic objects. Int. J. Mod. Phys. Conf. Ser. 2016, 44, 1660212. [Google Scholar] [CrossRef]
- Rantuch, P.; Chrebet, T. Thermal degradation of cellulose insulation. Cel. Chem. Tech. 2014, 48, 461–467. [Google Scholar]
- Santi, C.R.; Hage, E.; Vlachopoulos, J.; Correa, C.A. Rheology and processing of HDPE/wood flour composites. Int. Polym. Process. 2009, 24, 346–353. [Google Scholar] [CrossRef]



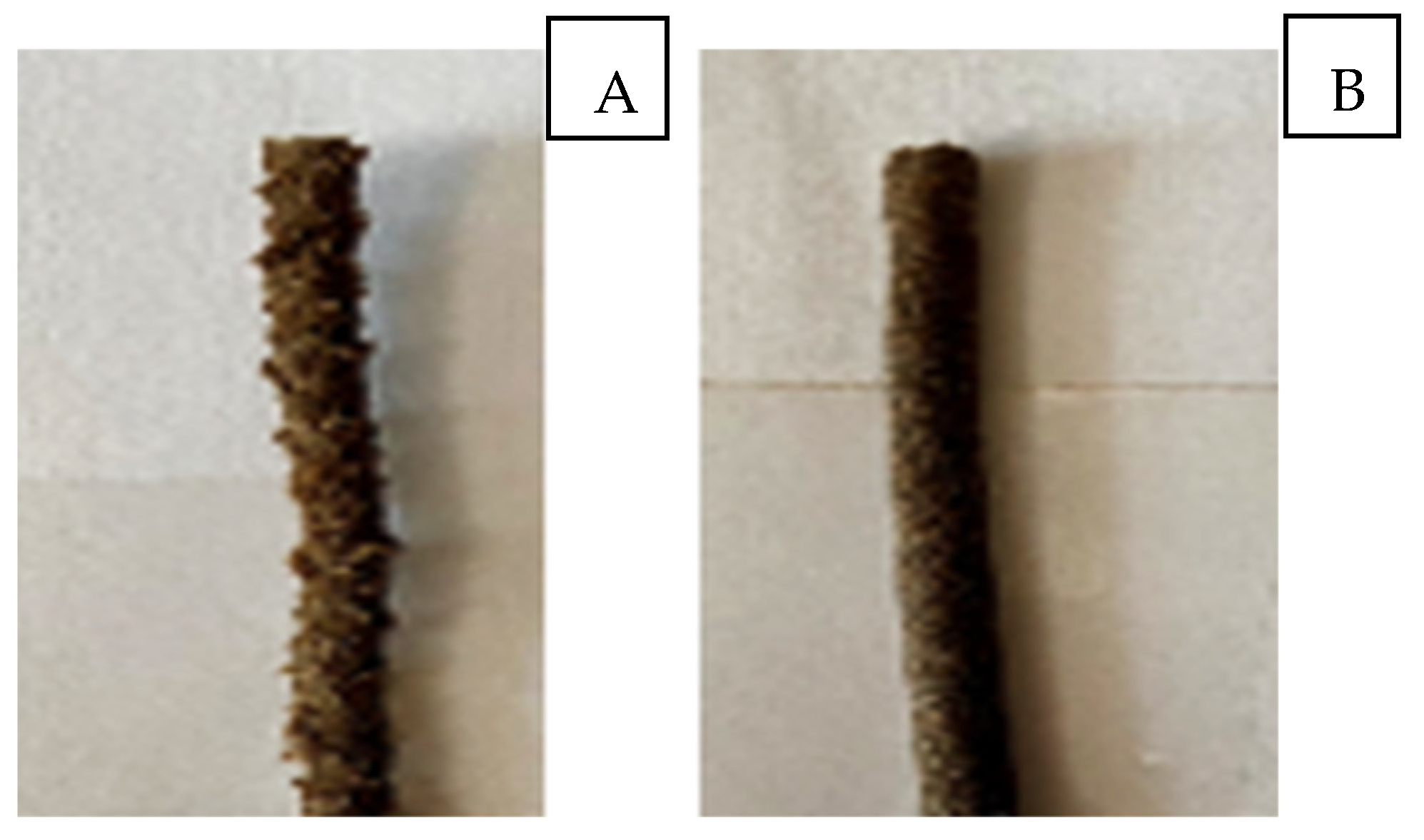
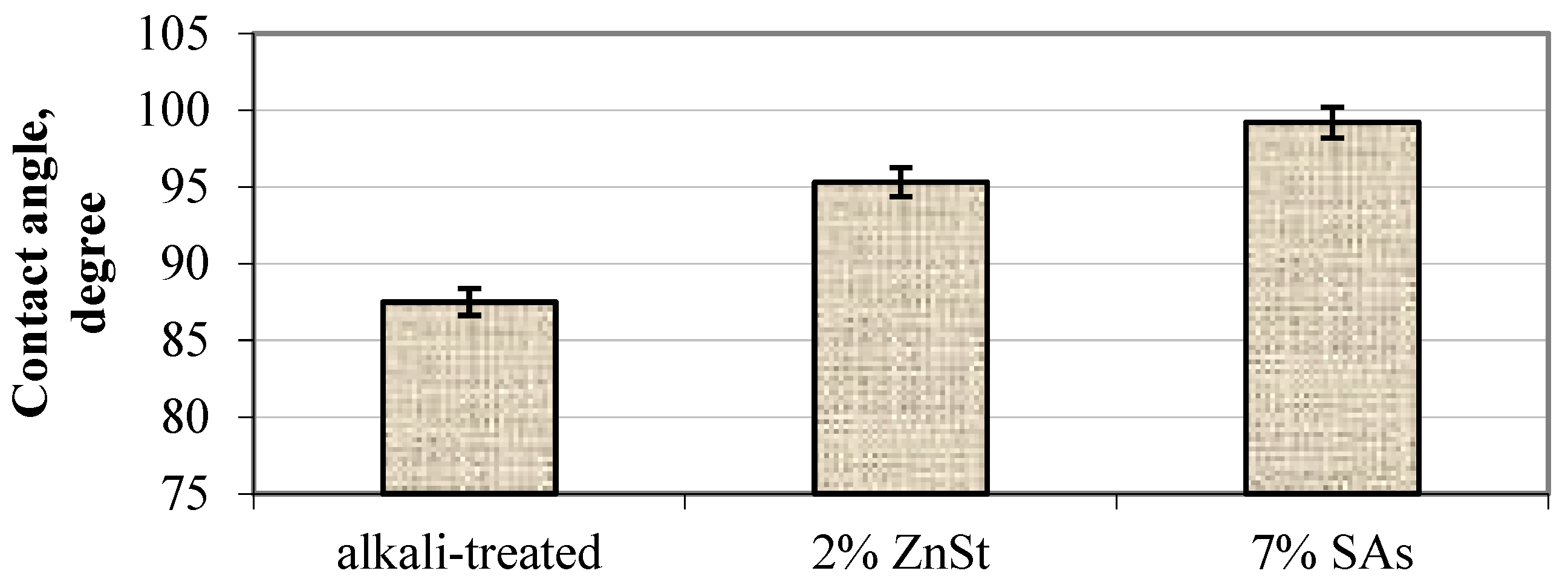
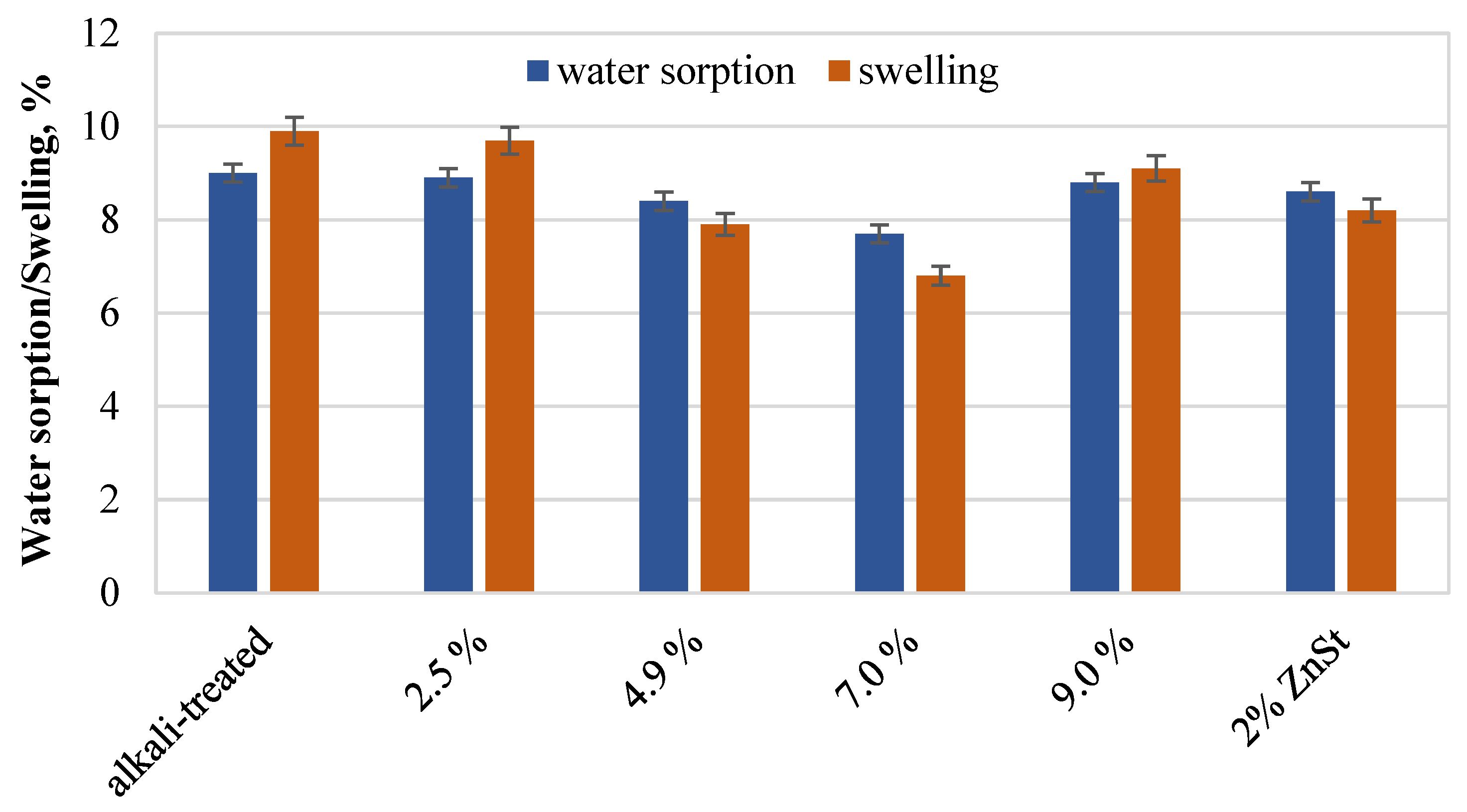
| Sample | C,
% | H,
% | O, % | N, % | O/C | Lignin,
% | Cellulose, % | Hemicelluloses, % |
|---|---|---|---|---|---|---|---|---|
| Initial | 45.9 | 6.2 | 47.5 | 0.4 | 1.03 | 21.5 | 47.9 | 26.1 |
| Alkali-treated | 44.1 | 6.3 | 49.3 | 0.3 | 1.12 | 22.3 | 58.3 | 18.2 |
| rPP/PLA, wt% | MBS, wt% | SAs, wt% |
|---|---|---|
| 50.0 | 50.0 | 0.0 |
| 50.0 | 48.0 | 1.2 |
| 50.0 | 47.5 | 2.5 |
| 50.0 | 45.1 | 4.9 |
| 50.0 | 43.0 | 7.0 |
| 50.0 | 40.7 | 9.3 |
| 50.0 | 38.0 | 12.0 |
| Lubricant | Minimal Torque, N cm | Injection Pressure, MPa |
|---|---|---|
| Without | 72 ± 3 | 690 ± 20 |
| 2 wt% ZnSt | 66 ± 4 | 550 ± 30 |
| 7 wt% SAs | 63 ± 4 | 520 ± 25 |
| Lubricant Content, wt% | Tensile Strength, MPa | Tensile Modulus, MPa | Deformation, % | Bending Strength, MPa | Bending Modulus, MPa | Deformation, mm |
|---|---|---|---|---|---|---|
| 0 | 17.5 (2.2) * | 686 (15) | 3.0 (0.3) | 18.7 (2.3) | 2990 (20) | 2.6 (0.3) |
| SAs | ||||||
| 2.3 | 17.8 (±2.1) | 732 (±12) | 2.6 (±0.2) | 20.6 (±2.3) | 3241 (±17) | 2.3 (±0.1) |
| 4.9 | 18.3 (±2.3) | 956 (±14) | 2.2 (±0.3) | 21.2 (±2.2) | 3358 (±18) | 1.9 (±0.2) |
| 7.0 | 19.5 (±2.0) | 988 (±12) | 2.1 (±0.2) | 22.3 (±2.2) | 3364 (±16) | 1.6 (±0.2) |
| 9.3 | 17.3 (±2.5) | 644 (±15) | 3.7 (±0.4) | 20.8 (±2.5) | 3139 (±23) | 2.2 (±0.3) |
| 12.0 | 15.2 (±2.4) | 458 (±17) | 4.6 (±0.4) | 18.5 (±2.8) | 3016 (±23) | 2.5 (±0.4) |
| ZnSt | ||||||
| 2.0 | 17.9 (±2.4) | 755 (±17) | 2.4 (±0.4) | 21.0 (±2.2) | 3175 (±16) | 1.8 (±0.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shulga, G.; Rizhikovs, J.; Neiberte, B.; Verovkins, A.; Vitolina, S.; Betkers, T.; Makars, R. Processing and Properties of Wood-Plastic Composite Containing Alkali-Treated Birch Wood Shavings and Bioadditive Obtained by Biorefinery of Birch Bark. Forests 2023, 14, 1906. https://doi.org/10.3390/f14091906
Shulga G, Rizhikovs J, Neiberte B, Verovkins A, Vitolina S, Betkers T, Makars R. Processing and Properties of Wood-Plastic Composite Containing Alkali-Treated Birch Wood Shavings and Bioadditive Obtained by Biorefinery of Birch Bark. Forests. 2023; 14(9):1906. https://doi.org/10.3390/f14091906
Chicago/Turabian StyleShulga, Galia, Janis Rizhikovs, Brigita Neiberte, Anrijs Verovkins, Sanita Vitolina, Talrits Betkers, and Raimonds Makars. 2023. "Processing and Properties of Wood-Plastic Composite Containing Alkali-Treated Birch Wood Shavings and Bioadditive Obtained by Biorefinery of Birch Bark" Forests 14, no. 9: 1906. https://doi.org/10.3390/f14091906
APA StyleShulga, G., Rizhikovs, J., Neiberte, B., Verovkins, A., Vitolina, S., Betkers, T., & Makars, R. (2023). Processing and Properties of Wood-Plastic Composite Containing Alkali-Treated Birch Wood Shavings and Bioadditive Obtained by Biorefinery of Birch Bark. Forests, 14(9), 1906. https://doi.org/10.3390/f14091906






