Altitudinal Variation in Soil Acid Phosphomonoesterase Activity in Subalpine Coniferous Forests in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Sampling and Sample Preparation
2.3. Analytical Methods
2.4. Statistical Analyses
3. Results
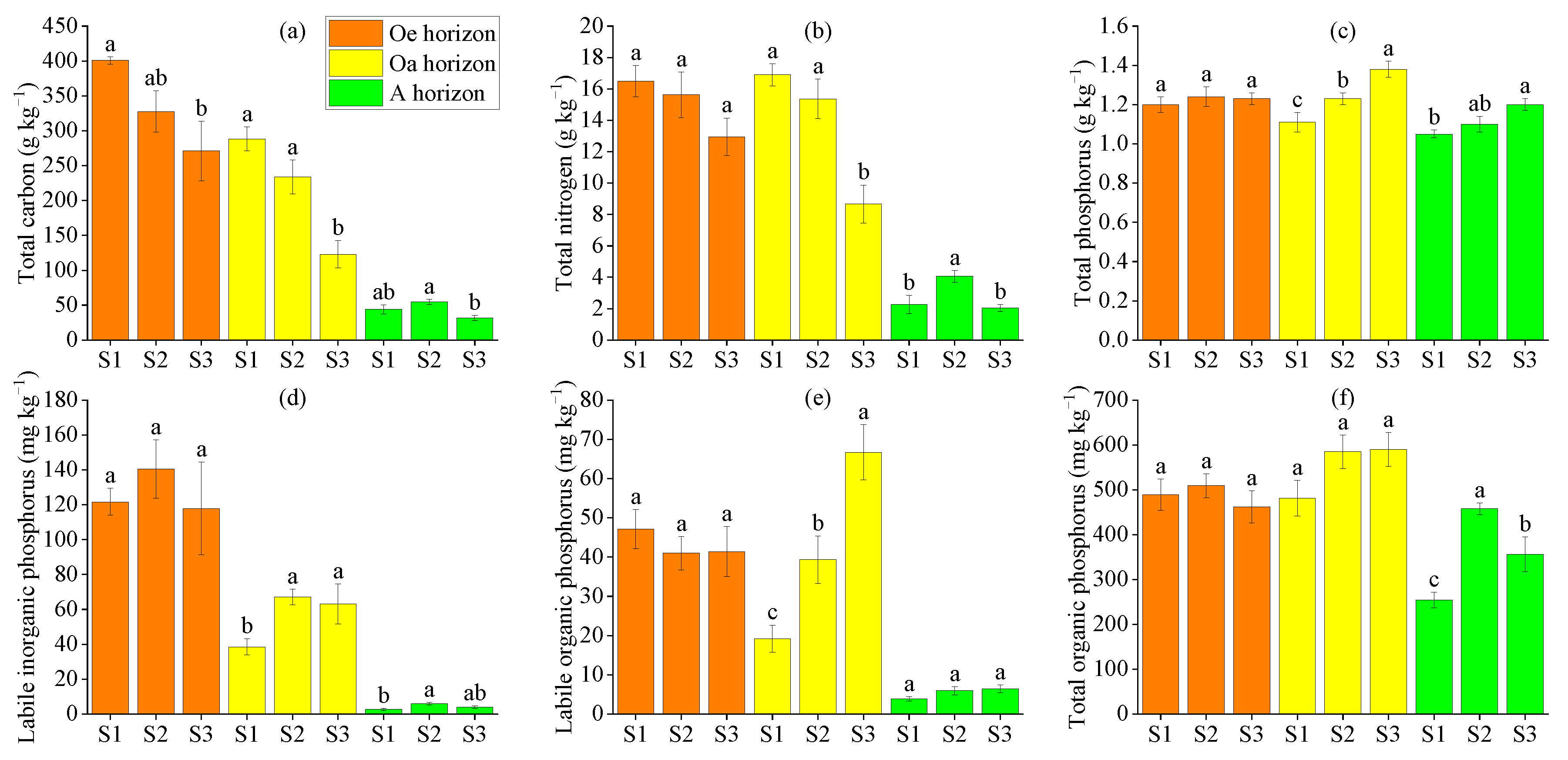
3.1. ACP Activity and Some Other Soil Properties
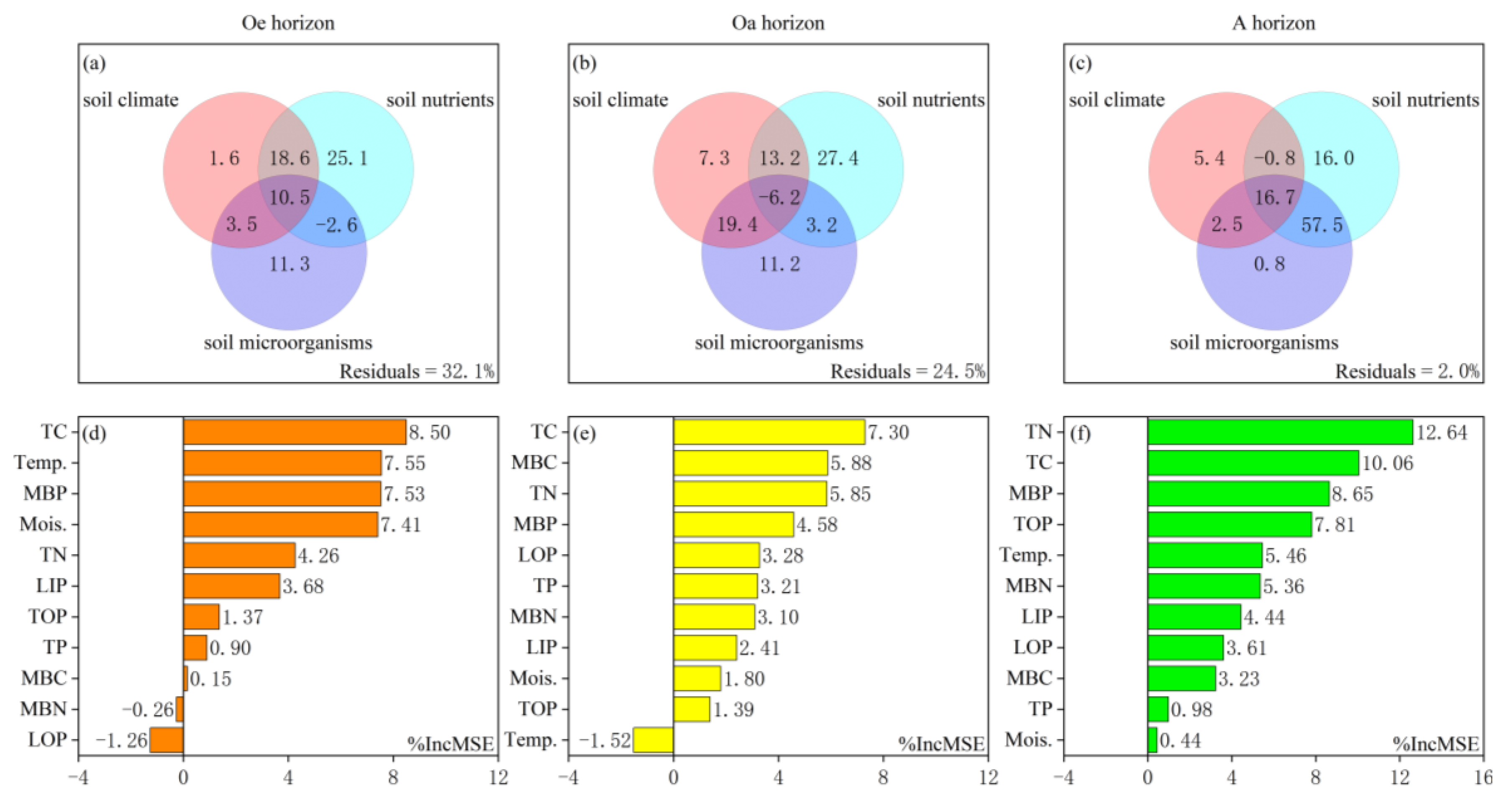
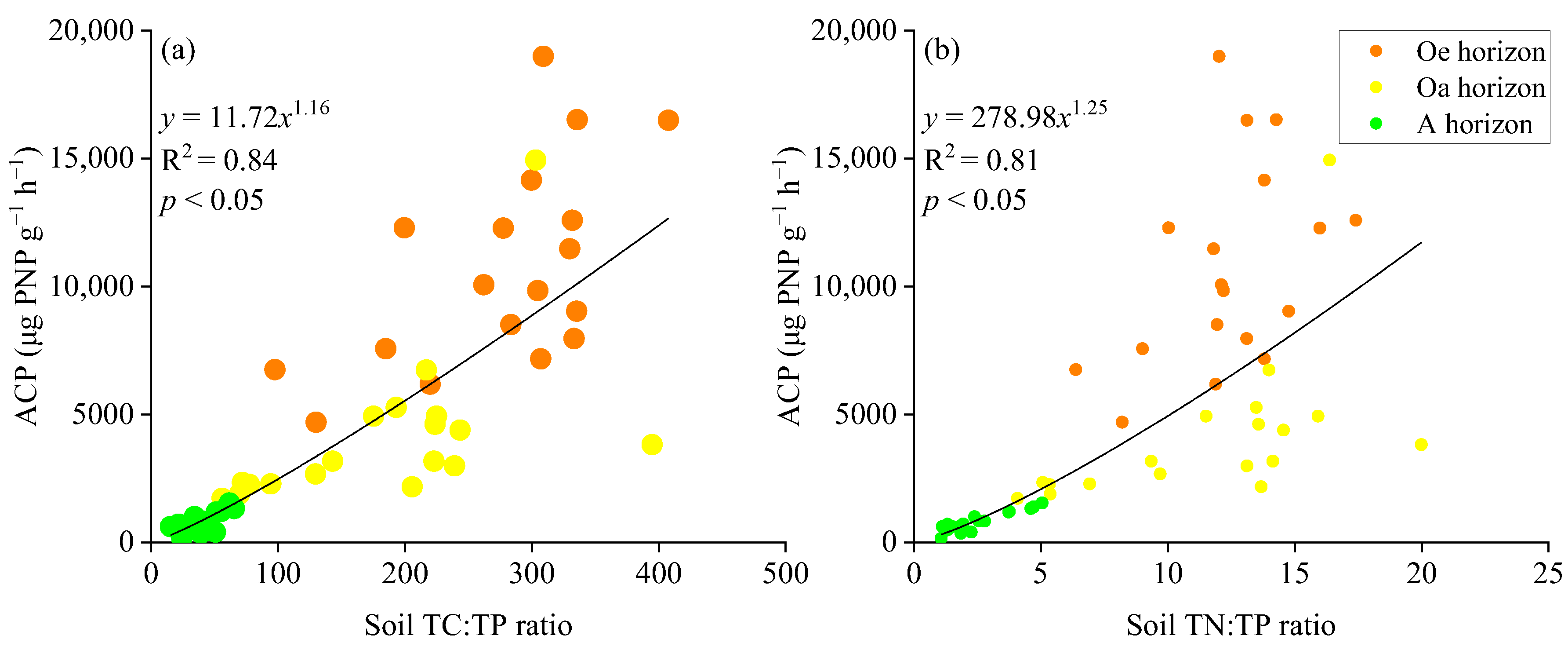
3.2. Impacts of Soil Properties on the ACP Activity
4. Discussion
4.1. Variation in Soil ACP Activity among Sites and Horizons
4.2. Drivers of Spatial Variation in Soil ACP Activity
4.2.1. Soil Climate
4.2.2. Soil Phosphorus Fractions
4.2.3. Soil TC and TN
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, M.; Qin, H.; Liang, Y.; Xiao, D.; Yan, P.; Yin, M.; Pan, F. The phoD-Harboring Microorganism Communities and Networks in Karst and Non-Karst Forests in Southwest China. Forests 2024, 15, 341. [Google Scholar] [CrossRef]
- Doolette, A.; Smernik, R.; McLaren, T. The Composition of Organic Phosphorus in Soils of the Snowy Mountains Region of South-Eastern Australia. Soil Res. 2017, 55, 10–18. [Google Scholar] [CrossRef]
- Prietzel, J.; Klysubun, W.; Werner, F. Speciation of Phosphorus in Temperate Zone Forest Soils As Assessed by Combined Wet-Chemical Fractionation and Xanes Spectroscopy. J. Plant Nutr. Soil Sci. 2016, 179, 168–185. [Google Scholar] [CrossRef]
- Li, M.; He, X.; Zhang, P.; Wang, R.; Wang, J.; Zhang, X.; Yin, H. Close Linkage Between Available and Microbial Biomass Phosphorus in the Rhizosphere of Alpine Coniferous Forests Along an Altitudinal Gradient. Rhizosphere 2024, 30, 100904. [Google Scholar] [CrossRef]
- Fetzer, J.; Loeppmann, S.; Frossard, E.; Manzoor, A.; Brödlin, D.; Kaiser, K.; Hagedorn, F. Leaching of Phosphomonoesterase Activities in Beech Forest Soils: Consequences for Phosphorus Forms and Mobility. Front. For. Glob. Chang. 2021, 4, 684069. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Xia, Y.; Cui, Z.; Cao, C. Soil Microbial Community Succession Based on PhoD and Gcd Genes along a Chronosequence of Sand-Fixation Forest. Forests 2021, 12, 1707. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Z.; Man, X.; Gao, M.; Zhao, J. Characteristics of Soil Enzyme Activity in Different Age Betula platyphylla Forests in the Cold Temperate Permafrost Region. J. Northeast. For. Univ. 2024, 52, 125–131. [Google Scholar]
- García-Saucedo, F.; García-Morote, F.A.; Picazo, M.; Wic, C.; Rubio, E.; López-Serrano, F.R.; Andrés-Abellán, M. Responses of Enzymatic and Microbiological Soil Properties to the Site Index and Age Gradients in Spanish Black Pine (Pinusnigra Arn ssp. salzmannii) Mediterranean Forests. Forests 2024, 15, 113. [Google Scholar] [CrossRef]
- Lucas-Borja, M.; Candel Pérez, D.; López-Serrano, F.; Andrés, M.; Bastida, F. Altitude-Related Factors but Not Pinus Community Exert a Dominant Role over Chemical and Microbiological Properties of a Mediterranean Humid Soil. Eur. J. Soil Sci. 2012, 63, 541–549. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, H.; Li, X.; Ren, Y.; Jin, C.; Xu, Z.; Lv, M.; Xie, J. Characteristics of Soil Organic Carbon Mineralization in Low Altitude and High Altitude Forests in Wuyi Mountains, Southeastern China. Chin. J. Appl. Ecol. 2018, 29, 748–756. [Google Scholar]
- Hofmann, K.; Lamprecht, A.; Pauli, H.; Illmer, P. Distribution of Prokaryotic Abundance and Microbial Nutrient Cycling Across a High-Alpine Altitudinal Gradient in the Austrian Central Alps is Affected By Vegetation, Temperature, and Soil Nutrients. Microb. Ecol. 2016, 72, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Wu, F.; Yang, W.; Xu, Z.; Tan, B.; Wang, B.; Li, J.; Chang, C. Effects of Altitudes on Soil Microbial Biomass and Enzyme Activity in Alpine-Gorge Regions. Chin. J. Appl. Ecol. 2016, 27, 1257–1264. [Google Scholar]
- He, Q.; Wu, Y.; Bing, H.; Zhou, J.; Wang, J. Vegetation Type Rather Than Climate Modulates the Variation in Soil Enzyme Activities and Stoichiometry in Subalpine Forests in the Eastern Tibetan Plateau. Geoderma 2020, 374, 114424. [Google Scholar] [CrossRef]
- Bach, L.; Grytnes, J.; Halvorsen, R.; Ohlson, M. Tree Influence on Soil Microbial Community Structure. Soil Biol. Biochem. 2010, 42, 1934–1943. [Google Scholar] [CrossRef]
- Yang, R.; Liu, S.; Wang, Z.; Cao, Y.; Zhao, Y.; He, W.; Geng, Z. Relationships Between the Soil Enzyme Activity and Soil Nutrients in Forest Soils Typical of the Qinling Mountain. Acta Pedol. Sin. 2016, 53, 1037–1046. [Google Scholar]
- Kunito, T.; Tobitani, T.; Moro, H.; Toda, H. Phosphorus Limitation in Microorganisms Leads to High Phosphomonoesterase Activity in Acid Forest Soils. Pedobiologia 2012, 55, 263–270. [Google Scholar] [CrossRef]
- Liu, J.; Chen, G.; Guo, J.; Yang, Z.; Li, Y.; Lin, C.; Yang, Y. Advances in Research on the Responses of Forest Soil Enzymes to Environmental Change. Acta Ecol. Sin. 2017, 37, 110–117. [Google Scholar]
- Feng, R.; Yang, W.; Zhang, J.; Deng, R.; Jian, Y.; Lin, J. Effects of Simulated Elevated Atmospheric Co2 Concentration and Temperature on Soil Enzyme Activity in the Subalpine Fir Forest. Acta Ecol. Sin. 2007, 27, 4019–4026. [Google Scholar]
- Zuccarini, P.; Asensio, D.; Ogaya, R.; Sardans, J.; Peuelas, J. Effects of Seasonal and Decadal Warming on Soil Enzymatic Activity in a P-Deficient Mediterranean Shrubland. Glob. Chang. Biol. 2020, 26, 3698–3714. [Google Scholar] [CrossRef]
- Olander, L.P.; Vitousek, P.M. Regulation of Soil Phosphatase and Chitinase Activity by N and P Availability. Biogeochemistry 2000, 49, 175–191. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Follstad Shah, J.J. Ecoenzymatic Stoichiometry and Ecological Theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 313–343. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Responses of Extracellular Enzymes to Simple and Complex Nutrient Inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Marklein, A.R.; Houlton, B.Z. Nitrogen Inputs Accelerate Phosphorus Cycling Rates Across a Wide Variety of Terrestrial Ecosystems. New Phytol. 2012, 193, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Naples, B.K.; Fisk, M.C. Belowground Insights into Nutrient Limitation in Northern Hardwood Forests. Biogeochemistry 2010, 97, 109–121. [Google Scholar] [CrossRef]
- Tarafdar, J.; Claassen, N. Organic Phosphorus Compounds As a Phosphorus Source for Higher Plants Through the Activity of Phosphatases Produced By Plant Roots and Microorganisms. Biol. Fertel. Soils 1988, 5, 308–312. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C. Stoichiometry of Soil Enzyme Activity at Global Scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- He, X.; Zhou, J.; Wu, Y.; Bing, H.; Sun, H.; Wang, J. Leaching Disturbed the Altitudinal Distribution of Soil Organic Phosphorus in Subalpine Coniferous Forests on Mt. Gongga, SW China. Geoderma 2018, 326, 144–155. [Google Scholar] [CrossRef]
- Yu, D. Vertical Zonal Characteristics of Soil in Gongga Mountain. Chin. J. Soil Sci. 1984, 15, 65–68. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Report No. 106; FAO: Rome, Italy, 2014; p. 156. [Google Scholar]
- Wang, J.; Huang, S.; He, Q.; Bing, H.; Chen, X.; Zhang, X.; Tian, X.; Zhou, J.; Wilcke, W.; Wu, Y. Microplate Fluorimetric Assay of Soil Leucine Aminopeptidase Activity: Alkalization is Not Needed before Fluorescence Reading. Biol. Fertil. Soils 2020, 56, 281–285. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Bowman, R.A.; Cole, C.V. An Exploratory Method for Fractionation of Organic Phosphorus from Grassland Soils. Soil Sci. 1978, 125, 95–101. [Google Scholar] [CrossRef]
- Sharpley, A.N.; Smith, S.J. Fractionation of Inorganic and Organic Phosphorus in Virgin and Cultivated Soils. Soil Sci. Soc. Am. J. 1985, 49, 127–130. [Google Scholar] [CrossRef]
- Ivanoff, D.B.; Reddy, K.R.; Robinson, S. Chemical Fractionation of Organic Phosphorus in Selected Histosols. Soil Sci. 1998, 163, 36–45. [Google Scholar] [CrossRef]
- Brookes, P.; Powlson, D.; Jenkinson, D. Measurement of Microbial Biomass Phosphorus in Soil. Soil Biol. Biochem. 1982, 14, 319–329. [Google Scholar] [CrossRef]
- Wu, J.; He, Z.; Wei, W.; O’Donnell, A.G.; Syers, J.K. Quantifying Microbial Biomass Phosphorus in Acid Soils. Biol. Fertil. Soils 2000, 32, 500–507. [Google Scholar] [CrossRef]
- Tabatabai, M. Soil enzymes. In Methods of Soil Analysis, Part 2: Microbiological and Biochemical Properties; Hart, S.C., Stark, J.M., Davidson, E.A., Firestone, M.K., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 775–833. [Google Scholar]
- Legendre, P. Studying Beta Diversity: Ecological Variati on Partitioning by Multiple Regression and Canonical Analysis. J. Plant Ecol. 2007, 31, 976–981. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Zhou, J.; Bing, H.; Sun, H. Carbon Demand Drives Microbial Mineralization of Organic Phosphorus During the Early Stage of Soil Development. Biol. Fertil. Soils 2016, 52, 825–839. [Google Scholar] [CrossRef]
- Hou, E.; Chen, C.; Wen, D.; Liu, X. Phosphatase Activity in Relation to Key Litter and Soil Properties in Mature Subtropical Forests in China. Sci. Total Environ. 2015, 515–516, 83–91. [Google Scholar] [CrossRef]
- Chen, C.R.; Condron, L.; Davis, M.R.; Sherlock, R.R. Effects of Afforestation on Phosphorus Dynamics and Biological Properties in a New Zealand Grassland Soil. Plant Soil 2000, 220, 151–163. [Google Scholar] [CrossRef]
- Pang, P.C.K.; Kolenko, H. Phosphomonoesterase Activity in Forest Soils. Soil Biol. Biochem. 1986, 18, 35–39. [Google Scholar] [CrossRef]
- Adams, M.A. Phosphatase Activity and Phosphorus Fractions in Karri (Eucalyptus diversicolor F. Muell) forest soils. Biol. Fertil. Soils 1992, 14, 200–204. [Google Scholar] [CrossRef]
- Zhang, A.; Chen, Z.; Zhang, G.; Chen, L.; Wu, Z. Soil Phosphorus Composition Determined By 31P NMR Spectroscopy and Relative Phosphatase Activities Influenced by Land Use. Eur. J. Soil Biol. 2012, 52, 73–77. [Google Scholar] [CrossRef]
- Zornoza, R.; Mataix-Solera, J.; Guerrero, C.; Arcenegui, V.; Mayoral, A.; Morales, J.; Mataix-Beneyto, J. Soil Properties under Natural Forest in the Alicante Province of Spain. Geoderma 2007, 142, 334–341. [Google Scholar] [CrossRef]
- Grierson, P.; Adams, M. Plant Species Affect Acid Phosphatase, Ergosterol and Microbial P in a Jarrah (Eucalyptus marginata Donn ex Sm.) Forest in South-Western Australia. Soil Biol. Biochem. 2000, 32, 1817–1827. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Maspons, J.; Molowny-Horas, R.; Fernandez-Martinez, M.; Janssens, I.; Richter, A.; Ciais, P.; Obersteiner, M.; Penuelas, J. The Effect of Global Change on Soil Phosphatase Activity. Glob. Chang. Biol. 2021, 27, 5989–6003. [Google Scholar] [CrossRef]
- Kitayama, K. The Activities of Soil and Root Acid Phosphatase in the Nine Tropical Rain Forests That Differ in Phosphorus Availability on Mount Kinabalu, Borneo. Plant Soil 2013, 367, 215–224. [Google Scholar] [CrossRef]
- Yang, L.; Wang, G.; Yang, Y.; Cao, Y.; Li, W.; Guo, J. Dynamics of Litter Fall in Abies Fabric Mature Forest at Gongga Mountain. Acta Agric. Univ. Jiangxiensis 2010, 32, 1163–1167. [Google Scholar]
- Lin, H.; Zhou, J.; Zeng, Q.; Sun, J.; Xie, H.; Liu, Y.; Mei, K.; Wu, Y.; Yuan, X.; Wu, J.; et al. Soil Enzyme Stoichiometry Revealed the Changes of Soil Microbial Carbon and Phosphorus Limitation Along an Elevational Gradient in a Pinus Taiwanensis Forest of Wuyi Mountains, Southeast China. Chin. J. Appl. Ecol. 2022, 33, 33–41. [Google Scholar]
- Yuan, X.; Niu, D.; Gherardi, L.; Liu, Y.; Wang, Y.; Elser, J.; Fu, H. Linkages of Stoichiometric Imbalances to Soil Microbial Respiration with Increasing Nitrogen Addition: Evidence from a Long-Term Grassland Experiment. Soil Biol. Biochem. 2019, 138, 107580. [Google Scholar] [CrossRef]
- Bing, H.; Wu, Y.; Zhou, J.; Sun, H.; Luo, J.; Wang, J.; Yu, D. Stoichiometric Variation of Carbon, Nitrogen, and Phosphorus in Soils and Its Implication for Nutrient Limitation in Alpine Ecosystem of Eastern Tibetan Plateau. J. Soils Sediments 2016, 16, 405–416. [Google Scholar] [CrossRef]
- Göransson, H.; Olde Venterink, H.; Bååth, E. Soil Bacterial Growth and Nutrient Limitation Along a Chronosequence From a Glacier Forefield. Soil Biol. Biochem. 2011, 43, 1333–1340. [Google Scholar] [CrossRef]
- Menge, D.N.; Chisholm, R.A.; Davies, S.J.; Abu Salim, K.; Allen, D.; Alvarez, M.; Bourg, N.; Brockelman, W.Y.; Bunyavejchewin, S.; Butt, N. Patterns of Nitrogen-Fixing Tree Abundance in Forests across Asia and America. J. Ecol. 2019, 107, 2598–2610. [Google Scholar] [CrossRef]
- Si, G.; Wang, J.; Xia, Y.; Yuan, Y.; Zhang, G.; Lei, T. Change Characteristics of Microbial Communities and Enzyme Activities in Soils of Marshes in Nyaiqentanglha Mountains with Heights above Sea Level. Wetl. Sci. 2014, 12, 340–348. [Google Scholar]




| Latitude and Longitude | Mean Annual Temperature (°C) | Monthly Mean Temperature (°C) | Mean Annual Precipitation (mm) | Mean Annual Humidity (%) | Mean Annual Potential Evaporation (mm) | |
|---|---|---|---|---|---|---|
| January | July | |||||
| 29°20′–30°20′ N, 101°30′–102°15′ E | 4.2 | −4.6 | 12.5 | 1947 | 90 | 327 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Dai, S.; Ma, T.; Zhang, T.; He, J.; Wu, Y. Altitudinal Variation in Soil Acid Phosphomonoesterase Activity in Subalpine Coniferous Forests in China. Forests 2024, 15, 1729. https://doi.org/10.3390/f15101729
He X, Dai S, Ma T, Zhang T, He J, Wu Y. Altitudinal Variation in Soil Acid Phosphomonoesterase Activity in Subalpine Coniferous Forests in China. Forests. 2024; 15(10):1729. https://doi.org/10.3390/f15101729
Chicago/Turabian StyleHe, Xiaoli, Shile Dai, Tingting Ma, Tao Zhang, Junbo He, and Yanhong Wu. 2024. "Altitudinal Variation in Soil Acid Phosphomonoesterase Activity in Subalpine Coniferous Forests in China" Forests 15, no. 10: 1729. https://doi.org/10.3390/f15101729







