Influence of Varied Phosphorus Fertilizer Ratios on the Rhizosphere Soil Microbial Community in Idesia polycarpa Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site Overview and Experimental Design
2.2. Measurement Items and Methods
2.2.1. Assessment of Soil Physicochemical Properties
2.2.2. Sampling and High-Throughput Sequencing of Rhizosphere Soil
2.3. Bioinformatics and Statistical Analysis
3. Results
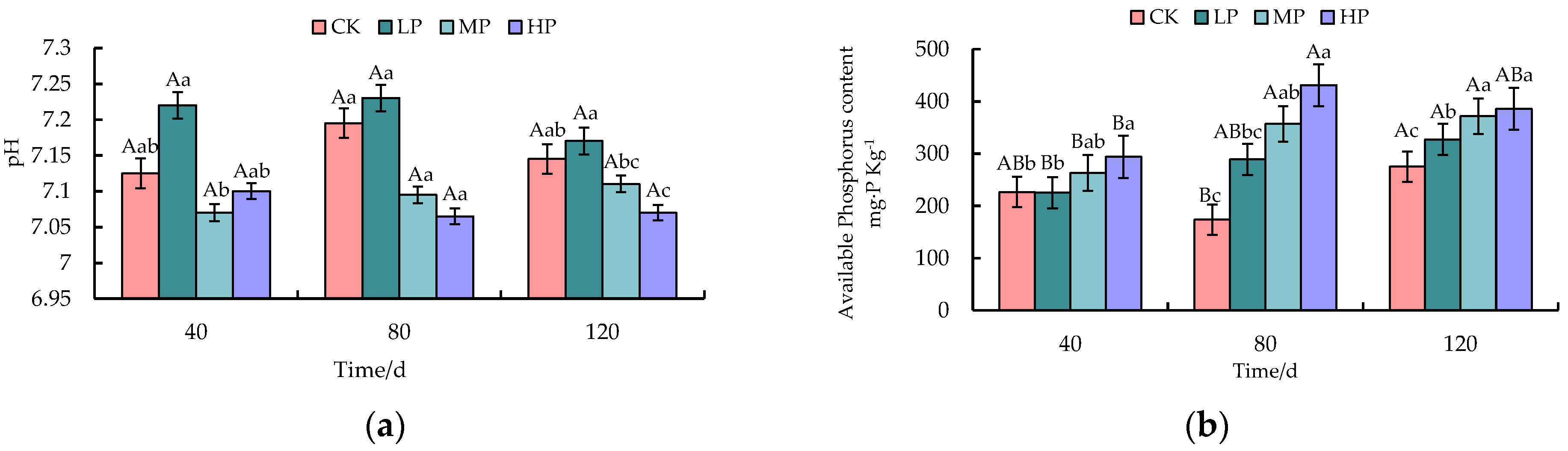
3.1. Effects of Different Phosphorus Levels on Soil Physicochemical Properties of Idesia polycarpa Seedlings
3.2. Effects of Different Phosphorus Levels on Microbial Community Composition in Rhizosphere Soil of Idesia polycarpa
3.3. Impact of Phosphorus Levels on the Relative Abundance of Microorganisms in Rhizosphere Soil of Idesia polycarpa Seedlings
3.3.1. Relative Abundance of Bacteria
3.3.2. Relative Abundance of Fungi
3.4. Analysis of Soil Microbial Community Diversity of Idesia polycarpa Seedlings at Different Phosphorus Levels
3.5. Correlation Analysis of AP and pH Value of Rhizosphere Soil of Idesia polycarpa on Bacteria and Fungi in Different Periods
3.6. Prediction and Analysis of Soil Microbial Community Function of Idesia polycarpa Seedlings at Different Phosphorus Levels
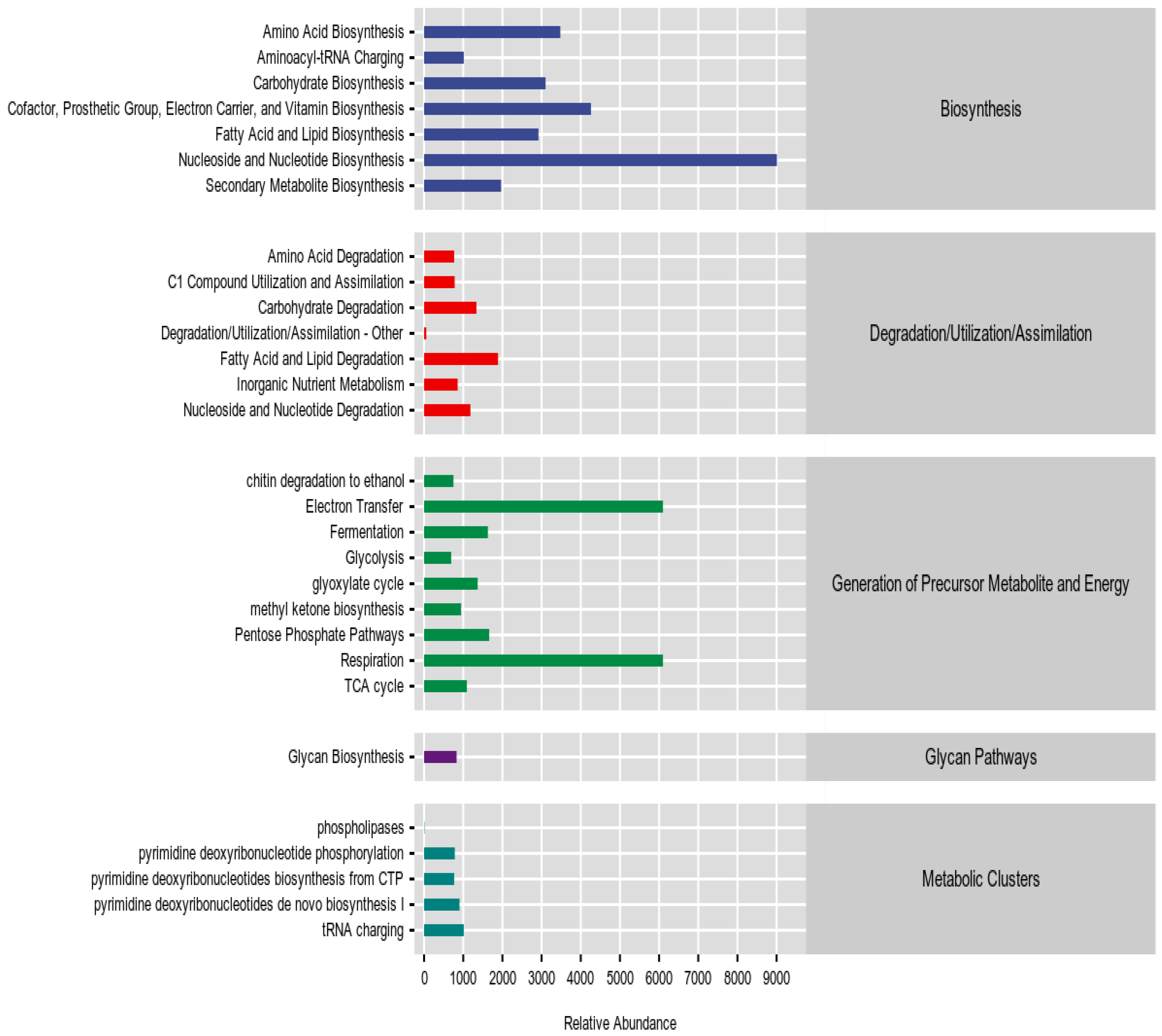
3.6.1. Metabolic Pathway Analysis
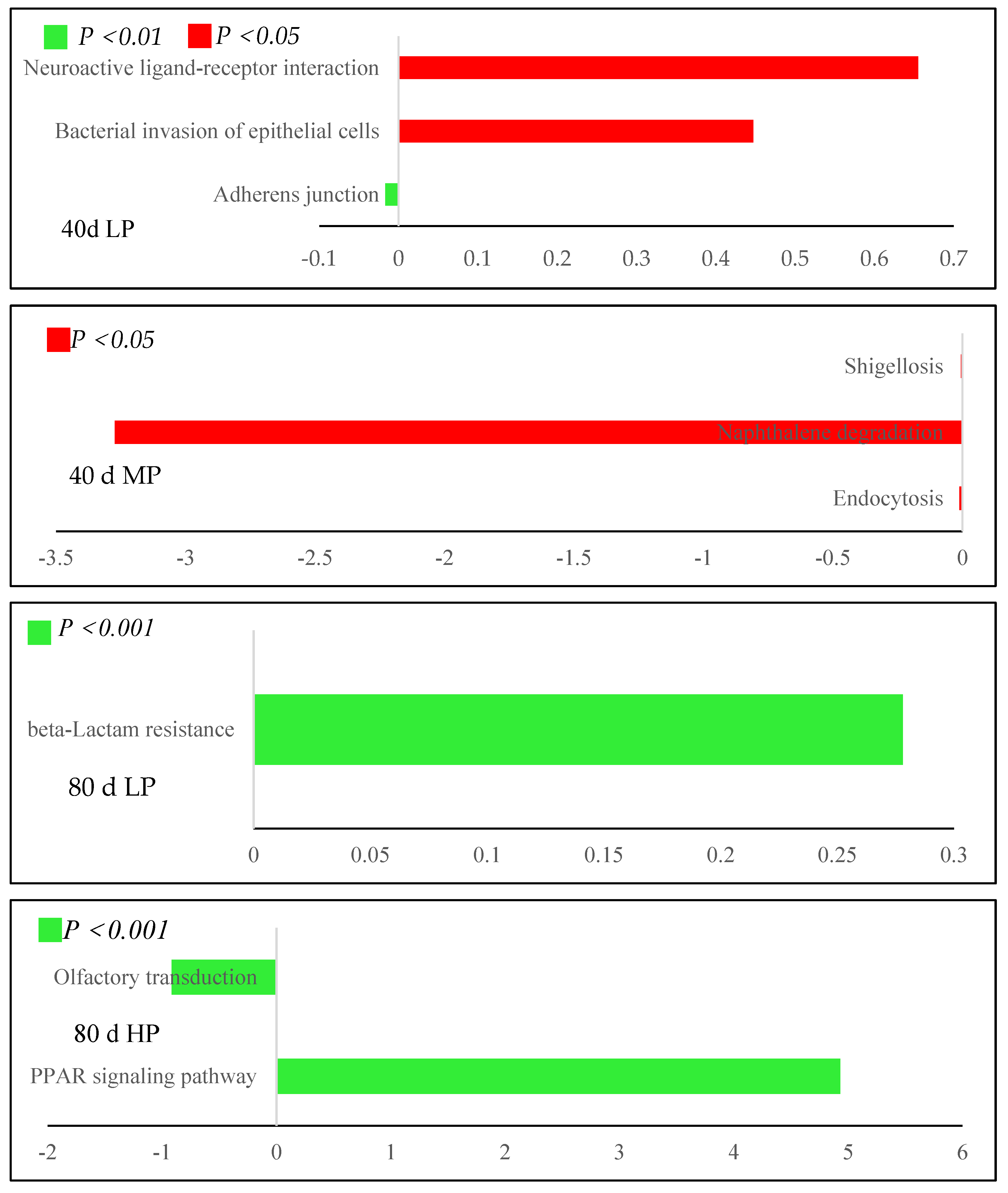
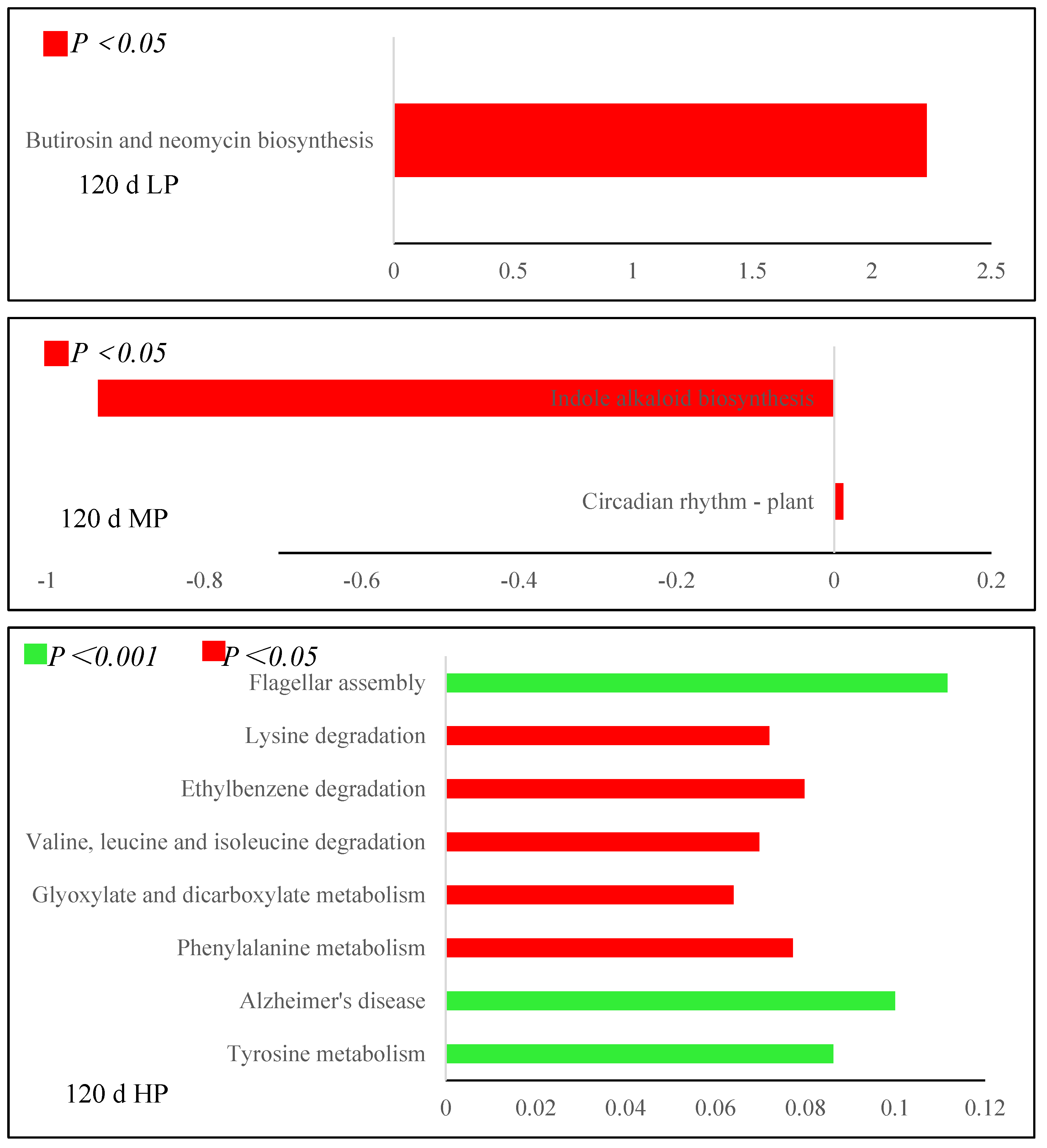
3.6.2. Metabolic Pathway Difference Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Z. Research on the Ecological Characteristics and Reproduction Technology of Idesia polycarpa. Master’s Thesis, Nanjing Forestry University, Nanjing, China, 2005. [Google Scholar]
- Liu, G.; Liang, Z. Review on the research of Idesia polycarpa. Jiangsu For. Sci. Technol. 2005, 5, 50–53. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Liu, Z. Analysis of oil content and fatty acid GC/MS in Idesia polycarpa seeds from different provenances. J. Henan Agric. Univ. 2011, 45, 530–533. [Google Scholar] [CrossRef]
- Baek, S.H.; Kim, D.; Lee, C. Idescarpin isolated from the fruits of Idesia polycarpa inhibits melanin biosynthesis. J. Microbiol. Biotechnol. 2006, 16, 667–672. [Google Scholar]
- Lee, M.; Lee, H.H.; Lee, J.K. Anti-adipogenic activity of compounds isolated from Idesia polycarpa on 3T3-L1 cells. Bioorg. Med. Chem. Lett. 2013, 23, 3170–3174. [Google Scholar] [CrossRef]
- Huang, L.; Zeng, Y.; Li, F. Polyphenolic compounds from Idesia polycarpa Maxim fruits ameliorate non-alcoholic fatty liver disease by modulating lipid metabolism in oleic acid-induced HepG2 cells and high-fat diet-induced mice. J. Funct. 2023, 108, 1756–4646. [Google Scholar] [CrossRef]
- Guo, J.; Wang, C.; Li, C.; Liu, Y. Effect of acetylation on the physical and mechanical performances of mechanical densified spruce wood. Forests 2022, 13, 1620. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, X.; Chen, G. Wood properties of Idesia polycarpa. J. Northwest A&F Univ. 2023, 51, 40–48+58. [Google Scholar]
- Li, H.; Wang, Y.; Xie, W. Preparation and characterization of soybean protein adhesives modified with an environmental-friendly tannin-based resin. Polymers 2023, 15, 2289. [Google Scholar] [CrossRef]
- Deng, Z.; Gao, M.; Wang, Y. Effects of chemical fertilizer reduction combined with organic fertilizer application on bacterial community structure in rhizosphere/non-rhizosphere soil of lemon. Chin. J. Environ. Sci. 2023, 44, 1074–1084. [Google Scholar] [CrossRef]
- Shao, Q.; Dong, C.; Han, Y. Research progress in the rhizosphere microbiome of plants. J. Plant Nutr. Fert. 2021, 27, 144–152. [Google Scholar]
- Dengzeng, Z.M.; Ma, W.; Zhou, Q. Research progress of rhizosphere soil microorganisms at home and abroad based on bibliometric analysis. Soil Fert. Sci. China. 2022, 302, 236–246. [Google Scholar] [CrossRef]
- Niu, X.; Sun, X.; Chen, D. Soil microorganisms, nutrients and enzyme activity of Larix kaempferi plantation under ages in mountainous region of eastern Liaoning Province, China. Chin. J. Appl. Ecol. 2015, 26, 2663–2672. [Google Scholar] [CrossRef]
- Li, Y.; Tremblay, J.; Bainard, L.D. Long-term effects of nitrogen and phosphorus fertilization on soil microbial community structure and function under continuous wheat production. Environ. Microbiol. 2020, 22, 1066–1088. [Google Scholar] [CrossRef]
- Xun, W.; Zhao, J.; Xue, C. Significant alteration of soil bacterial communities and organic carbon decomposition by different long-term fertilization management conditions of extremely low-productivity arable soil in South China. Environ. Microbiol. 2016, 18, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus uptake by plants: From soil to cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Wang, Y.; Guo, J. Absorption and utilization of phosphorus by plants. Rural Sci. Tech. 2022, 13, 71–75. [Google Scholar] [CrossRef]
- Saleem, M.; Hu, J.; Jousset, A. More than the sum of its parts: Microbiome biodiversity as a driver of plant growth and soil health. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 145–168. [Google Scholar] [CrossRef]
- Wu, J.Q. Effects of Vegetation Degradation on Soil Physicochemical Properties and Enzyme Activities in Gahai Wet Meadow. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2021. [Google Scholar] [CrossRef]
- Ren, B.; Hu, Y.; Bu, R. Vertical distribution patterns and drivers of soil bacterial communities across the continuous permafrost region of northeastern China. Ecol. Process. 2022, 11, 1–13. [Google Scholar] [CrossRef]
- Wang, B.; Xu, M.; Wen, S. The effect of long term fertilizer application on phosphorus in red upland soil. Chin. Agric. Sci. Bull. 2007, 3, 254–259. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zhang, P.; Lin, Q. Response of bacterial communities in rubber plantations to different fertilizer treatments. 3 Biotech 2019, 9, 293. [Google Scholar] [CrossRef]
- Gryta, A.; Frąc, M.; Oszust, K. Genetic and metabolic diversity of soil microbiome in response to exogenous organic matter amendments. Agronomy 2020, 10, 546. [Google Scholar] [CrossRef]
- Peng, Q.; He, H.; Fan, C. The interaction of phosphate and selenite in alkaline soil and accumulation by alfalfa (Medicago sativa L.). Arch. Agron. Soil Sci. 2020, 67, 122–135. [Google Scholar] [CrossRef]
- Liu, J.; Ma, Q.; Hui, X. Long-term high-P fertilizer input decreased the total bacterial diversity but not phoD-harboring bacteria in wheat rhizosphere soil with available-P deficiency. Soil Biol. Biochem. 2020, 149, 107918. [Google Scholar] [CrossRef]
- Lv, L. Effects of Exogenous Phosphorus on Ginseng Quality and Soil Physicochemical and Biological Characteristics. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2019. [Google Scholar]
- Sun, R.; Zhang, X.; Guo, X. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, W.; Guo, Z. Effects of long-term application of phosphorus fertilizer on fungal community diversity, composition, and intraspecific interactions and variation with application rate in a lime concretion black soil. Acta Ecol. Sin. 2019, 39, 4158–4167. [Google Scholar]
- Li, S.; Sun, Y.; Liu, X. Effects of phosphate fertilizer on rhizosphere soil microenvironment and hay yield of alfalfa under drip irrigation. Acta Agrestia Sin. 2022, 30, 495–502. [Google Scholar] [CrossRef]
- Shi, Y.; Ziadi, N.; Hamel, C. Soil microbial biomass, activity and community structure as affected by mineral phosphorus fertilization in grasslands. Appl. Soil Ecol. 2020, 146, 103391. [Google Scholar] [CrossRef]
- Rana, S. Study on the Influence Mechanism of Water and Fertilizer Coupling on the Growth and Development of Idesia polycarpa Seedlings. Ph.D. Thesis, College of Forestry, Henan Agricultural University, Zhengzhou, China, 2024. [Google Scholar]
- Rana, S.; Liu, Z. Study on the pattern of vegetative growth in young dioecious trees of Idesia polycarpa maxim. Trees 2021, 35, 69–80. [Google Scholar] [CrossRef]
- Rana, S.; Jemim, R.S.; Li, Z.; Geng, X.D.; Wang, Y.M.; Cai, Q.F.; Liu, Z. Study on the pattern of reproductive allocation and fruit development in young dioecious trees of Idesia polycarpa Maxim. S. Afr. J. Bot. 2022, 146, 472–480. [Google Scholar] [CrossRef]
- Bao, S. Soil Agrochemical Analysis; China Agriculture Press: Beijing, China, 2000; pp. 263–270. [Google Scholar]
- Martin, M. CUTADAPT removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 2011, 17. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.J.; Rasmussen, L.; Asplund, M.; Knudsen, S.W.; Clausen, M.L.; Agner, T.; Hansen, A.J. Comparing DADA2 and OTU clustering approaches in studying the bacterial communities of atopic dermatitis. J. Med. Microbiol. 2020, 69, 1293–1302. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [PubMed]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. ACM SIGMOBILE Mob. Comput. Commun. Rev. 2001, 5, 3–55. [Google Scholar] [CrossRef]
- Zgadzaj, R.; Garrido-Oter, R.; Jensen, D.B.; Koprivova, A.; Schulze-Lefert, P.; Radutoiu, S. Root nodule symbiosis in Lotus japonicus drives the establishment of distinctive rhizosphere, root, and nodule bacterial communities. Proc. Natl. Acad. Sci. USA 2016, 113, E7996–E8005. [Google Scholar] [CrossRef]
- Douglas, G.; Maffei, V.; Zaneveld, J.; Yurgel, S.; Brown, J.; Taylor, C.; Huttenhower, C.; Langille, M. PICRUSt2: An improved and customizable approach for metagenome inference. BioRxiv 2019. [Google Scholar] [CrossRef]
- Cao, S.; Ouyang, M.; Zhou, W. Soil pH and main nutrient characteristics of citrus orchards and their correation in Hunan Province. Soil Fert. Sci. China 2020, 285, 31–38. [Google Scholar] [CrossRef]
- Peng, T. Effects of Phosphorus Addition on Soil Nutrient Elements and Microorganisms in Young Phoebe bournei Plantations. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2022. [Google Scholar]
- Richardson, A.E.; Simpson, R.J. Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ishimoto, K.; Kuriyama, Y. Ninety-year-, but not single, application of phosphorus fertilizer has a major impact on arbuscular mycorrhizal fungal communities. Plant Soil 2013, 365, 397–407. [Google Scholar] [CrossRef]
- Tischer, A.; Blagodatskaya, E. Microbial community structure and resource availability drive the catalytic efficiency of soil enzymes under land-use change conditions. Soil Biol. Biochem. 2015, 89, 226–237. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, B.; Liu, C. Soil fertility, enzyme activity, and microbial community structure diversity among different soil textures under different land use types in coastal saline soil. J. Soils Sediments 2021, 21, 2240–2252. [Google Scholar] [CrossRef]
- Gao, P.; Tian, H.; Wang, Y. Spatial isolation and environmental factors drive distinct bacterial and archaeal communities in different types of petroleum reservoirs in China. Sci. Rep. 2016, 6, 20174. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Zhou, X. Effects of long-term different fertilization on soil fungal communities in black soil based on the Illumina Mi Seq platform. Acta Microbiol. Sin. 2018, 58, 1658–1671. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, P.; Zeng, Z. Dynamics of bacterial communities in a 30-year fertilized paddy field under different organic-inorganic fertilization strategies. Agronomy 2019, 9, 14. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, Y.; Gao, J. Long-term combined application of manure and chemical fertilizer sustained higher nutrient status and rhizospheric bacterial dibersity in reddish paddy soil of Central South China. Sci. Rep. 2018, 8, 16554. [Google Scholar] [CrossRef]
- He, Q. Effects of Phosphorus Application Rate and Combined Application of Organic and Inorganic Fertilizer on Soil Microorganism, Phosphorus Status and Yield in Cotton Field. Master’s Thesis, Xinjiang Agricultural University, Xinjiang, China, 2022. [Google Scholar] [CrossRef]
- Yabe, S.; Aiba, Y.; Sakai, Y. Thermosporothrix hazakensis gen. nov., sp.nov., isolated from compost, description of Thermosporotrichaceae fam. Nov. within the class Ktedonobacteria Cavaletti et al. 2007 and emended description of the class Ktedonobacteria. Int. J. Syst. 2010, 60, 1794–1801. [Google Scholar] [CrossRef]
- Hou, T. Effects of Phosphate Fertilizer Application on Phosphorus Uptake and Soil Microbial Community Structure of Cotton under Mulch Drip Irrigation. Master’s Thesis, Xinjiang Agricultural University, Xinjiang, China, 2022. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Nie, S.; Wang, Y.; Lei, X. Responses of fungal community structure and functional group to fertilization in yellow clayey soil. Chin. J. Appl. Ecol. 2018, 29, 2721–2729. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, S.; Chen, Y. Effects of short-term fertilization on soil fungal community structure and functional group in eucalyptus artificial forest. Acta Ecol. Sin. 2020, 40, 3813–3821. [Google Scholar]


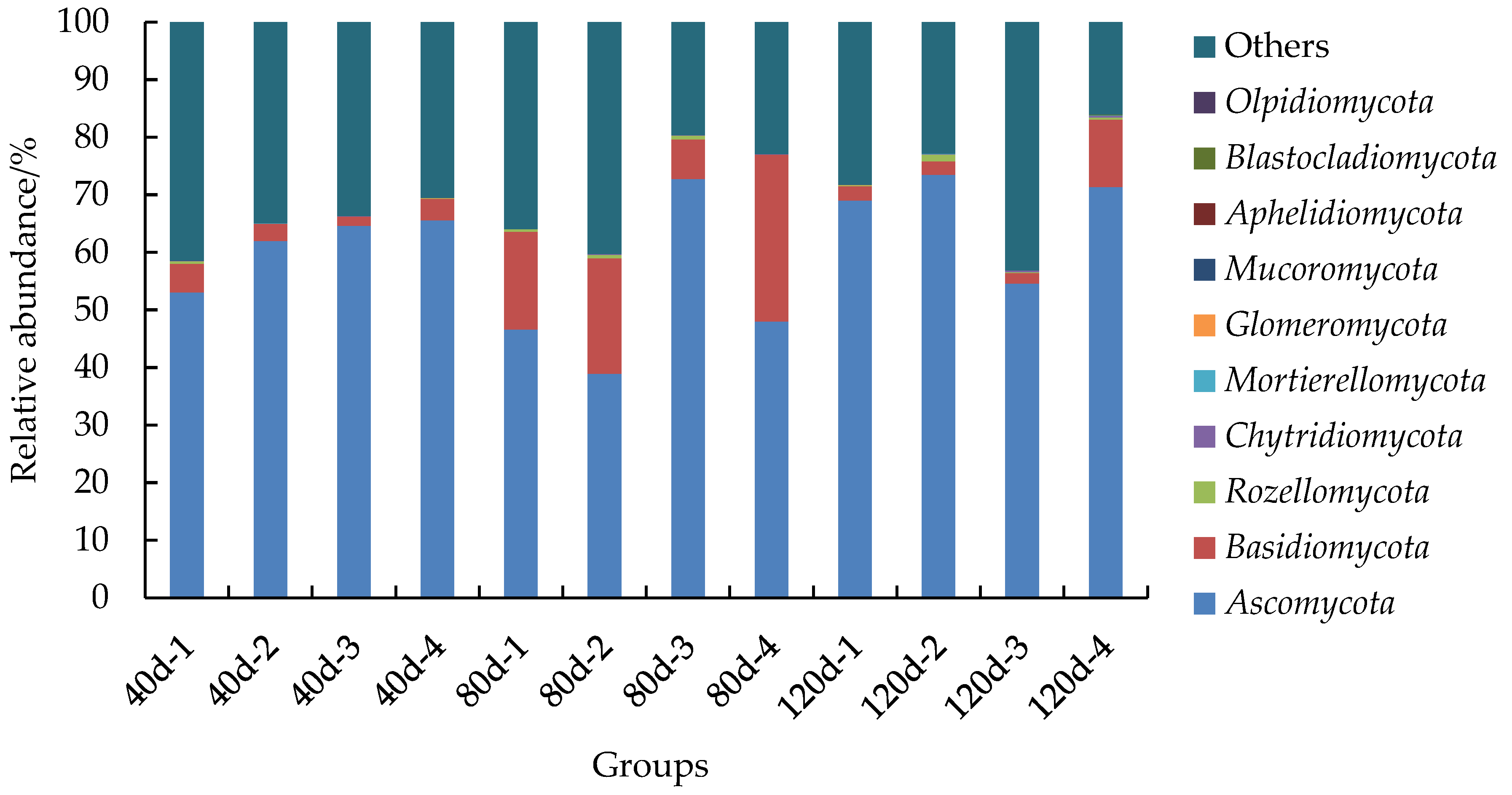



| Treatment (g/Plant × Times) | N | P | K |
|---|---|---|---|
| CK | 2.4 | 0 | 1.29 |
| LP | 2.4 | 0.92 | 1.29 |
| MP | 2.4 | 1.83 | 1.29 |
| HP | 2.4 | 2.75 | 1.29 |
| Time/d | Treatment | Bacteria | Fungus | ||||
|---|---|---|---|---|---|---|---|
| OTUs | Original Sequence | Significant Sequence | OTUs | Original Sequence | Significant Sequence | ||
| 40 d | CK | 8669 | 422,347 | 365,990 | 959 | 368,513 | 303,709 |
| LP | 10,334 | 384,480 | 326,901 | 975 | 445,732 | 352,752 | |
| MP | 9405 | 426,817 | 367,978 | 874 | 422,679 | 342,421 | |
| HP | 9039 | 383,246 | 328,198 | 768 | 359,271 | 267,004 | |
| 80 d | CK | 8530 | 272,643 | 235,049 | 489 | 222,056 | 202,600 |
| LP | 9320 | 293,613 | 253,278 | 614 | 194,790 | 177,251 | |
| MP | 9155 | 423,011 | 370,504 | 552 | 247,558 | 218,145 | |
| HP | 7885 | 420,825 | 371,096 | 556 | 219,004 | 192,487 | |
| 120 d | CK | 7542 | 339,478 | 308,449 | 683 | 210,495 | 188,659 |
| LP | 7563 | 326,329 | 296,977 | 675 | 212,991 | 185,018 | |
| MP | 6780 | 341,323 | 312,835 | 779 | 209,846 | 182,647 | |
| HP | 7907 | 319,213 | 289,002 | 650 | 210,875 | 186,959 | |
| Groups | Genus Level | Family Level | Order Level | Class Level | Phylum Level |
|---|---|---|---|---|---|
| 40 d | 1178 | 545 | 305 | 114 | 37 |
| 80 d | 1182 | 564 | 324 | 127 | 43 |
| 120 d | 1260 | 578 | 331 | 130 | 44 |
| Groups | Genus Level | Family Level | Order Level | Class Level | Phylum Level |
|---|---|---|---|---|---|
| 40 d | 246 | 147 | 71 | 33 | 10 |
| 80 d | 164 | 107 | 61 | 30 | 10 |
| 120 d | 213 | 131 | 67 | 31 | 11 |
| Time/d | Groups | Pielou’s Evenness | Goods Coverage | Chao1 | Shannon | Simpson |
|---|---|---|---|---|---|---|
| 40 d | CK | 0.78 ± 0.03 Ba | 0.99 ± 0.01 Ca | 4124.03 ± 259.46 Aa | 9.27 ± 0.38 Aa | 0.98 ± 0.01 Aa |
| LP | 0.82 ± 0.03 Aa | 0.99 ± 0.01 Ba | 4276.08 ± 560.56 Aa | 9.75 ± 0.48 Aa | 0.99 ± 0.01 Aa | |
| MP | 0.81 ± 0.01 Aa | 0.98 ± 0.01 Ba | 4263.78 ± 327.12 Aa | 9.59 ± 0.082 Aa | 0.99 ± 0.01 Aa | |
| HP | 0.81 ± 0.03 ABa | 0.99 ± 0.01 Ba | 4006.08 ± 387.39 Aa | 9.57 ± 0.45 Aa | 0.99 ± 0.01 Aa | |
| 80 d | CK | 0.82 ± 0.01 ABab | 0.99 ± 0.01 Ba | 3586.52 ± 97.07 Ab | 9.68 ± 0.11 Aab | 0.99 ± 0.01 Aab |
| LP | 0.83 ± 0.01 Aa | 0.99 ± 0.01 Ba | 3889.86 ± 38.18 Ab | 9.90 ± 0.04 Aa | 0.99 ± 0.01 Aa | |
| MP | 0.82 ± 0.01 Aab | 0.98 ± 0.01 Cc | 4537.89 ± 92.01 Aa | 9.83 ± 0.17 Aab | 0.99 ± 0.01 Aa | |
| HP | 0.76 ± 0.04 Bb | 0.98 ± 0.01 Cb | 3776.05 ± 320.50 Ab | 8.86 ± 0.50 Ab | 0.98 ± 0.01 Ab | |
| 120 d | CK | 0.86 ± 0.01 Aa | 0.99 ± 0.01 Aa | 3804.44 ± 231.67 Aa | 10.22 ± 0.22 Aa | 0.99 ± 0.01 Aa |
| LP | 0.87 ± 0.01 Aa | 0.99 ± 0.01 Aa | 3788.07 ± 41.08 Aa | 10.31 ± 0.05 Aa | 0.99 ± 0.01 Aa | |
| MP | 0.79 ± 0.04 Aa | 0.99 ± 0.01 Aa | 3263.82 ± 258.38 Ba | 9.21 ± 0.59 Aa | 0.98 ± 0.01 Aa | |
| HP | 0.87 ± 0.01 Aa | 0.99 ± 0.01 Aa | 3741.39 ± 176.37 Aa | 10.29 ± 0.19 Aa | 0.99 ± 0.01 Aa |
| Time/d | Groups | Pielou’s Evenness | Goods Coverage | Chao1 | Shannon | Simpson |
|---|---|---|---|---|---|---|
| 40 | CK | 0.61 ± 0.03 Aa | 0.99 ± 0.01 Aa | 444.90 ± 17.46 Aa | 5.36 ± 0.21 Aa | 0.91 ± 0.02 Aa |
| LP | 0.54 ± 0.06 Aa | 0.99 ± 0.01 Aa | 421.73 ± 92.64 Aa | 4.68 ± 0.69 Aa | 0.88 ± 0.04 Aa | |
| MP | 0.53 ± 0.05 ABa | 0.99 ± 0.01 Ba | 381.83 ± 67.86 Aa | 4.54 ± 0.52 ABa | 0.87 ± 0.02 ABa | |
| HP | 0.52 ± 0.04 Aa | 0.99 ± 0.01 Aa | 332.91 ± 41.23 Aa | 4.35 ± 0.32 Aa | 0.85 ± 0.06 Aa | |
| 80 | CK | 0.50 ± 0.13 Aa | 0.99 ± 0.01 Aa | 229.29 ± 19.74 Cb | 3.96 ± 1.03 Aa | 0.75 ± 0.18 Aa |
| LP | 0.61 ± 0.042 Aa | 0.99 ± 0.01 Aa | 298.65 ± 12.26 Aa | 4.99 ± 0.38 Aa | 0.89 ± 0.03 Aa | |
| MP | 0.46 ± 0.06 Ba | 0.99 ± 0.01 ABa | 256.29 ± 5.01 Aab | 3.67 ± 0.46 Ba | 0.75 ± 0.07 Ba | |
| HP | 0.55 ± 0.01 Aa | 0.99 ± 0.01 Aa | 257.58 ± 19.78 Aab | 4.41 ± 0.05 Aa | 0.86 ± 0.02 Aa | |
| 120 | CK | 0.57 ± 0.04 Aa | 0.99 ± 0.01 Aa | 349.34 ± 15.56 Ba | 4.81 ± 0.37 Aa | 0.88 ± 0.04 Aa |
| LP | 0.52 ± 0.09 Aa | 0.99 ± 0.01 Aa | 316.74 ± 59.04 Aa | 4.35 ± 0.85 Aa | 0.82 ± 0.07 Aa | |
| MP | 0.64 ± 0.03 Aa | 0.99 ± 0.01 Aa | 382.44 ± 35.81 Aa | 5.52 ± 0.34 Aa | 0.93 ± 0.02 Aa | |
| HP | 0.55 ± 0.06 Aa | 0.99 ± 0.01 Aa | 313.61 ± 26.06 Aa | 4.59 ± 0.51 Aa | 0.86 ± 0.06 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Rana, S.; Zhang, T.; Wang, Y.; Liu, Z.; Cai, Q.; Geng, X.; Yuan, Q.; Yang, Y.; Miao, C.; et al. Influence of Varied Phosphorus Fertilizer Ratios on the Rhizosphere Soil Microbial Community in Idesia polycarpa Seedlings. Forests 2024, 15, 1686. https://doi.org/10.3390/f15101686
Wang S, Rana S, Zhang T, Wang Y, Liu Z, Cai Q, Geng X, Yuan Q, Yang Y, Miao C, et al. Influence of Varied Phosphorus Fertilizer Ratios on the Rhizosphere Soil Microbial Community in Idesia polycarpa Seedlings. Forests. 2024; 15(10):1686. https://doi.org/10.3390/f15101686
Chicago/Turabian StyleWang, Shasha, Sohel Rana, Tao Zhang, Yanmei Wang, Zhen Liu, Qifei Cai, Xiaodong Geng, Qiupeng Yuan, Yi Yang, Chao Miao, and et al. 2024. "Influence of Varied Phosphorus Fertilizer Ratios on the Rhizosphere Soil Microbial Community in Idesia polycarpa Seedlings" Forests 15, no. 10: 1686. https://doi.org/10.3390/f15101686
APA StyleWang, S., Rana, S., Zhang, T., Wang, Y., Liu, Z., Cai, Q., Geng, X., Yuan, Q., Yang, Y., Miao, C., Zhou, H., Dai, L., Peng, H., & Li, Z. (2024). Influence of Varied Phosphorus Fertilizer Ratios on the Rhizosphere Soil Microbial Community in Idesia polycarpa Seedlings. Forests, 15(10), 1686. https://doi.org/10.3390/f15101686







