Genome-Wide Identification of the Auxin Response Factor Gene Family in Maple (Acer truncatum) and Transcriptional Expression Analysis at Different Coloration Stages of Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Sampling
2.2. Total RNA Extraction
2.3. Library Construction and On-Board Sequencing
2.4. Identification and Bioinformatics Analysis of the AtARF Gene Family
2.5. Phylogenetic Evolutionary Tree and Cis-Acting Elements on the AtARFs
2.6. Chromosome Distribution and Collinearity Analysis of the AtARF Gene Family
2.7. qRT-PCR Analysis
3. Results
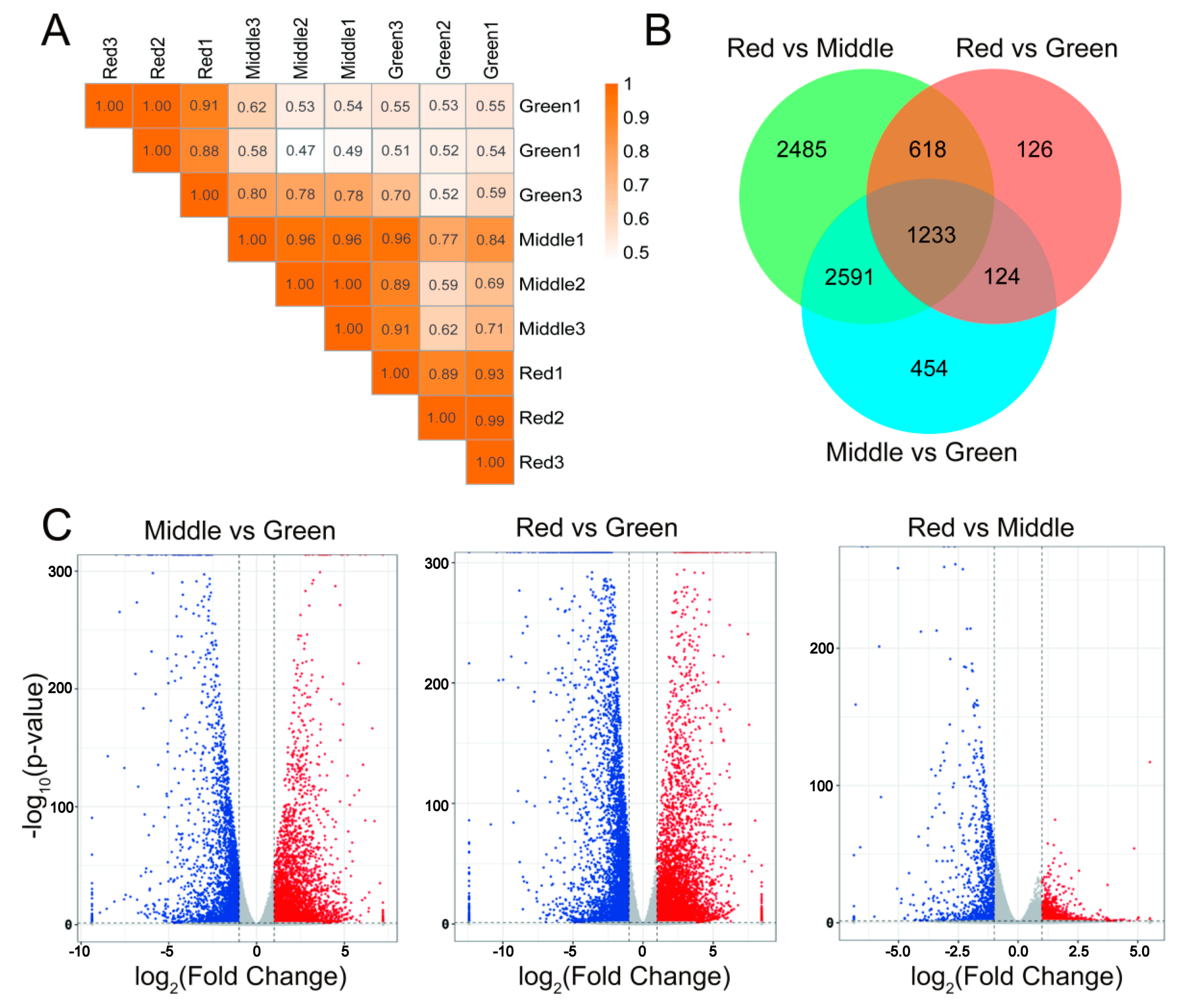
3.1. Full Transcriptome Data Construction
3.2. Transcriptome Results Analysis
3.3. Functional Annotation Analysis
3.4. Identification and Analysis of the AtARF Gene Family
3.5. Protein Structure Analysis of AtARF Gene Family
3.6. Analysis of the Gene Structure and Conserved Protein Sequence of AtARF Gene Family
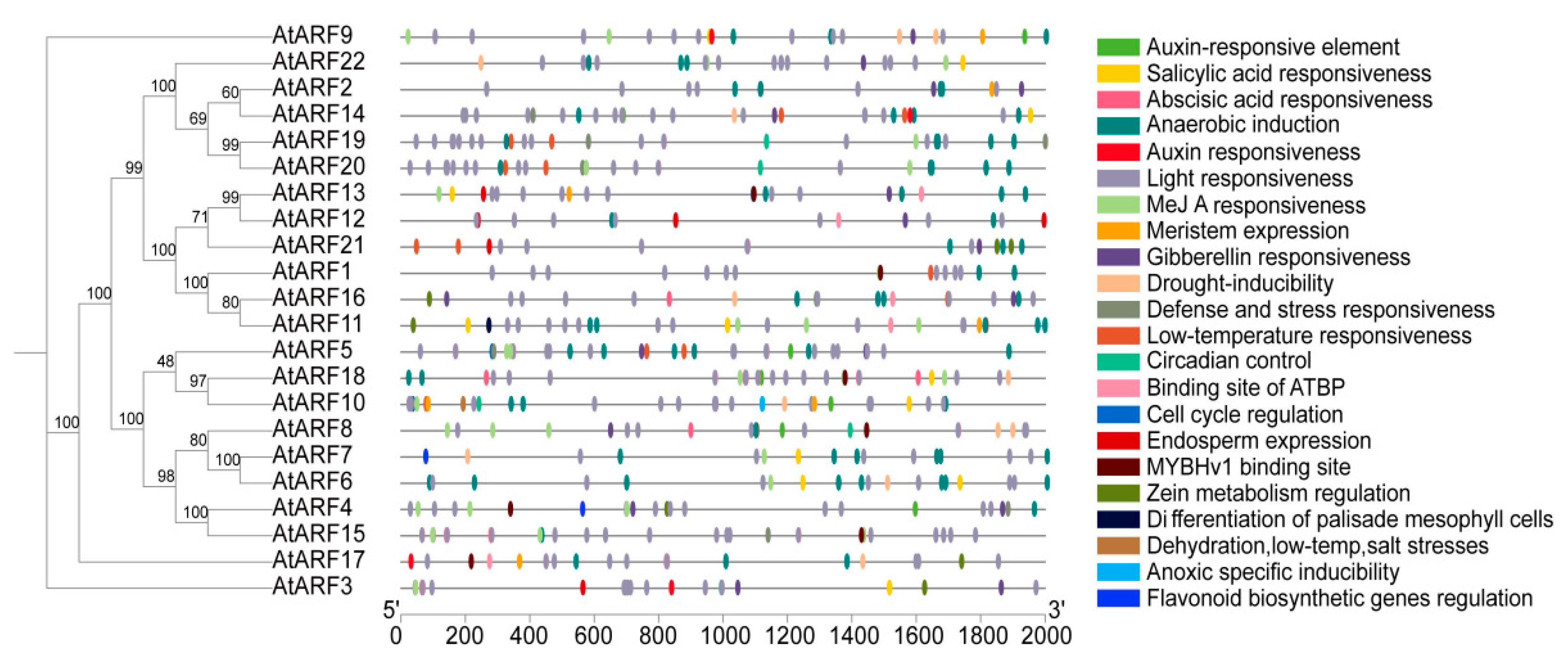
3.7. Identification of Cis-Acting Elements of the AtARF Gene Family
3.8. Chromosomal Location and Gene Collinearity Analysis of the AtARF Gene Family
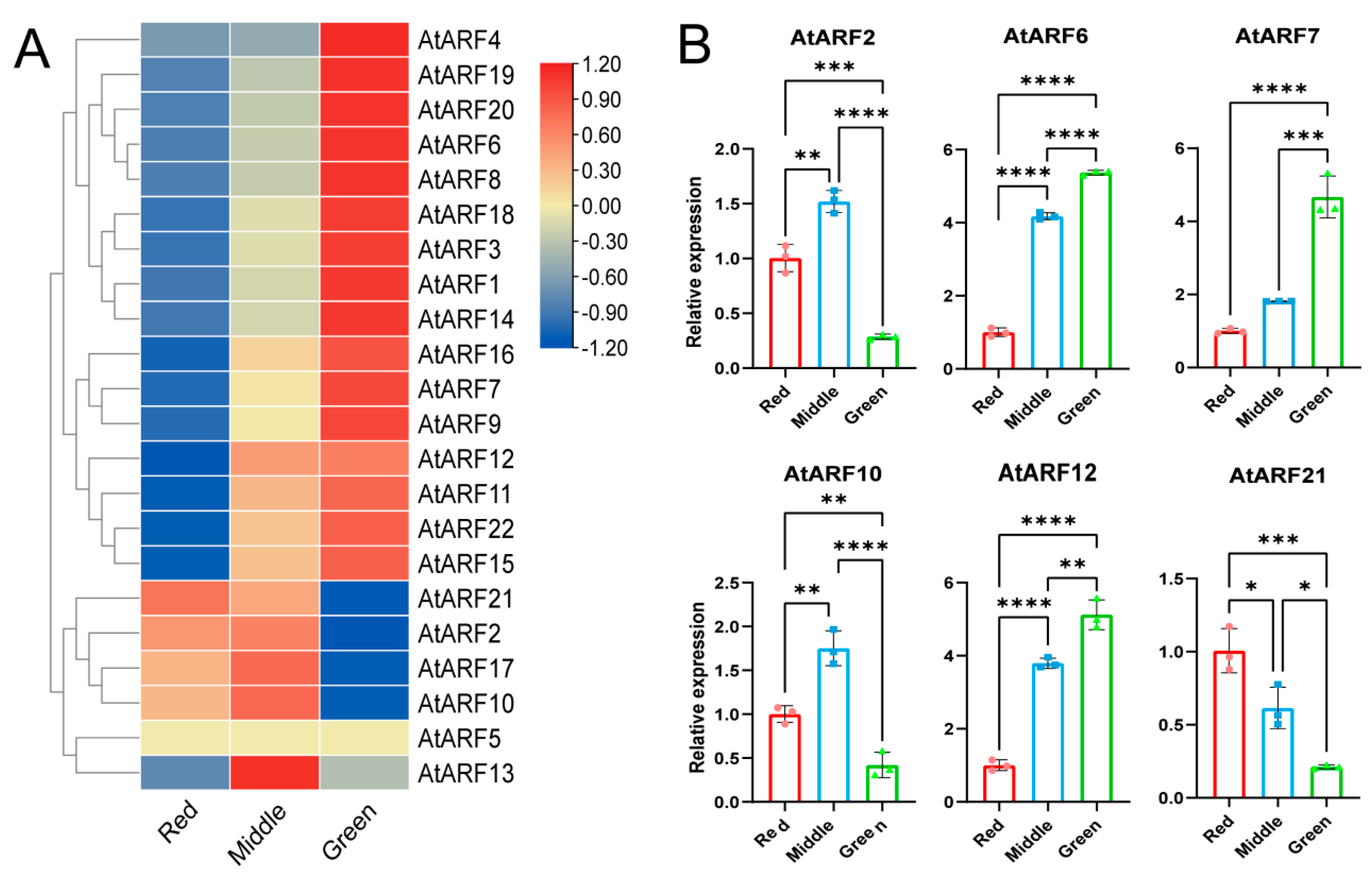
3.9. Expression Analysis of the AtARF Gene Family
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Archetti, M.; Döring, T.F.; Hagen, S.B.; Hughes, N.M.; Leather, S.R.; Lee, D.W.; Lev-Yadun, S.; Manetas, Y.; Ougham, H.J.; Schaberg, P.G.; et al. Unravelling the evolution of autumn colours: An interdisciplinary approach. Trends Ecol. Evol. 2009, 24, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.Y.; Hao, P.Y.; Dong, L. Color characteristics of Beijing’s regional woody vegetation based on Natural Color System. Color. Res. Appl. 2019, 44, 595–612. [Google Scholar] [CrossRef]
- Li, C.-F.; Xu, Y.-X.; Ma, J.-Q.; Jin, J.-Q.; Huang, D.-J.; Yao, M.-Z.; Ma, C.-L.; Chen, L. Biochemical and transcriptomic analyses reveal different metabolite biosynthesis profiles among three color and developmental stages in ‘Anji Baicha’ (Camellia sinensis). BMC Plant Biol. 2016, 16, 195. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Klaering, H.-P.; Kroh, L.W.; Krumbein, A. Cool-cultivated red leaf lettuce accumulates cyanidin-3-O-(6″-O-malonyl)-glucoside and caffeoylmalic acid. Food Chem. 2014, 146, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Lev-Yadun, S.; Yamazaki, K.; Holopainen, J.K.; Sinkkonen, A. Spring versus autumn leaf colours: Evidence for different selective agents and evolution in various species and floras. Flora—Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 80–85. [Google Scholar] [CrossRef]
- Primka, E.J.; Smith, W.K. Synchrony in fall leaf drop: Chlorophyll degradation, color change, and abscission layer formation in three temperate deciduous tree species. Am. J. Bot. 2019, 106, 377–388. [Google Scholar] [CrossRef]
- Junker, L.V.; Ensminger, I.; Niinemets, Ü. Relationship between leaf optical properties, chlorophyll fluorescence and pigment changes in senescing Acer saccharumleaves. Tree Physiol. 2016, 36, 694–711. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, N.; Zhang, Q.; Pei, Z.; Chang, M.; Zhou, H.; Ge, Y.; Yang, Q.; Li, G. Anthocyanin Biosynthesis Associated with Natural Variation in Autumn Leaf Coloration in Quercus aliena Accessions. Int. J. Mol. Sci. 2022, 23, 12179. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, Q.; Yu, Y.; Feng, J.; Liu, C.; Yang, J. Leaf Coloration in Acer palmatum Is Associated with a Positive Regulator ApMYB1 with Potential for Breeding Color-Leafed Plants. Plants 2022, 11, 759. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Feng, S.; Yan, S.; Li, Y.; Huang, Y.; Yang, M. Combined whole transcriptome analysis and physical-chemical reveals the leaf color change mechanism of Ulmus pulima under heat stress. Environ. Exp. Bot. 2023, 210, 105347. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, Q.; Yan, K.; Wang, X.; Xu, P. Transcriptomic and metabolomic analyses reveal how girdling promotes leaf color expression in Acer rubrum L. BMC Plant Biol. 2022, 22, 498. [Google Scholar] [CrossRef]
- Li, W.; Wang, L.; He, Z.; Lu, Z.; Cui, J.; Xu, N.; Jin, B.; Wang, L. Physiological and Transcriptomic Changes during Autumn Coloration and Senescence in Ginkgo biloba Leaves. Hortic. Plant J. 2020, 6, 396–408. [Google Scholar] [CrossRef]
- Lo Piccolo, E.; Landi, M.; Massai, R.; Remorini, D.; Guidi, L. Girled-induced anthocyanin accumulation in red-leafed Prunus cerasifera: Effect on photosynthesis, photoprotection and sugar metabolism. Plant Sci. 2020, 294, 110456. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, P.; Zheng, Y.; Gu, M.; Lin, X.; Wang, S.; Jin, S.; Ye, N. Comparison of Metabolome and Transcriptome of Flavonoid Biosynthesis Pathway in a Purple-Leaf Tea Germplasm Jinmingzao and a Green-Leaf Tea Germplasm Huangdan reveals Their Relationship with Genetic Mechanisms of Color Formation. Int. J. Mol. Sci. 2020, 21, 4167. [Google Scholar] [CrossRef]
- Guo, P.; Huang, Z.; Zhao, W.; Lin, N.; Wang, Y.; Shang, F. Mechanisms for leaf color changes in Osmanthus fragrans ‘Ziyan Gongzhu’ using physiology, transcriptomics and metabolomics. BMC Plant Biol. 2023, 23, 453. [Google Scholar] [CrossRef]
- Perico, C.; Tan, S.; Langdale, J.A. Developmental regulation of leaf venation patterns: Monocot versus eudicots and the role of auxin. New Phytol. 2022, 234, 783–803. [Google Scholar] [CrossRef]
- Verma, S.; Attuluri, V.P.S.; Robert, H.S. An Essential Function for Auxin in Embryo Development. Cold Spring Harb. Perspect. Biol. 2021, 13, a039966. [Google Scholar] [CrossRef]
- Cavallari, N.; Artner, C.; Benkova, E. Auxin-Regulated Lateral Root Organogenesis. Cold Spring Harb. Perspect. Biol. 2021, 13, a039941. [Google Scholar] [CrossRef]
- Goldental-Cohen, S.; Israeli, A.; Ori, N.; Yasuor, H. Auxin Response Dynamics During Wild-Type and entire Flower Development in Tomato. Plant Cell Physiol. 2017, 58, 1661–1672. [Google Scholar] [CrossRef]
- Luo, W.-G.; Liang, Q.-W.; Su, Y.; Huang, C.; Mo, B.-X.; Yu, Y.; Xiao, L.-T. Auxin inhibits chlorophyll accumulation through ARF7-IAA14-mediated repression of chlorophyll biosynthesis genes in Arabidopsis. Front. Plant Sci. 2023, 14, 1172059. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, X.; Gong, Z.; Tang, Y.; Wu, M.; Yan, F.; Zhang, X.; Zhang, Q.; Yang, F.; Hu, X.; et al. Auxin response factor 6A regulates photosynthesis, sugar accumulation, and fruit development in tomato. Hortic. Res. 2019, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-K.; Han, P.-L.; Zhao, Y.-W.; Yu, J.-Q.; You, C.-X.; Hu, D.-G.; Hao, Y.-J. Genome-wide analysis of auxin response factor (ARF) genes and functional identification of MdARF2 reveals the involvement in the regulation of anthocyanin accumulation in apple. N. Z. J. Crop Hortic. Sci. 2020, 49, 78–91. [Google Scholar] [CrossRef]
- Maritim, T.K.; Masand, M.; Seth, R.; Sharma, R.K. Transcriptional analysis reveals key insights into seasonal induced anthocyanin degradation and leaf color transition in purple tea (Camellia sinensis (L.) O. Kuntze). Sci. Rep. 2021, 11, 1244. [Google Scholar] [CrossRef]
- Duan, W.; Li, X.; Li, Q.; Jing, R.; Zhang, S.; Zhang, B.; Hamel, C.; Sheng, M. Arbuscular mycorrhizal fungal community association determines the production of flavonoids and chlorogenic acid in Acer truncatum Bunge. Ind. Crops Prod. 2024, 208, 117858. [Google Scholar] [CrossRef]
- Fan, H.; Sun, L.; Yang, L.; Zhou, J.; Yin, P.; Li, K.; Xue, Q.; Li, X.; Liu, Y. Assessment of the bioactive phenolic composition of Acer truncatum seed coat as a byproduct of seed oil. Ind. Crops Prod. 2018, 118, 11–19. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, W.; Yuan, F.; Liu, X.; Li, D.; Yang, K.Q. Characterization of yuanbaofeng (Acer truncatum Bunge) samaras: Oil, fatty acid, and phytosterol content. Ind. Crops Prod. 2019, 135, 344–351. [Google Scholar] [CrossRef]
- Gu, R.; Rybalov, L.; Negrin, A.; Morcol, T.; Long, W.; Myers, A.K.; Isaac, G.; Yuk, J.; Kennelly, E.J.; Long, C. Metabolic Profiling of Different Parts of Acer truncatum from the Mongolian Plateau Using UPLC-QTOF-MS with Comparative Bioactivity Assays. J. Agric. Food Chem. 2019, 67, 1585–1597. [Google Scholar] [CrossRef]
- Ma, Q.; Sun, T.; Li, S.; Wen, J.; Zhu, L.; Yin, T.; Yan, K.; Xu, X.; Li, S.; Mao, J.; et al. The Acer truncatum genome provides insights into nervonic acid biosynthesis. Plant J. 2020, 104, 662–678. [Google Scholar] [CrossRef]
- Zhai, Y.; Shen, X.; Sun, Y.; Liu, Q.; Ma, N.; Zhang, X.; Jia, Q.; Liang, Z.; Wang, D. Genome-wide investigation of ARF transcription factor gene family and its responses to abiotic stress in Coix (Coix lacryma-jobi L.). Protoplasma 2023, 260, 1389–1405. [Google Scholar] [CrossRef]
- Niu, X.; Guan, Y.; Chen, S.; Li, H. Genome-wide analysis of basic helix-loop-helix (bHLH) transcription factors in Brachypodium distachyon. BMC Genom. 2017, 18, 619. [Google Scholar] [CrossRef]
- Mo, Z.; Zhang, Y.; Hu, L.; Zhai, M.; Xuan, J. Genome-wide identification and expression analysis of auxin response factor (ARF) gene family in pecan indicates its possible roles during graft union formation. Sci. Hortic. 2023, 322, 112401. [Google Scholar] [CrossRef]
- Mei, M.; Ai, W.; Liu, L.; Xu, X.; Lu, X. Genome-wide identification of the auxin response factor (ARF) gene family in Magnolia sieboldii and functional analysis of MsARF5. Front. Plant Sci. 2022, 13, 958816. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, L.; Li, W.; Dang, Z.; Xie, Y.; Zhao, W.; Zhao, L.; Li, W.; Yang, C.; Xu, C.; et al. Genome-Wide Identification and Expression Analysis of Auxin Response Factor Gene Family in Linum usitatissimum. Int. J. Mol. Sci. 2023, 24, 11006. [Google Scholar] [CrossRef]
- Okushima, Y.; Overvoorde, P.J.; Arima, K.; Alonso, J.M.; Chan, A.; Chang, C.; Ecker, J.R.; Hughes, B.; Lui, A.; Nguyen, D.; et al. Functional Genomic Analysis of theAUXIN RESPONSE FACTORGene Family Members in Arabidopsis thaliana: Unique and Overlapping Functions ofARF7andARF19. Plant Cell 2005, 17, 444–463. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, F.; Zhu, Y.; Lan, D.; Yan, P.; Wang, Y.; Hu, Z.; Zhang, X.; Hu, J.; Niu, F.; et al. Auxin signaling module OsSK41-OsIAA10-OsARF regulates grain yield traits in rice. J. Integr. Plant Biol. 2023, 65, 1753–1766. [Google Scholar] [CrossRef]
- Suri, G.S.; Tiwari, M. Frontline Warrior microRNA167: A Battle of Survival. Int. J. Plant Biol. 2022, 13, 598–600. [Google Scholar] [CrossRef]
- Gambhir, P.; Singh, V.; Parida, A.; Raghuvanshi, U.; Kumar, R.; Sharma, A.K. Ethylene response factor ERF.D7 activates auxin response factor 2 paralogs to regulate tomato fruit ripening. Plant Physiol. 2022, 190, 2775–2796. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Wang, L.; Li, W.; Zhao, L.; Huang, X.; Azam, S.M.; Qin, Y. Genome-Wide Identification of Auxin Response Factor (ARF) Genes Family and its Tissue-Specific Prominent Expression in Pineapple (Ananas comosus). Trop. Plant Biol. 2017, 10, 86–96. [Google Scholar] [CrossRef]
- Feng, X.; Gao, G.; Yu, C.; Zhu, A.; Chen, J.; Chen, K.; Wang, X.; Abubakar, A.S.; Chen, P. Transcriptome and metabolome analysis reveals anthocyanin biosynthesis pathway associated with ramie (Boehmeria nivea (L.) Gaud.) leaf color formation. BMC Genom. 2021, 22, 684. [Google Scholar] [CrossRef]
- Zou, J.; Gong, Z.; Liu, Z.; Ren, J.; Feng, H. Investigation of the Key Genes Associated with Anthocyanin Accumulation during Inner Leaf Reddening in Ornamental Kale (Brassica oleracea L. var. acephala). Int. J. Mol. Sci. 2023, 24, 2837. [Google Scholar] [CrossRef]
- Arpita, K.; Sharma, S.; Srivastava, H.; Kumar, K.; Mushtaq, M.; Gupta, P.; Jain, R.; Gaikwad, K. Genome-wide survey, molecular evolution and expression analysis of Auxin Response Factor (ARF) gene family indicating their key role in seed number per pod in pigeonpea (C. cajan L. Millsp.). Int. J. Biol. Macromol. 2023, 253, 126833. [Google Scholar] [CrossRef]
- Chen, G.; Yue, Y.; Li, L.; Li, Y.; Li, H.; Ding, W.; Shi, T.; Yang, X.; Wang, L. Genome-Wide Identification of the Auxin Response Factor (ARF) Gene Family and Their Expression Analysis during Flower Development of Osmanthus fragrans. Forests 2020, 11, 245. [Google Scholar] [CrossRef]
- Shen, C.; Yue, R.; Sun, T.; Zhang, L.; Xu, L.; Tie, S.; Wang, H.; Yang, Y. Genome-wide identification and expression analysis of auxin response factor gene family in Medicago truncatula. Front. Plant Sci. 2015, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, U.C.; DiFazio, S.P.; Brunner, A.M.; Tuskan, G.A. Genome-wide analysis of Aux/IAA and ARF gene families in Populus trichocarpa. BMC Plant Biol. 2007, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, H.; Chen, W.; Qian, Y.; Ma, Q.; Cheng, B.; Zhu, S. Genome-wide analysis of the auxin response factor (ARF) gene family in maize (Zea mays). Plant Growth Regul. 2010, 63, 225–234. [Google Scholar] [CrossRef]
- Babenko, V.N. Prevalence of intron gain over intron loss in the evolution of paralogous gene families. Nucleic Acids Res. 2004, 32, 3724–3733. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Fang, T.; Zhang, Y.; Zhang, M.; Zeng, L. Genome-Wide Identification and Expression Analysis of Auxin Response Factor (ARF) Gene Family in Longan (Dimocarpus longan L.). Plants 2020, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Roosjen, M.; Paque, S.; Weijers, D. Auxin Response Factors: Output control in auxin biology. J. Exp. Bot. 2018, 69, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, R.; Yu, J.; Huang, S.; Zhang, Y.; Wei, H.; Wei, Z. Genome-Wide Identification and Characterization of Auxin Response Factor (ARF) Gene Family Involved in Wood Formation and Response to Exogenous Hormone Treatment in Populus trichocarpa. Int. J. Mol. Sci. 2023, 24, 740. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Quan, W.; Ding, G. Effects of PmaIAA27 and PmaARF15 genes on drought stress tolerance in pinus massoniana. BMC Plant Biol. 2023, 23, 478. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, X.; Lu, J.; Song, F.; Sun, J.; Wang, C.; Lian, J.; Zhao, L.; Zhao, B. OsIAA20, an Aux/IAA protein, mediates abiotic stress tolerance in rice through an ABA pathway. Plant Sci. 2021, 308, 110903. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, Y.; Zhang, S.; Shui, D.; Xia, Z.; Sun, J. Genome-wide identification and expression analysis of the AUX/IAA gene family in turnip (Brassica rapa ssp. rapa). BMC Plant Biol. 2023, 23, 342. [Google Scholar] [CrossRef] [PubMed]
- Aerts, N.; Pereira Mendes, M.; Van Wees, S.C.M. Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J. 2020, 105, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Xia, Y.; Su, X.; Pang, Y. ARF2 positively regulates flavonols and proanthocyanidins biosynthesis in Arabidopsis thaliana. Planta 2022, 256, 44. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Liu, Z.Y.; Wang, X.N.; Han, Y.; You, C.X.; An, J.P. E3 ubiquitin ligases SINA4 and SINA11 regulate anthocyanin biosynthesis by targeting the IAA29-ARF5-1-ERF3 module in apple. Plant Cell Environ. 2023, 46, 3902–3918. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.; Vivian-Smith, A.; Johnson, S.D.; Koltunow, A.M. AUXIN RESPONSE FACTOR8 Is a Negative Regulator of Fruit Initiation in Arabidopsis. Plant Cell 2006, 18, 1873–1886. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, T.; Sun, Y.; Yu, R.; Liu, J.; Zhang, X.; Gong, Z. Genome-Wide Identification of the Auxin Response Factor Gene Family in Maple (Acer truncatum) and Transcriptional Expression Analysis at Different Coloration Stages of Leaves. Forests 2024, 15, 1723. https://doi.org/10.3390/f15101723
Qu T, Sun Y, Yu R, Liu J, Zhang X, Gong Z. Genome-Wide Identification of the Auxin Response Factor Gene Family in Maple (Acer truncatum) and Transcriptional Expression Analysis at Different Coloration Stages of Leaves. Forests. 2024; 15(10):1723. https://doi.org/10.3390/f15101723
Chicago/Turabian StyleQu, Tongbao, Yinglun Sun, Ran Yu, Jian Liu, Xinyu Zhang, and Zaixin Gong. 2024. "Genome-Wide Identification of the Auxin Response Factor Gene Family in Maple (Acer truncatum) and Transcriptional Expression Analysis at Different Coloration Stages of Leaves" Forests 15, no. 10: 1723. https://doi.org/10.3390/f15101723




