Growing Jatropha curcas L. Improves the Chemical Characteristics of Degraded Tropical Soils
Abstract
:1. Introduction
2. Materials and Methods
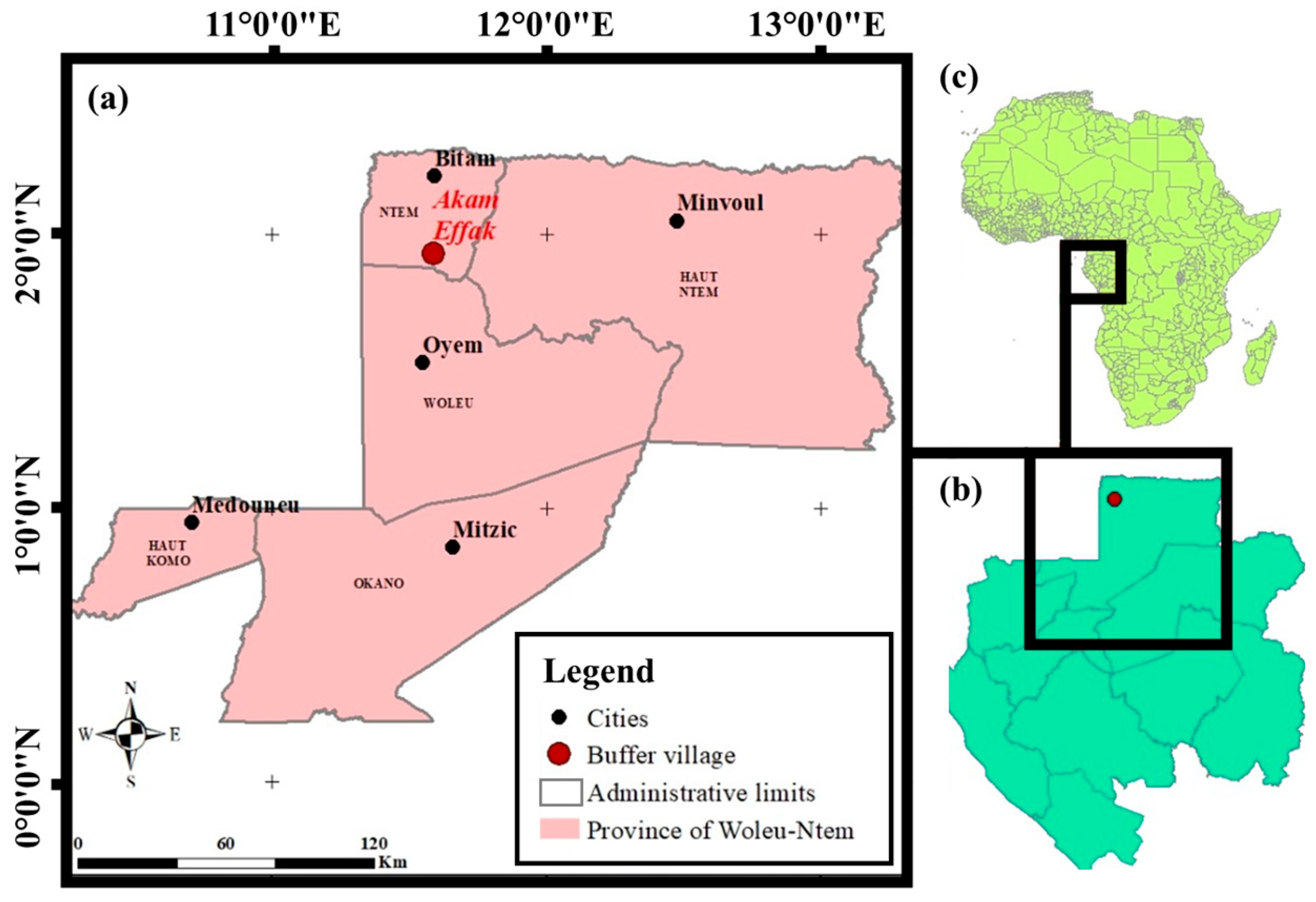
2.1. Description of the Study Site and Experimental Set-Up
2.2. Soil Sampling and Analysis
2.3. Statistical Analysis
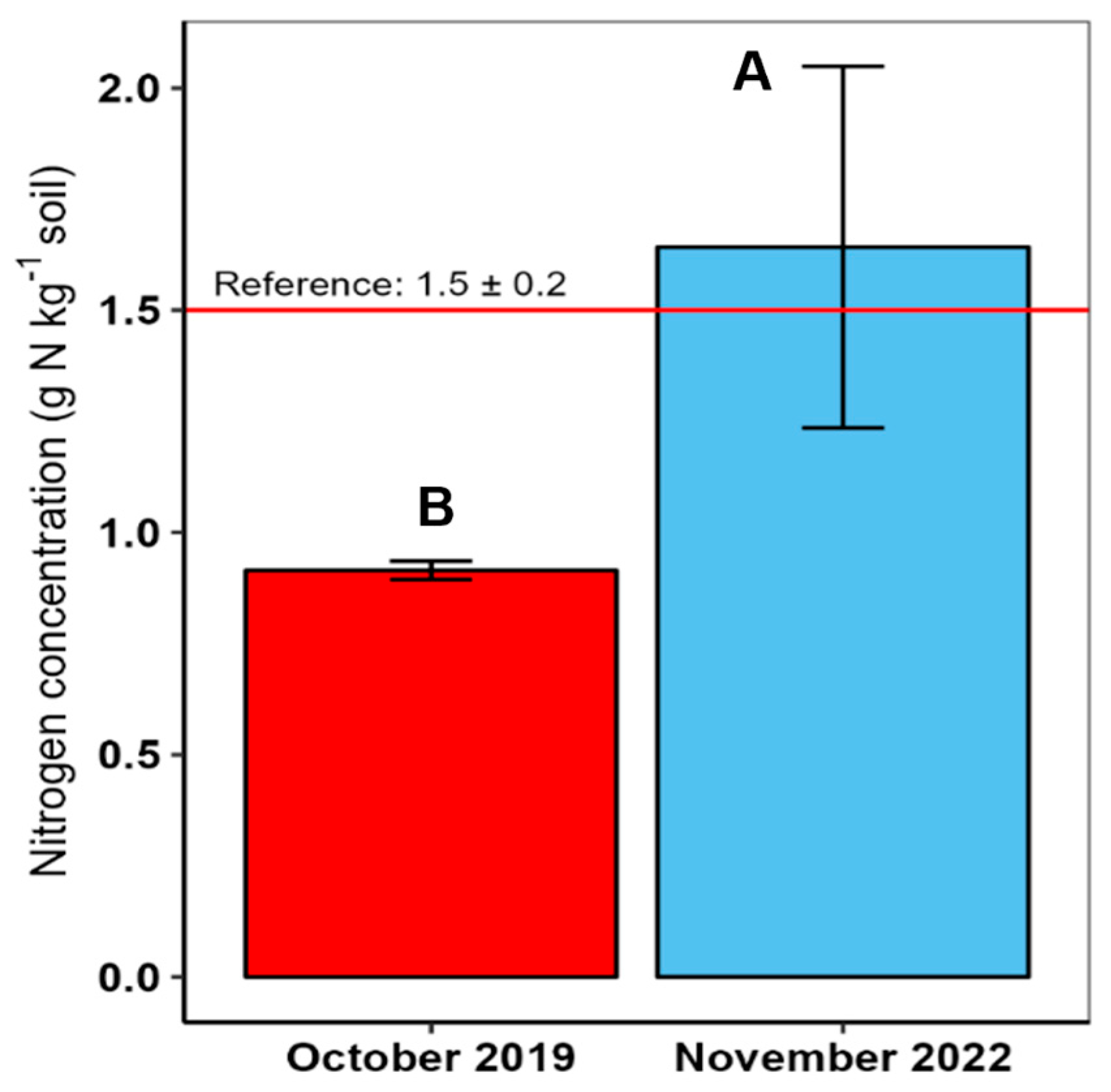
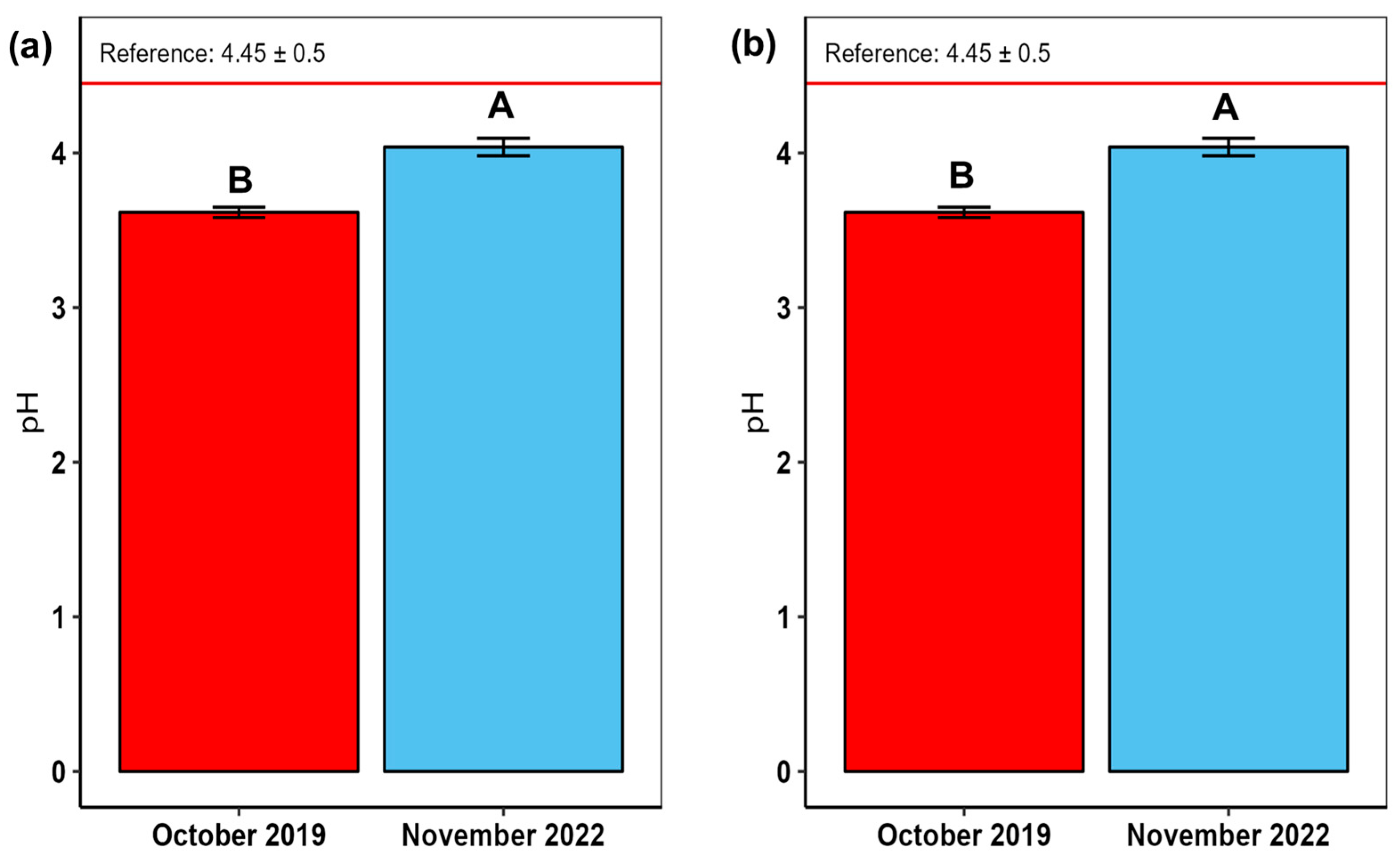
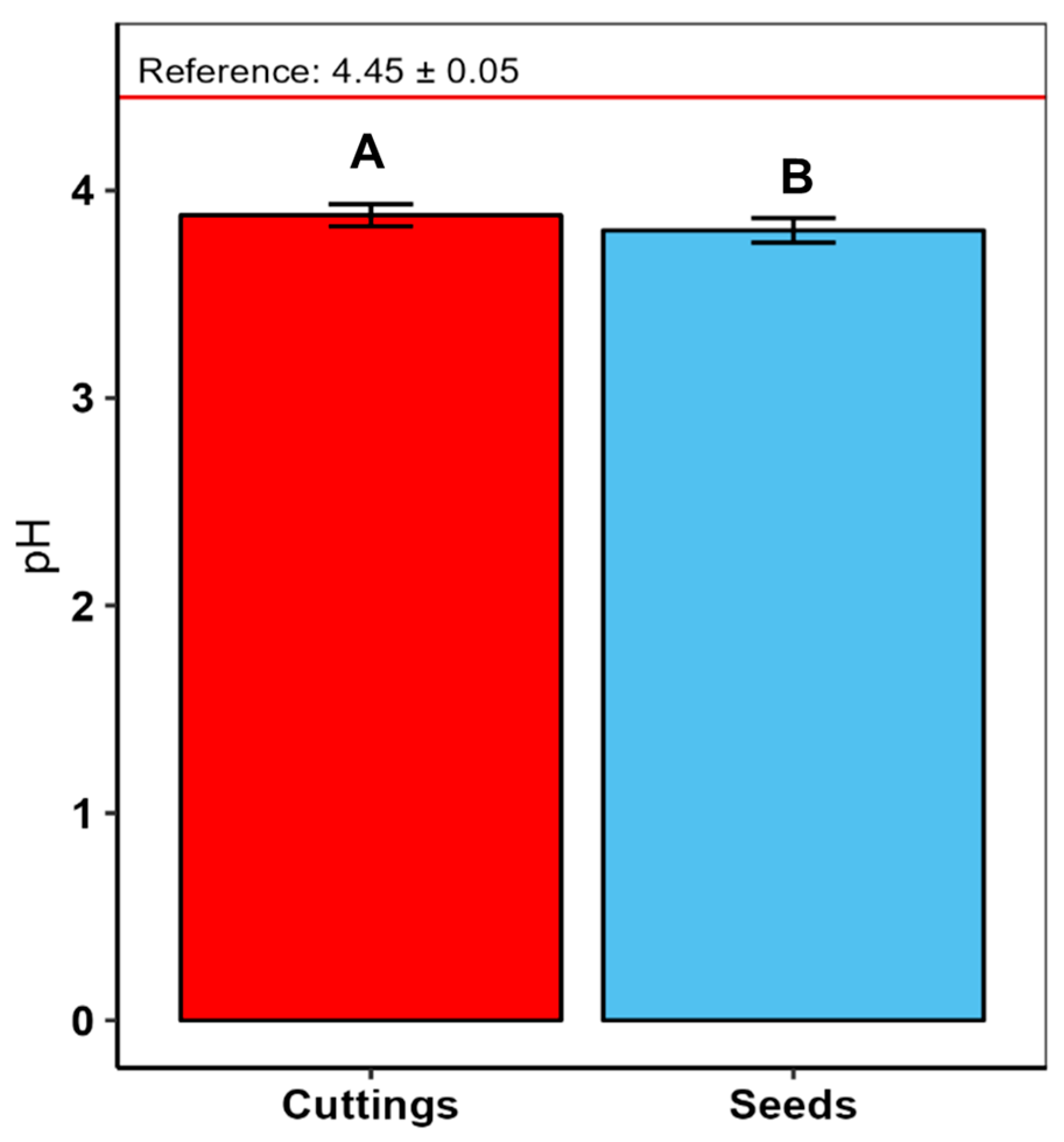
3. Results
4. Discussion
4.1. Soil Organic Carbon Concentration
4.2. Total Soil Nitrogen Concentration
4.3. Soil Acidity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lal, R. Soil degradation as a reason for inadequate human nutrition. Food Secur. 2009, 1, 45–57. [Google Scholar] [CrossRef]
- Lal, R. Restoring soil quality to mitigate soil degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Chenu, C.; Klumpp, K.; Bispo, A.; Angers, D.; Colnenne, C.; Metay, A. Stocker du carbone dans les sols agricoles: Évaluation de leviers d’action pour la France. Innov. Agron. 2014, 37, 23–37. Available online: https://hal.science/hal-01173319 (accessed on 13 September 2024).
- Lal, R. Soil degradation by erosion. Land Degrad. Dev. 2001, 12, 519–539. [Google Scholar] [CrossRef]
- Benbrahim, K.F.; Ismaili, M.; Benbrahim, S.F.; Tribak, A. Land degradation by desertification and deforestation in Morocco. Sci. Chang. Planétaires/Sécheresse 2004, 15, 307–320. [Google Scholar]
- Lahmar, R.; Ruellan, A. Dégradation des sols et stratégies coopératives en Méditerranée: La pression sur les ressources naturelles et les stratégies de développement durable. Cah. Agric. 2007, 16, 318–323. [Google Scholar] [CrossRef]
- Rajot, J.L.; Karambiri, H.; Ribolzi, O.; Planchon, O.; Thiebaux, J.-P. Interaction entre érosions hydrique et éolienne sur sols sableux pâturés au Sahel: Cas du bassin-versant de Katchari au nord du Burkina Faso. Sci. Chang. Planétaires/Sécheresse 2009, 20, 17–30. [Google Scholar] [CrossRef]
- Khalid, N.; Majid, A.; Bilal Tahir, M.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]
- Al-Awadhi, J.M.; Misak, R.F.; Omar, S.S. Causes and consequences of desertification in Kuwait: A case study of land degradation. Bull. Eng. Geol. Environ. 2003, 62, 107–115. [Google Scholar] [CrossRef]
- Walter, C.; Lemercier, B. Les sols bretons sont-ils menacés? Bretagnes 2008, 9, 30–35. Available online: https://hal.science/hal-02060271 (accessed on 18 September 2024).
- Ahononga, F.; Gouwakinnou, G.; Biaou, S.; Ahouandjinou, O.; Biaou, S.; Sonounameto, R. Facteurs d’affectation des terres et effets sur les services écosystémiques et la biodiversité: Synthèse bibliographique. Bull. Rech. Agron. Bénin 2020, 30, 1–9. [Google Scholar]
- El Mazi, M.; Hmamouchi, M.; Houari, A. Impact de l’évolution de l’utilisation des terres sur la dégradation des ressources en sols dans le Rif Central méridional, Maroc. Rev. Marocaine Sci. Agron. Vétérinaires 2021, 9, 567–572. [Google Scholar]
- Maier, C.; Kress, L. Soil CO2 evolution and root respiration in 11 year-old loblolly pine (Pinus taeda) plantations as affected by moisture and nutrient availability. Can. J. For. Res. 2000, 30, 347–359. [Google Scholar] [CrossRef]
- Thibodeau, L.; Raymond, P.; Camiré, C.; Munson, A.D. Impact of precommercial thinning in balsam fir stands on soil nitrogen dynamics, microbial biomass, decomposition, and foliar nutrition. Can. J. For. Res. 2000, 30, 229–238. [Google Scholar] [CrossRef]
- Calvaruso, C.; Blanchart, A.; Bertin, S.; Grand, C.; Pierart, A.; Eglin, T. Quels paramètres du sol mesurer pour évaluer les fonctions et les services écosystémiques associés? Étude Gest. Sols 2020, 27, 36–376. [Google Scholar]
- Eswaran, H.; Lal, R.; Reich, P. Land degradation: An overview. In Response to Land Degradation; Padmanabhan USDA Natural Resources Conservation Service: Washington, DC, USA; University of Puerto Rico: Mayaguez, Puerto Riuco; Universiti Technologi Sumber: Kuching, Malaysia, 2019; pp. 20–35. [Google Scholar]
- Eswaran, H.; Reich, P. Human impact on land systems of the world. Soil Surv. Horiz. 2007, 48, 11–15. [Google Scholar] [CrossRef]
- St. Clair, S.B.; Sharpe, W.E.; Lynch, J.P. Key interactions between nutrient limitation and climatic factors in temperate forests: A synthesis of the sugar maple literature. Can. J. For. Res. 2008, 38, 401–414. [CrossRef]
- Mitchard, E.T. The tropical forest carbon cycle and climate change. Nature 2018, 559, 527–534. [Google Scholar] [CrossRef]
- Patel, D.; Saraf, M. Influence of soil ameliorants and microflora on induction of antioxidant enzymes and growth promotion of Jatropha curcas L. under saline condition. Eur. J. Soil Biol. 2013, 55, 47–54. [Google Scholar] [CrossRef]
- Théodore, A.K.; Gnénakan, Y.; Justin, K.Y. Etude de l’influence de Jatropha curcas sur le rendement du maïs (Zea mays L.) en association culturale dans la région de Bingerville en Côte d’Ivoire. Int. J. Innov. Appl. Stud. 2021, 34, 532–542. [Google Scholar]
- Bazongo, P.; Ouattara, B.; Traore, K.; Traore, O. Effet de Jatropha curcas sur les propriétés physiques et chimiques des sols dans la zone sud-soudanienne du Burkina Faso. Int. J. Innov. Appl. Stud. 2021, 32, 35–42. [Google Scholar]
- Bazongo, P.; Traore, K.; Traore, O.; Bilgo, A.; Yelemou, B.; Sanon, K.B.; Hien, V.; Nacro, B.H. Caractérisation des systèmes de production de Jatropha dans les exploitations agricoles de la zone Ouest du Burkina Faso. Int. J. Biol. Chem. Sci. 2015, 9, 2432–2445. [Google Scholar] [CrossRef]
- Divakara, B.; Upadhyaya, H.; Wani, S.; Gowda, C.L. Biology and genetic improvement of Jatropha curcas L.: A review. Appl. Energy 2010, 87, 732–742. [Google Scholar] [CrossRef]
- Domergue, M.; Pirot, R. Jatropha curcas L. Rapport de Synthèse Bibliographique; CIRAD: Paris, France, 2008; 111p. [Google Scholar]
- Sunil, N.; Sivaraj, N.; Anitha, K.; Abraham, B.; Kumar, V.; Sudhir, E.; Vanaja, M.; Varaprasad, K. Analysis of diversity and distribution of Jatropha curcas L. germplasm using Geographic Information System (DIVA-GIS). Genet. Resour. Crop Evol. 2009, 56, 115–119. [Google Scholar] [CrossRef]
- Wang, X.-R.; Ding, G.-J. Reproductive biology characteristic of Jatropha curcas (Euphorbiaceae). Rev. Biol. Trop. 2012, 60, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Peng, D.; Duan, Z.; Wang, Z.; Sun, Q. Study on pollination biology of Jatropha curcas (Euphorbiaceae). J. South China Agric. Univ. 2007, 28, 62–66. [Google Scholar]
- Massoukou Pamba, R.; Poirier, V.; Nguema Ndoutoumou, P. L’écartement de plantation affecte différemment la croissance végétative du Jatropha curcas L. en culture sur un sol surexploité au nord du Gabon. Int. J. Biol. Chem. Sci. 2022, 16, 158–172. [Google Scholar] [CrossRef]
- Minengu, J.d.D.; Mobambo, P.; Mergeai, G. Influence de l’environnement et des pratiques culturales sur la productivité de Jatropha curcas L. en Afrique subsaharienne (synthèse bibliographique). Biotechnol. Agron. Société Environ. 2014, 18, 290–300. Available online: https://hdl.handle.net/2268/171574 (accessed on 18 September 2024).
- Ogunwole, J.; Chaudhary, D.; Ghosh, A.; Daudu, C.; Chikara, J.; Patolia, J. Contribution of Jatropha curcas to soil quality improvement in a degraded Indian entisol. Acta Agric. Scand. Sect. B–Soil Plant Sci. 2008, 58, 245–251. [Google Scholar] [CrossRef]
- Srivastava, P.; Kumar, A.; Behera, S.K.; Sharma, Y.K.; Singh, N. Soil carbon sequestration: An innovative strategy for reducing atmospheric carbon dioxide concentration. Biodivers. Conserv. 2012, 21, 1343–1358. [Google Scholar] [CrossRef]
- Wani, S.P.; Chander, G.; Sahrawat, K.L.; Rao, C.S.; Raghvendra, G.; Susanna, P.; Pavani, M. Carbon sequestration and land rehabilitation through Jatropha curcas (L.) plantation in degraded lands. Agric. Ecosyst. Environ. 2012, 161, 112–120. [Google Scholar] [CrossRef]
- Singh, B.; Singh, K.; Rao, G.R.; Chikara, J.; Kumar, D.; Mishra, D.; Saikia, S.P.; Pathre, U.V.; Raghuvanshi, N.; Rahi, T. Agro-technology of Jatropha curcas for diverse environmental conditions in India. Biomass Bioenergy 2013, 48, 191–202. [Google Scholar] [CrossRef]
- Behera, S.K.; Srivastava, P.; Tripathi, R.; Singh, J.; Singh, N. Evaluation of plant performance of Jatropha curcas L. under different agro-practices for optimizing biomass–a case study. Biomass Bioenergy 2010, 34, 30–41. [Google Scholar] [CrossRef]
- Singh, K.; Singh, B.; Tuli, R. Sodic soil reclamation potential of Jatropha curcas: A long-term study. Ecol. Eng. 2013, 58, 434–440. [Google Scholar] [CrossRef]
- Achten, W.M.; Nielsen, L.R.; Aerts, R.; Lengkeek, A.G.; Kjær, E.D.; Trabucco, A.; Hansen, J.K.; Maes, W.K.; Graudal, L.; Akinnifesi, F.K. Towards domestication of Jatropha curcas. Biofuels 2010, 1, 91–107. [Google Scholar] [CrossRef]
- Achten, W.M.; Verchot, L.; Franken, Y.J.; Mathijs, E.; Singh, V.P.; Aerts, R.; Muys, B. Jatropha bio-diesel production and use. Biomass Bioenergy 2008, 32, 1063–1084. [Google Scholar] [CrossRef]
- Diop, B.; Samba, S.; Akpo, L.E. Caractéristiques morphologiques et croissance de jeunes plants de Jatropha curcas L. Int. J. Biol. Chem. Sci. 2012, 6, 677–691. [Google Scholar] [CrossRef]
- Geiger, R. Klassifikation der Klimate nach W. Köppen. In Classification des Climats d’après W. Köppen; Landolt-Börnstein—Zahlenwerte und Funktionen aus Physik, Chemie, Astronomie, Geophysik und Technik, Alte Serie; Springer: Berlin, Germany, 1954; Volume 3, pp. 603–607. [Google Scholar]
- Geiger, R. Überarbeitete Neuausgabe von Geiger, R.: Köppen-Geiger/Klima der Erde. (Wandkarte 1:16 Mill.); Klett-Perthes: Gotha, Germany, 1961. [Google Scholar]
- Nguema Ndoutoumou, P.; Bouanga, E.P.; Massounga, Y.C.; Boussiengui Boussiengu, G. Étude comparée de trois méthodes de multiplication de Jatropha curcas L. dans les conditions climatiques du sud-est du Gabon. J. Appl. Biosci. 2013, 65, 4989–4998. [Google Scholar]
- Nguema Ndoutoumou, P.; Ndong, A.N.; Anda, C.C.O.; Midoumbou, F.P.N.; Ognalaga, M.; Missang, C.E. Régénération du cacaoyer (Theobroma cacao L.) sur un substrat à base de compost de Jatropha curcas L. Int. J. Biol. Chem. Sci. 2019, 13, 1043–1053. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Hendershot, W.; Lalande, H.; Duquette, M. Chapter 16—Soil reaction and exchangeable acidity. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2006; 8p. [Google Scholar]
- Kroetsch, D.; Wang, C. Particle Size Distribution. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2006; 16p. [Google Scholar]
- R Core Team. Platform: x86_64-w64-mingw32/x64 (64-bit); The R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Block, R.; Van Rees, K.; Knight, J. A review of fine root dynamics in Populus plantations. Agrofor. Syst. 2006, 67, 73–84. [Google Scholar] [CrossRef]
- Chomel, M.; DesRochers, A.; Baldy, V.; Larchevêque, M.; Gauquelin, T. Non-additive effects of mixing hybrid poplar and white spruce on aboveground and soil carbon storage in boreal plantations. For. Ecol. Manag. 2014, 328, 292–299. [Google Scholar] [CrossRef]
- Marone, D.; Poirier, V.; Coyea, M.; Olivier, A.; Munson, A.D. Carbon storage in agroforestry systems in the semi-arid zone of Niayes, Senegal. Agrofor. Syst. 2017, 91, 941–954. [Google Scholar] [CrossRef]
- Sartori, F.; Lal, R.; Ebinger, M.H.; Eaton, J.A. Changes in soil carbon and nutrient pools along a chronosequence of poplar plantations in the Columbia Plateau, Oregon, USA. Agric. Ecosyst. Environ. 2007, 122, 325–339. [Google Scholar] [CrossRef]
- Atchada, C.C.; Zoffoun, A.G.; Akplo, T.M.; Azontonde, A.H.; Tente, A.B.; Djego, J.G. Modes d’utilisation des terres et stock de carbone organique du sol dans le bassin supérieur de Magou au Bénin. Int. J. Biol. Chem. Sci. 2018, 12, 2818–2829. [Google Scholar] [CrossRef]
- Lawrey, J.D. The relative decomposition potential of habitats variously affected by surface coal mining. Can. J. Bot. 1977, 55, 1544–1552. [Google Scholar] [CrossRef]
- Leye, E.; Ndiaye, M.; Ndiaye, F.; Diallo, B.; Sarr, A.; Diouf, M.; Diop, T. Effet de la mycorhization sur la croissance et le développement de Jatropha curcas L. J. Renew. Energy 2009, 12, 269–278. [Google Scholar]
- Anguessin, B.; Mapongmetsem, P.M.; Ibrahima, A.; Fawa, G. Effet de la fertilisation organique à base de litière foliaire de Jatropha curcas L. et Jatropha gossypifolia L. sur la culture de tomate (Lycopersicon esculentum Mill.) à Guider (Nord/Cameroun). Int. J. Biol. Chem. Sci. 2021, 15, 524–535. [Google Scholar] [CrossRef]
- Haynes, R.; Beare, M. Influence of six crop species on aggregate stability and some labile organic matter fractions. Soil Biol. Biochem. 1997, 29, 1647–1653. [Google Scholar] [CrossRef]
- Stäuble, N.I. Etude ethnobotanique des Euphorbiacées d’Afrique de l’Ouest. J. Ethnopharmacol. 1986, 16, 23–103. [Google Scholar] [CrossRef]
- Gbemavo, D.S.J.C.; Gnangle, P.C.; Azontonde, A.; Glele Kakai, R.L. Modélisation du stock de biomasse et dynamique de séquestration minérale et carbone de Jatopha curcas L. sous différent types de sol au Bénin. Ann. Sci. Agron. 2014, 18, 1–20. [Google Scholar]
- Paterson, E. Importance of rhizodeposition in the coupling of plant and microbial productivity. Eur. J. Soil Sci. 2003, 54, 741–750. [Google Scholar] [CrossRef]
- Chaudhary, D.R.; Saxena, J.; Lorenz, N.; Dick, L.K.; Dick, R.P. Microbial profiles of rhizosphere and bulk soil microbial communities of biofuel crops switchgrass (Panicum virgatum L.) and jatropha (Jatropha curcas L.). Appl. Environ. Soil Sci. 2012, 2012, 906864. [Google Scholar] [CrossRef]
- Koranda, M.; Schnecker, J.; Kaiser, C.; Fuchslueger, L.; Kitzler, B.; Stange, C.F.; Sessitsch, A.; Zechmeister-Boltenstern, S.; Richter, A. Microbial processes and community composition in the rhizosphere of European beech—The influence of plant C exudates. Soil Biol. Biochem. 2011, 43, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Studies on arbuscular mycorrhizal fungi associated with Jatropha curcas L. Mycorrhiza News 2006, 18, 12–14. [Google Scholar]
- Madhaiyan, M.; Peng, N.; Te Ngoh, S.; Hsin, C.; Lin, C.; Lin, F.; Reddy, C.; Yan, H.; Ji, L. Improvement of plant growth and seed yield in Jatropha curcas by a novel nitrogen-fixing root associated Enterobacter species. Biotechnol. Biofuels 2013, 6, 140. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Panwar, J.; Jha, P.N. Natural occurrence of Pseudomonas aeruginosa, a dominant cultivable diazotrophic endophytic bacterium colonizing Pennisetum glaucum (L.) R. Br. Appl. Soil Ecol. 2013, 64, 252–261. [Google Scholar] [CrossRef]
- Patel, D.; Saraf, M. Comparative study of different soil amendments and microbes for integrated nutrient management and growth promotion of Jatropha curcas. J. Plant Nutr. 2014, 37, 2209–2226. [Google Scholar] [CrossRef]
- Kumar, C.M.; Ghoshal, N. Variation in Soil physicochemical properties in dry tropics: Effect of land-use change. Archives 2017, 17, 1404–1410. [Google Scholar]
- Silva, E.; Alleoni, L.R.F.; Santos, S.; Magalhães, S.; Farnezi, M. Response of physic nut trees to liming of acidic soils. Commun. Soil Sci. Plant Anal. 2016, 47, 1023–1032. [Google Scholar] [CrossRef]
- Santos, A.A.; Agustini, J.A.; Maltoni, K.L.; Cassiolato, A.M. Addition of residues and reintroduction of microorganisms in Jatropha curcas cultivated in degraded soil. Rev. Bras. Eng. Agrícola E Ambient. 2016, 20, 372–377. [Google Scholar] [CrossRef]
- Butterly, C.R.; Wang, X.; Sale, P.; Li, G.; Tang, C. Liming effect of non-legume residues promotes the biological amelioration of soil acidity via nitrate uptake. Plant Soil 2021, 464, 63–73. [Google Scholar] [CrossRef]
- Ritchie, G.; Dolling, P. The role of organic matter in soil acidification. Aust. J. Soil Res. 1985, 23, 569–576. [Google Scholar] [CrossRef]
- Butterly, C.R.; Baldock, J.A.; Tang, C. The contribution of crop residues to changes in soil pH under field conditions. Plant Soil 2013, 366, 185–198. [Google Scholar] [CrossRef]
- Serpantié, G.; Ouattara, B. Fertilité et jachères en Afrique de l’Ouest. La Jachère En Afr. Trop. 2001, 2, 21–83. [Google Scholar]
- Medza Mve, S.D.; Mergeai, G.; Baudoin, J.-P.; Toussaint, A. Culture in vitro de Jatropha curcas L. Biotechnol. Agron. Société Environ. 2011, 15, 567–574. Available online: https://hdl.handle.net/2268/138817 (accessed on 18 September 2024).
- Jones, D.L.; Nguyen, C.; Finlay, R.D. Carbon flow in the rhizosphere: Carbon trading at the soil–root interface. Plant Soil 2009, 321, 5–33. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef]
- Boyer, J. L’aluminium échangeable: Incidences agronomiques, évaluation et correction de sa toxicité dans les sols tropicaux. Cah. ORSTOM Sér. Pédol. 1976, 14, 259–269. [Google Scholar]

| C | N | pH | ||||
|---|---|---|---|---|---|---|
| Source of Variation | F Value | Pr (>F) | F Value | Pr (>F) | F Value | Pr (>F) |
| 0–10 cm depth | ||||||
| D | 0.33 | 0.7201 | 0.80 | 0.4565 | 1.458 | 0.2458 |
| M | 2.11 | 0.1554 | 1.14 | 0.2920 | 0.003 | 0.9555 |
| P | 21.37 | 0.0001 | 3.08 | 0.0872 | 19.426 | 0.0001 |
| D × M | 0.30 | 0.7412 | 0.97 | 0.3886 | 0.106 | 0.9002 |
| D × P | 0.36 | 0.6976 | 0.64 | 0.5347 | 1.614 | 0.2134 |
| M × P | 0.09 | 0.7647 | 0.74 | 0.3943 | 0.177 | 0.6758 |
| D × M × P | 0.05 | 0.9549 | 0.86 | 0.4333 | 0.147 | 0.8642 |
| 10–20 cm depth | ||||||
| D | 0.517 | 0.6010 | 0.890 | 0.4185 | 0.934 | 0.4024 |
| M | 1.720 | 0.1975 | 0.930 | 0.3413 | 6.928 | 0.0123 |
| P | 47.446 | 0.0001 | 2.628 | 0.1142 | 54.206 | 0.0001 |
| D × M | 0.032 | 0.9688 | 1.074 | 0.3523 | 2.263 | 0.1184 |
| D × P | 0.122 | 0.8845 | 0.921 | 0.4068 | 1.754 | 0.1875 |
| M × P | 0.096 | 0.7588 | 0.772 | 0.3863 | 0.688 | 0.4121 |
| D × M × P | 0.032 | 0.9687 | 1.012 | 0.3732 | 0.854 | 0.4343 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massoukou Pamba, R.; Poirier, V.; Nguema Ndoutoumou, P.; Epule, T.E. Growing Jatropha curcas L. Improves the Chemical Characteristics of Degraded Tropical Soils. Forests 2024, 15, 1709. https://doi.org/10.3390/f15101709
Massoukou Pamba R, Poirier V, Nguema Ndoutoumou P, Epule TE. Growing Jatropha curcas L. Improves the Chemical Characteristics of Degraded Tropical Soils. Forests. 2024; 15(10):1709. https://doi.org/10.3390/f15101709
Chicago/Turabian StyleMassoukou Pamba, Renaud, Vincent Poirier, Pamphile Nguema Ndoutoumou, and Terence Epule Epule. 2024. "Growing Jatropha curcas L. Improves the Chemical Characteristics of Degraded Tropical Soils" Forests 15, no. 10: 1709. https://doi.org/10.3390/f15101709








