Abstract
Flowering plants require normal pollen germination and growth to be fertilized, but studies on the mechanism regulating pollen tube growth in Fraxinus mandshurica are limited. Here, we used transcriptomic data to study the oxidative phosphorylation pathway during pollen tube growth in Fraxinus mandshurica. Our study identified 8,734 differentially expressed genes during the stages S1 to S3 of pollen tube growth. Significant enrichment of the oxidative phosphorylation pathway, amino acid synthesis, protein processing in the ER, carbon metabolism, pyruvate metabolism, citrate cycle (TCA cycle), and glycolysis/gluconeogenesis were examined using the Kyoto Encyclopedia of Genes and Genomes, and 58 genes linked to ROS synthesis and scavenging during the S1–S3 stages were identified. Also, H2DCFDA staining confirmed ROS formation in the pollen and the pollen tubes, and treatment with copper (II) chloride (CuCl2) and diphenyleneiodonium (DPI) was shown to reduce ROS in the pollen tube. Reduction in ROS content caused decreased pollen germination and pollen tube length. Furthermore, FmRbohH (respiratory burst oxidase homolog H) expression was detected in the pollen and pollen tube, and an antisense oligodeoxynucleotide assay demonstrated reduced ROS and pollen tube growth in Fraxinus mandshurica. This study shed more light on the RbohH gene functions during pollen tube growth.
1. Introduction
In higher plants, fertilization is completed by transfer of the male nuclei into the ovules of a flower. This process involves pollen–pistil interaction, hydration, pollen tube growth, and release of male nuclei into the plant ovary [1]. These processes are essential for successful fertilization in plants.
Many factors influence pollen tube growth. After pollen tube germination, pollen tube extension is completed by swift development of the pollen tube tip [2]. Mutation of the pollen coat proteins cause defects and failure of the pollen tubes to germinate and grow normally [3]. This process is largely dependent on the actin cytoskeleton, which regulates organelle movement, vesicle trafficking, and cytoplasmic organization in pollen tubes [4,5]. The vacuole protein sorting genes VPS11, VPS 16, VPS18, and VPS33 are major players in organelle transport and are essential for plant normal pollen tube growth. Also, pollen tube development in Atvps18 mutant was severely impaired [6]. Studies have shown that in Arabidopsis, extracellular small proteins (RAPID ALKALINIZATION FACTOR) RALF4 and RALF 19 secreted from pollen tubes interact with the Catharanthus RLK1L (RLK1-like receptor kinases), ANX (ANXUR), and BUPS (Buddha’s Paper Seal) receptor complex for pollen tube cell vitality. Mutation of ANX1/ANX2 and Buddha’s Paper Seal proteins BUPS1/BUPS2 lead to abnormal pollen tube growth and premature pollen tip rupture [7,8]. The function of Rboh in pollen tubes is ROS (Reactive Oxygen Species) generation and homeostasis [9,10]. The use of exogenous agents or genetic knockdown of OeRbohH in olives inhibited pollen tube growth [11]. Cellular Ca2+ activates the Rboh (respiratory burst oxidase homolog) or NOXs (NADPH OXIDASES) genes to produce ROS [9,12]. Consequently, ROS is an important player in pollen tube growth.
Numerous pieces of evidence have demonstrated the importance of ROS in all phases of plant reproduction [13]. Also, pollen growth and pollen tip rupture require ROS [14,15]. In kiwifruit, pollen tube initiation was significantly influenced by ROS [16]. Growth of pollen tubes in tobacco decreased by AS-NbRboh (antisense oligodeoxynucleotide) treatment, and the pollen tube growth defect was rescued by H2O2 application, indicating Rboh-mediated ROS generation for proper growth of the pollen tube [17]. Also, the growth of the pollen tube was affected by AtRbohH/J knockout in Arabidopsis, which inhibited ROS generation [9]. Additionally, knocking out AtABCG28 in Arabidopsis showed inability to transport polyamines and ROS to the pollen tube tip, which led to abnormal pollen tube development and inability to discharge male nuclei [18].
Plant Rboh genes are characterized by NADPH-binding domains, a cytosolic FAD domain, two heme groups, and two highly conserved EF-hand calcium-binding domains regulating their activity [9,19]. Ten Rboh were identified in the genome of Arabidopsis thaliana [20], rice encodes 9 [21], barley encodes 6 [22], common grape vine encodes 7 [23], apple encodes 6 [24], and alfalfa encodes 7 Rboh members [25]. The Arabidopsis RbohA and RbohI exhibited root expression, while AtRbohD and AtRbohF showed expression in various tissues, and AtRbohH and AtRbohJ showed expression in the stamen and pollen [26]. Rice RbohA, RbohB, RbohC, RbohE, RbohF, RbohG, and RbohI genes have root, leaf, shoot, and calli expression, respectively [27], while OsRbohD is mainly expressed in the shoot and calli, and OsRbohH was detected in the panicle during rice reproductive development [28]. Although Rboh is widely present in plant tissues, functional studies of Rboh genes in the pollen tube growth of Fraxinus mandshurica are yet to be reported.
Fraxinus mandshurica belongs to the Oleaceae family, reaching up to 30 m tall; it is an economical tree plant with monetary value and medical properties [29]. F. mandshurica is native to Northeast China, Southeast Russia, Korea, and Japan. It has separate male and female flowers, and it is usually propagated by seed [30]. Climate change exacerbates the temperature, which greatly affects the plant’s male flower, and this in turn affects its pollination and poses the risk of self-incompatibility [31]. The plant also has a high seed abortion rate due to its peculiar physiological characteristics affecting pollination and fertilization [32]. Despite its economic value, the reproductive physiology of this plant is poorly understood. To address this gap, transcriptomic analysis was carried out to examine the transcript profile of the ROS pathway at various stages of pollen tube growth. Also, exogenous treatment of ROS scavengers (CuCl2 and DPI) and AS-ODN knockdown assay demonstrated that the RbohH protein is needed for pollen tube growth in Fraxinus mandshurica. This provides useful information for F. mandshurica breeding and its general conservation.
2. Materials and Methodology
2.1. Flower Collection and Pollen Morphological Analyses
F. mandshurica flowers were collected from experimental forest areas of the Northeast Forestry University (Harbin, China). After the stamens of F. mandshurica were dispersed, the mature pollen (S1) of F. mandshurica was collected. A total of 0.3 g of pollen was added to the pollen germination medium, and the mixture was cultured for 1 h (S2) and 5 h (S3), respectively. The pollen germination media contained 135 g/L sucrose, 100 mg/L CaCl2, and 200 mg/L H3BO3 at pH = 6.2. Pollens from three different germination stages were collected by centrifugation at 3000 rpm for 2 min, and three biological and technical repeats were set in each stage. Liquid nitrogen was used to flash-freeze the samples, which were kept at −80 °C for RNA extraction. Pollen from three different stages was visualized by Leica DM4 B microscope (Wetzlar, Germany).
2.2. RNA Sequencing Library Preparation
For RNA extraction, pollen and pollen tubes of F. mandshurica were collected and processed with the E.Z.N.A.®Plant RNA Kit (Omega Bio-tek Inc., Norcross, GA, USA) using the manufacturer’s protocol. A total of 9 RNA libraries from three biological replicates were prepared for the sequencing library. The Illumina HiSeq 2000 platform was used for the sequencing process (San Diego, CA, USA). The differentially expressed genes (DEGs) were extracted using the DESeq2 R program (https://rdrr.io/bioc/DESeq2/src/R/core.R accessed on 20 January 2024) with parameters set to |log2Fold Change| ≥ 1 and a false discovery rate < 0.05 [33].
2.3. Real-Time Quantitative PCR Analyses
Primers for the qPCR were generated from the Primer-Blast program (https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi accessed on 23 January 2024 ) (Table S2). The cDNA was prepared with a One-Step-TransScript-gDNA Removal cDNA kit (TransGen, Beijing, China), and the LightCycler480 (Roche, Basel, Switzerland) qPCR system was used to carry out the real-time qPCR with SYBR green Mix (TransGen, Beijing, China). The Livak method (2−ΔΔCT) was applied for the relative gene expression calculation [34], and FmTUB was used for normalization. This experiment was carried out in three replicates.
2.4. ROS Detection
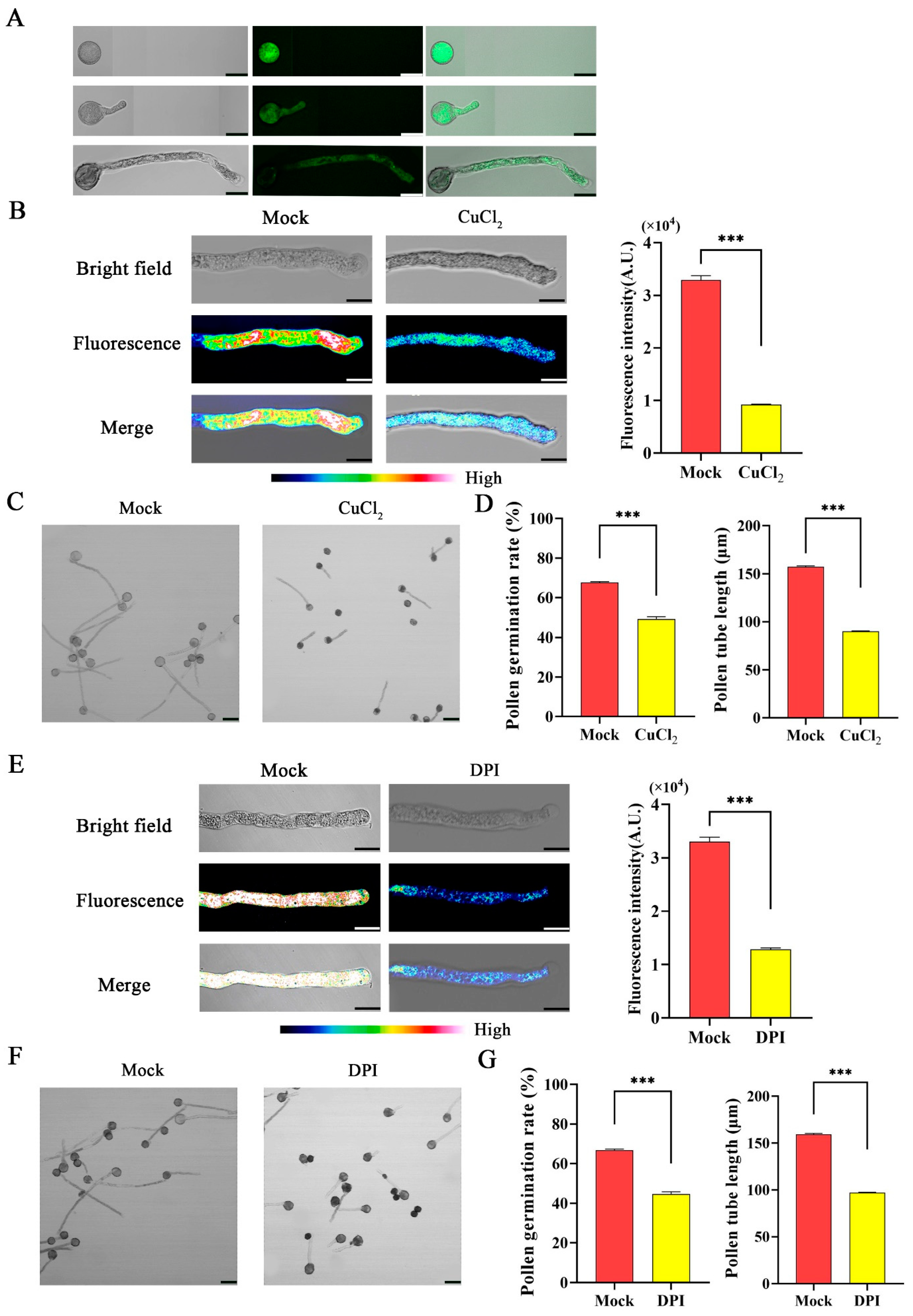
The level of ROS in the pollen of F. mandshurica was quantified by using H2DCFDA (2,7-dichlorodihydrofluorescein diacetate). The pollen was exposed to 100 μM H2DCF-DA followed by 30 min dark incubation at 37 °C, then centrifugation of the pollen at 3000 rpm for 2 min [35]. Pollen samples were washed with PBS buffer three times (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4; pH 7.2) [36]. All samples were observed using an LSM900 confocal microscope (Zeiss, Germany), and ROS content was calculated using Zeiss tools. Sixty pollen tubes were visualized in each sample, and pollen germination rate was calculated as follows:
Pollen germination rate = Number of pollen germination/Total number of pollen
2.5. Treating the Pollen with Exogenous Reagents Copper (II) Chloride (CuCl2) and Diphenylene Iodonium (DPI)
The pollen was added to the previously mentioned pollen germination media and pre-cultured for 45 min, followed by the addition of 1000 μM copper (II) chloride (CuCl2) or 150 μM diphenylene iodonium (DPI) to the pre-cultured pollen, and the mixture continued to culture for 5 h [14]. The samples were visualized with a confocal microscope LSM900 (Zeiss, Jena, Germany), and at least lengths of 300 pollen tubes were calculated using Zeiss tools.
2.6. Transfection of Oligonucleotide (ODN) Chain into Pollen
Antisense and sense of oligodeoxyribonucleotides (AS-ODN and S-ODN) were designed to generate AS-FmRbohH and S-FmRbohH [14,37]. Table S3 contains the AS-FmRbohH and S-FmRbohH sequences. The ODN sequences were synthesized at the Beijing Genomics Institution (BGI), followed by phosphorothioate modification of three nucleotides at the 5′ and 3′ ends for stability. A Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) treatment was applied to 50 μM AS-FmRbohH and S-FmRbohH primers and 15 min incubation at 65 °C temperature, then followed by another round of 5 h incubation on pollen germination medium. The samples were visualized using an LSM900 confocal microscope (Zeiss, Germany), and Zeiss tools were used to calculate the lengths of at least 300 pollen tubes.
2.7. Statistical Analysis
For statistical analysis, Student’s t-test was used, and the significance is shown just above the error bars as * p < 0.05, ** p < 0.01, and *** p < 0.001. The GraphPadPrism software 8.0.1 was used to plot the means and SEM (standard error of the mean) for the pollen number, pollen tube length, and ROS signal intensity.
3. Results
3.1. Transcriptomic Analysis Revealed Enriched Pathways during the Pollen Tube Growth in F. mandshurica
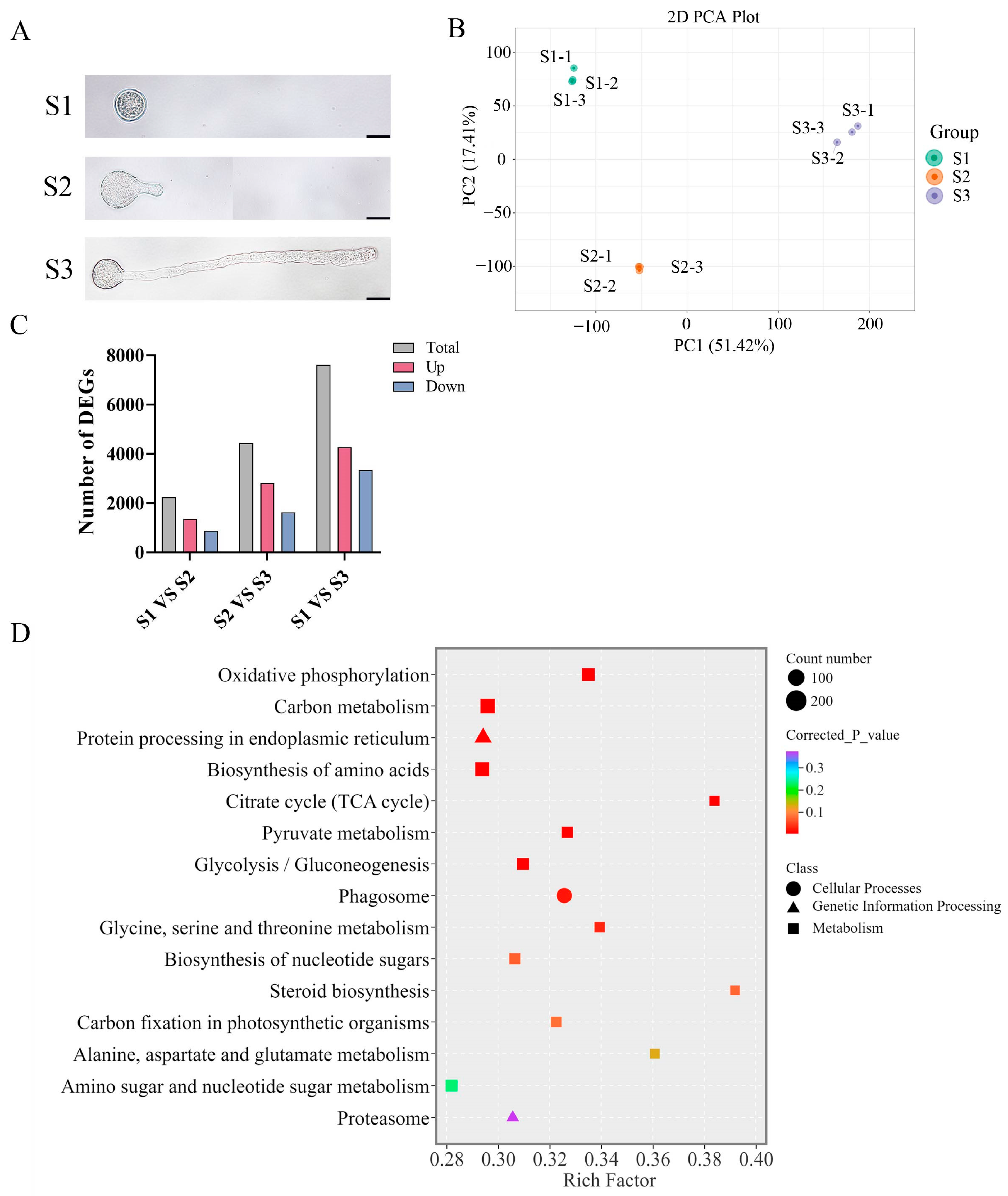
To examine the pollen morphology of F. mandshurica, the pollen was grown on pollen germination media, and our results revealed stage S1, where the pollen is not undergoing germination; stage S2, where the pollen tube begins to germinate after 1 h; and stage S3, where the pollen grows after 5 h (Figure 1A).
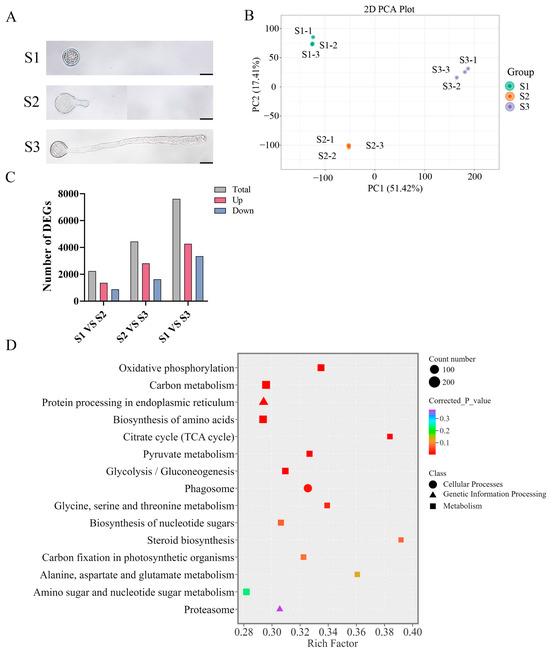
Figure 1.
Transcriptome analysis of F. mandshurica pollen at different growth stages. (A) Morphological observation of F. mandshurica pollen at S1, S2, and S3 stages. Scale bar = 20 µm. (B) PCA data showing a clear distinction between S1, S2, and S3 stages of pollen tube growth in F. mandshurica. (C) Statistics of differentially expressed genes between different pollen tube growth stages. (D) The DEG KEGG enrichment analysis of pathways during pollen tube growth.
Total RNA from the S1, S2, and S3 stages was used to generate the transcriptome data. The Illumina HiSeq platform generated 53.4–97 million raw reads from nine RNA samples, with 52.2–93.6 million clean reads retained, accounting for 95% of the total data. Also, 36,541 unigenes were successfully annotated in one or more databases. The data generated satisfied the downstream analysis requirements (Table S1). Subsequent analyses were conducted using |log2Fold Change| ≥ 1 and FDR < 0.05 as significance cut-offs, and the PCA (principal component analysis) revealed distinctive differences between different stages and showed minimal differences between the biological replicates (Figure 1B). Differential expression analysis during pollen tube growth identified 2240 (1355 upregulated and 885 downregulated) in S1 vs. S2, 4446 (2818 upregulated and 1628 downregulated) in S1 vs. S3, and 7610 (4263 upregulated and 3347 downregulated) in S2 vs. S3 DEGs (Figure 1C). For KEGG enrichment analysis, the data from S1, S2, and S3 were pulled together to enhance statistical power, observe core changes, and explore shared biological pathways across all comparisons. Our results showed that DEGs were associated with cellular processing, genetic information processing, and metabolism. The top 15 pathways with the smallest adjusted p-values and higher rich factor were considered significant and are listed as follows: oxidative phosphorylation, carbon metabolism, protein processing in endoplasmic reticulum, biosynthesis of amino acids, citrate cycle (TCA), pyruvate metabolism, glycosis/gluconeogenesis, phagosome, glycine serine and threonine metabolism, biosynthesis of nucleotide sugars, steroid biosynthesis, carbon fixation in photosynthetic organisms, alanine aspartate and glutamate metabolism, amino sugar and nucleotide sugar metabolism, and proteasome (Figure 1D).
3.2. Oxidative Phosphorylation Pathway Complexes Influenced Pollen Tube Growth of F. mandshurica
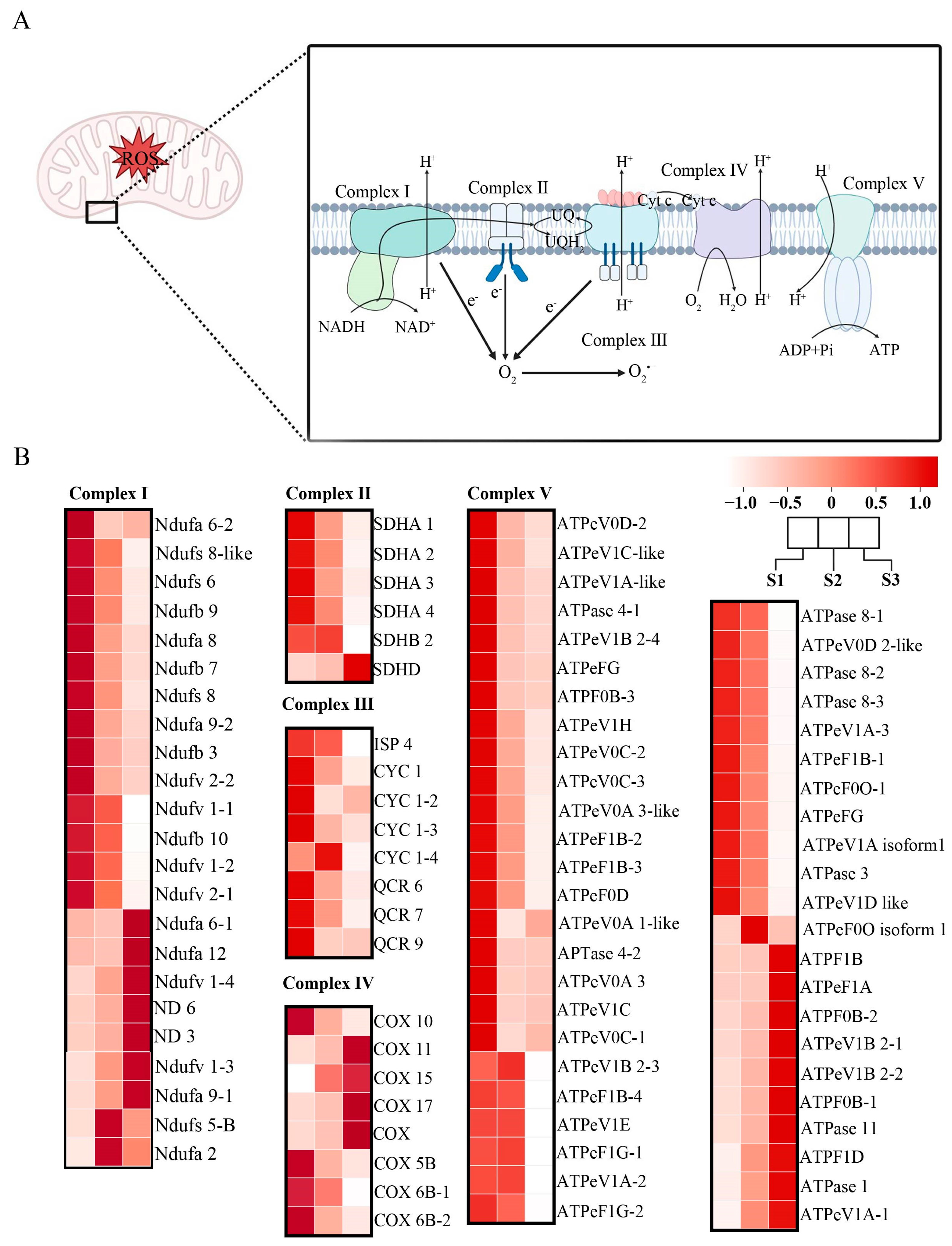
Oxidative phosphorylation occurs in the mitochondria, where several complexes work together to produce ATP, generating ROS as a byproduct (Figure 2A). Complex I consists of Ndufa, Ndufb, Ndufs, Ndufv, and ND genes, differentially expressed in all the stages of pollen tube growth. Moreover, complex I is important for catalyzing the conversion of NADH to NAD+, as well as the electron transfer to oxygen in the mitochondria. The members of complex II in all eukaryotic cells were inherited from the Alphaproteobacteria [38]; they regulate electron transfer and mitochondrial oxidative phosphorylation. This complex comprises succinate dehydrogenase enzymes (SDHs); SDHA 1, SDHA 2, SDHA 3, SDHA 4, SDHB 2, and SDHD. They are expressed more in the S1 stage, except for SDHD, which is more expressed in the S3 stage. Complex III consists of Cytochrome C (CYCs); CYC 1, CYC 1-2, CYC 1-3, CYC 1-4, ISP 4, QCR 6, QCR 7, and QCR 9; they are also more expressed in the S1 stage, except for CYC 1-4, which is more expressed in stage S2. These complexes are crucial for energy production, catalyzing electron transfer from dihydroubiquinone (QHz) to cytochrome c, a process that is coupled to transmembrane proton transfer and ROS generation as a byproduct. Complex IV members mainly are the the cyclooxygenases (COXs); COX gene family, COX 5B, COX 6B-1, COX 6B-2, COX 10, COX 11, COX 15, and COX 17. They catalyze electron transfer by using molecular oxygen as a substrate. Complex V consists of mainly ATPase genes, which carry out the last step of oxidative phosphorylation in the cell. Most of the genes were more expressed in the S1–S2 stages and decreased in the S3 stage (Figure 2B).
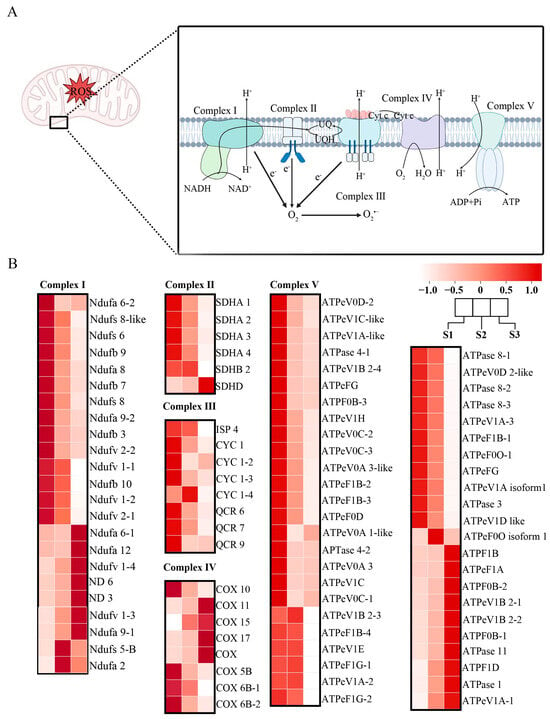
Figure 2.
Cluster analysis of genes in the oxidative phosphorylation pathway during pollen tube growth in F. mandshurica. (A) The oxidative phosphorylation pathway in F. mandshurica. (B) Heatmap of the oxidative phosphorylation pathway during pollen tube growth in F. mandshurica.
Similarly, the Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis showed the TCA (tricarboxylic acid) cycle and glycolysis/gluconeogenesis to be among the enriched pathways during pollen tube growth. The TCA cycle and glycolysis/gluconeogenesis genes showed different expression levels during pollen tube growth. Cluster analysis revealed the key genes in the TCA cycle and glycolysis/gluconeogenesis expressed higher during the S1–S2 stages.
3.3. ROS-Related Genes Were Differentially Expressed in Apoplast, Cytoplasm, Chloroplast, Mitochondrion, and Peroxisome during Pollen Tube Growth in F. mandshurica
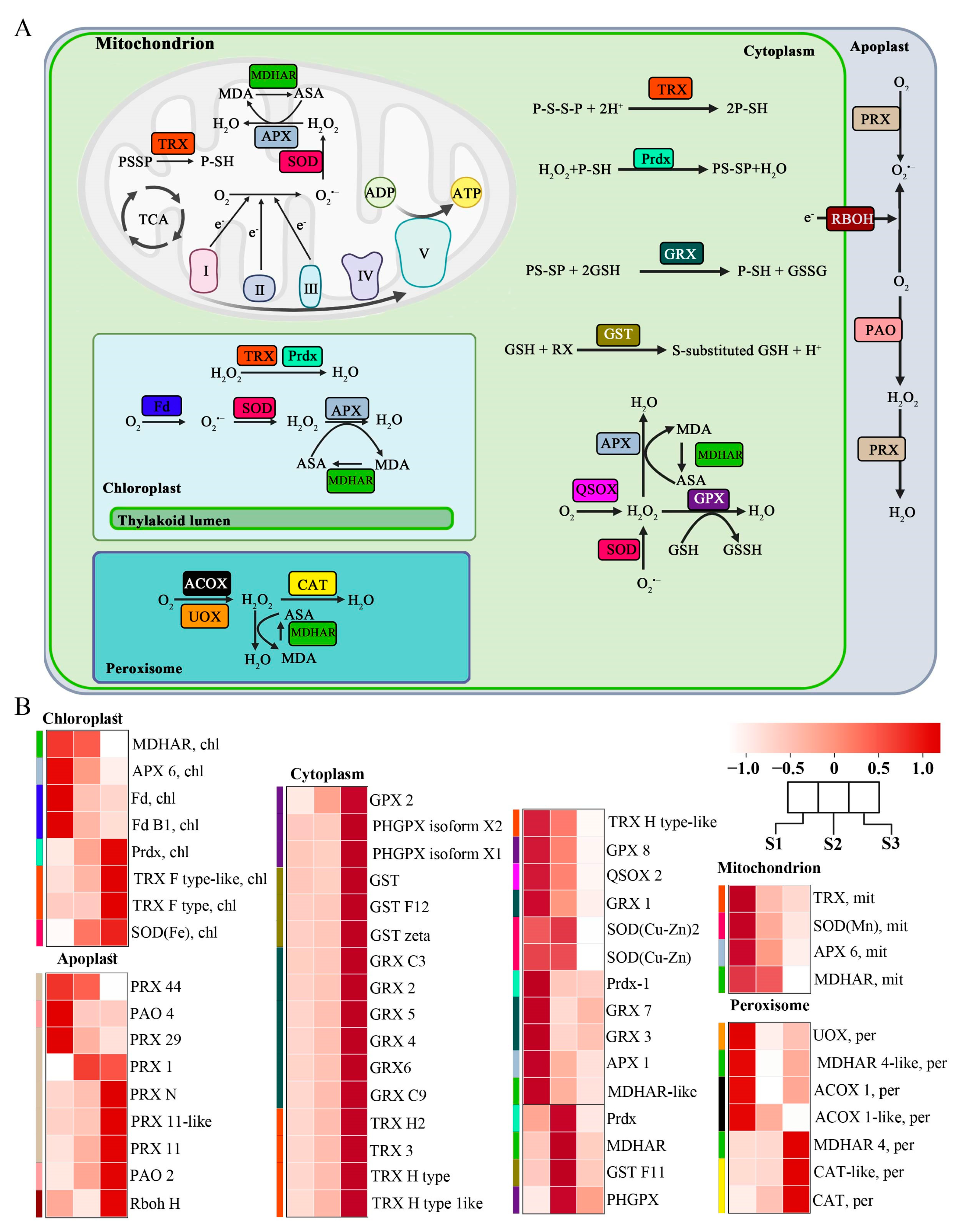
Plants’ subcellular compartments are the main sites for ROS production, and therefore an efficient mechanism to maintain their optimum level is required for proper cell function. Our transcriptome data showed the transcript level of genes related to ROS synthesis and sequestration in different subcellular compartments. Our analysis found 59 genes expressed differentially in the apoplast, chloroplast, cytoplasm, mitochondria, and peroxisome, including genes that catalyze ROS biosynthesis and enzymes that catalyze ROS scavenging (Figure 3A).
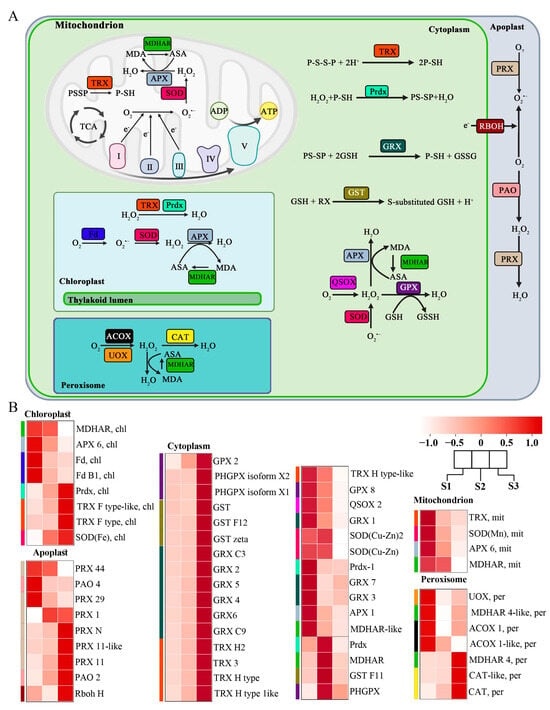
Figure 3.
Different ROS-related genes exhibiting differential expression patterns in different cellular compartments and stages of pollen tube growth. (A) ROS-related genes in various cellular compartments. (B) Heatmap expression of differentially expressed genes in various cellular compartments at various stages of pollen tube growth in F. mandshurica. The blue, brown, cyan, green, and magenta colors represent the genes in the cellular compartments.
In the chloroplast, ferredoxin (FD) is an enzyme that generates ROS and is highly expressed in the S1 period. At the same time, monodehydroascorbate reductase (MDHAR) and ascorbate peroxidase (APX), enzymes that scavenge ROS, are also highly expressed in the S1 period. Three other enzymes that can scavenge ROS, peroxiredoxin (Prdx), thioredoxin (TRX), and superoxide dismutase (SOD), were found to be highly expressed in S3.
In the apoplast, peroxiredoxin (PRX), respiratory burst oxidase homologs (Rboh: NADPH oxidase), and polyamine oxidases (PAO) were found to be significantly expressed; they catalyze conversion of O2 to O2·− (superoxide ion) and H2O2 (hydrogen peroxide). PRX, PAO, and RbohH were more expressed in the S3 stage.
In the cytoplasm, TRX, Prdx, glutaredoxin (GRX), glutathione-s-transferases (GST), APX, MDHAR, quiescin sulfhydryl oxidase (QSOX), glutathione peroxidase (GPX), and SOD were significantly expressed. GPX, GST, TRX, MDHAR, Prdx, and GRX are enzymes that mainly scavenge ROS in the cytoplasm. Cluster analysis revealed elevated expression of these genes in the S3 stage. QSOX is the enzyme that mainly generates ROS in the cytoplasm, and its expression level was highest in the S1 stage.
In the mitochondrion, the expressions of TRX, SOD, APX, and MDHAR were both highly expressed in the S1 stage. The expression of acyl-CoA oxidase (ACOX) and urate oxidase (UOX) decreased significantly in peroxisome as the pollen tube growth progressed. Catalase (CAT) in particular was found to be highly expressed; it also catalyzed the scavenging of the ROS in the cell (Figure 3B).
3.4. Genes Associated with ROS Exhibited Different Expression Levels at Various Pollen Tube Growth Stages in F. mandshurica
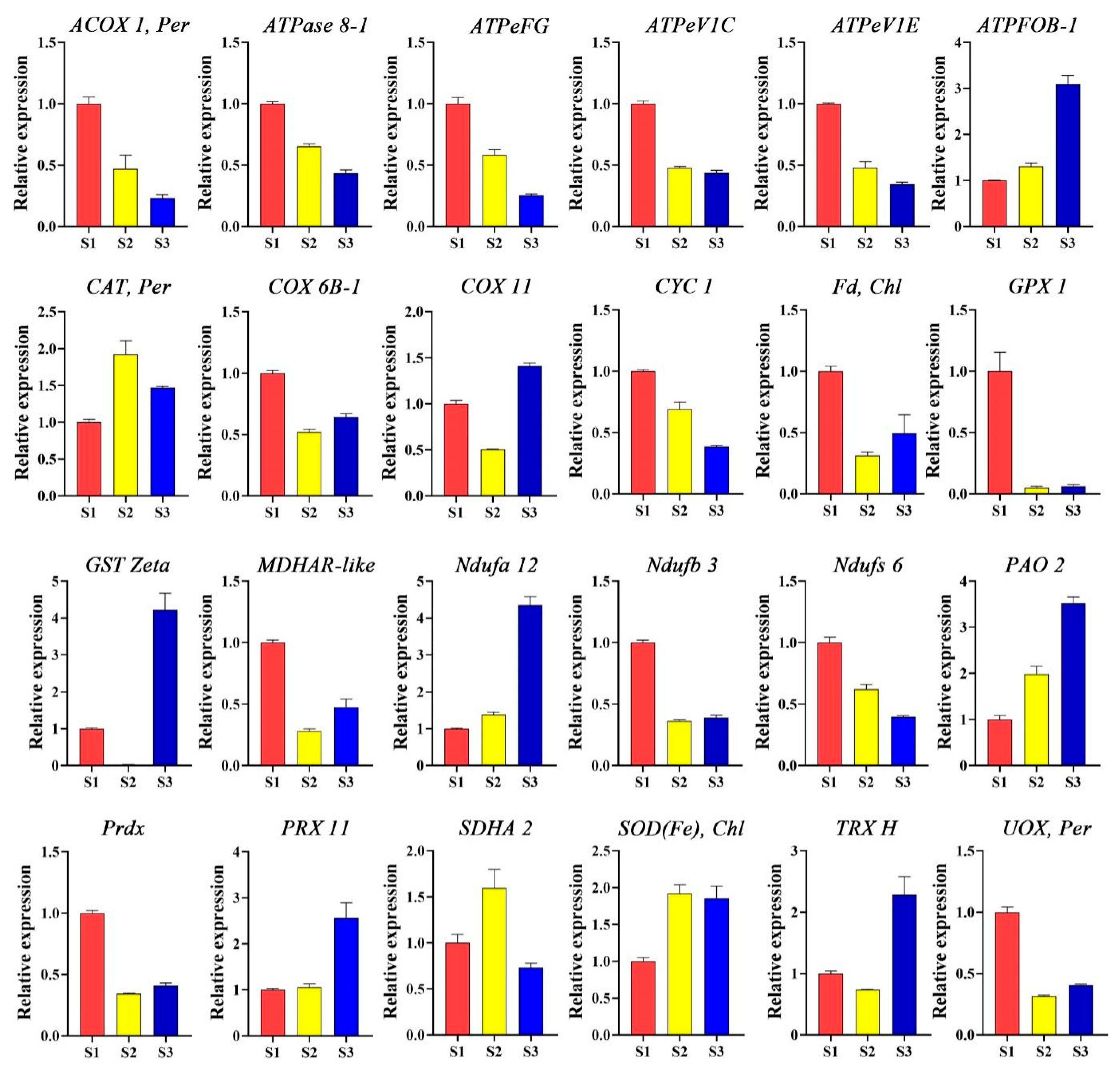
Many ROS-related genes regulate pollen tube growth in different ways, maintaining optimum concentration of the ROS in the cell. To explore how these genes mediate ROS sequestration, their expression levels were examined, and the data revealed that they exhibit different expression patterns at various stages of pollen tube growth. The ACOX 1, per, ATPase 8-1, ATPeFG, ATPeVIC, ATPeVIE, COX 6B-1, CYC1, Fd, Chl, GPX 1, MDHAR-like, Ndufb 3, Ndufs 6, Prdx, and UOX, per were upregulated during the S1 stage. Also, CAT, per, SDHA 2, and SOD(Fe) Chl were found to have more expression in the S2 stage. Furthermore, AFPFOB-1, COX 11, GST-Zeta, Ndufa 12, PAO 2, PRX 11, and TRX H showed higher expression levels in the S3 stage (Figure 4). These data suggest that ROS-related genes play different roles in maintaining optimum ROS levels for proper pollen tube growth in F. mandshurica.
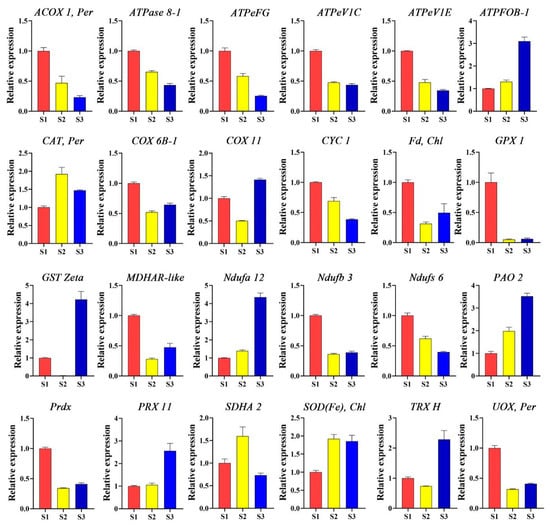
Figure 4.
Expression levels of ROS-related genes at stages S1, S2, and S3 of pollen tube growth in F. mandshurica. ACOX 1, acyl-CoA oxidase; ATPase 8-1, ATP synthase protein 8-1; ATPaseFG, ATP synthase protein FG; ATPaseVIC, ATP synthase protein VIC; ATPaseVIE, ATP synthase protein VIE; ATPFOB, ATPase F type; CAT, catalase 6B-1; COX, cytochrome C oxidase 6B-1; COX 11, cytochrome C oxidase 11; CYC 1, cytochrome C oxidoreductase 1; Fd, ferredoxin; GPX 1, glutathione peroxidase 1; GSTZeta, glutathione S-transferase zeta; MDHAR-like, monodehydroascorbate reductase like; Ndufa 12, NADH:ubiquinone oxidoreductase a 12; Ndufb 3, NADH:ubiquinone oxidoreductase b 3; Ndufs 6, NADH:ubiquinone oxidoreductase s 6; PAO 2, polyamine oxidase 2; Prdx, peroxiredoxin; PRX 11, peroxidase 11; SDHA 2, succinate dehydrogenase A 2; SOD, superoxide dismutase; TRX H, thioredoxin H; UOX, urate oxidase.
3.5. ROS Generation Was Crucial for F. mandshurica Pollen Tube Growth
To determine the ROS generation during pollen tube growth in F. mandshurica, pollen was grown on pollen germination media and treated with 2,7-dichlorodihydrofluorescein diacetate (H2DCFDA) at S1, S2, and S3. The result showed ROS fluorescence, and the ROS continued to accumulate as the pollen tube growth progressed (Figure 5A). The pollen grains were then treated with ROS scavenger copper (II) chloride (CuCl2) to examine the function of ROS in pollen tube germination. After the treatment with CuCl2, ROS fluorescence intensity and the pollen tube germination significantly decreased (Figure 5B–D). Furthermore, to examine the function of FmRbohH in pollen tube growth, pollen grains were treated with the Rboh inhibitor diphenyleneiodonium chloride (DPI), and the result revealed a noticeable decrease in ROS fluorescence intensity, with the rate of germination in the mock being 68% compared to the treated pollen with about 50%. The pollen tube length was 150 µm in the mock compared to the treated with 93 µm (Figure 5E–G), indicating that the function of Rboh in pollen tubes is to generate ROS and enhance pollen tube growth.
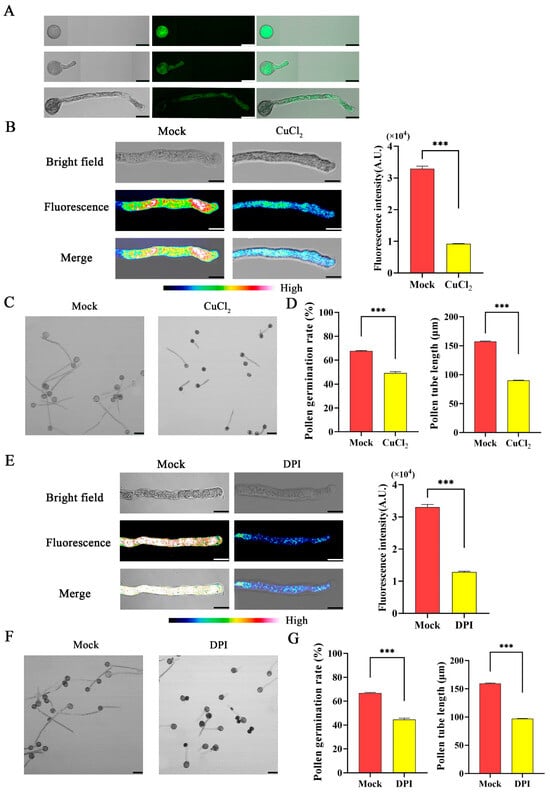
Figure 5.
ROS enhanced pollen tube growth in F. mandshurica. (A) H2DCFDA staining to detect ROS in S1, S2, and S3 of pollen tube growth; scale bar = 20 µm. (B) ROS fluorescence after treatment with CuCl2 (ROS scavenger); scale bar = 10 µm. (C) Pollen tube germination after treatment with CuCl2 (ROS scavenger); scale bar = 50 µm. (D) Pollen tube length in micro-meters (µm) and pollen germination rate as a percentage (%). (E) ROS fluorescence after treatment with DPI (NADPH oxidase inhibitor); scale bar = 10 μm. (F) Pollen tube growth after treatment with NADPH oxidase inhibitor (DPI); scale bar = 50 µm. (G) Pollen tube germination rate as a percentage (%) and length in micro-meters (µm). The *** indicates a p-value < 0.001 by Student’s t-test.
3.6. FmRbohH Exhibited Higher Transcript Level in the Pollen and Pollen Tubes of F. mandshurica
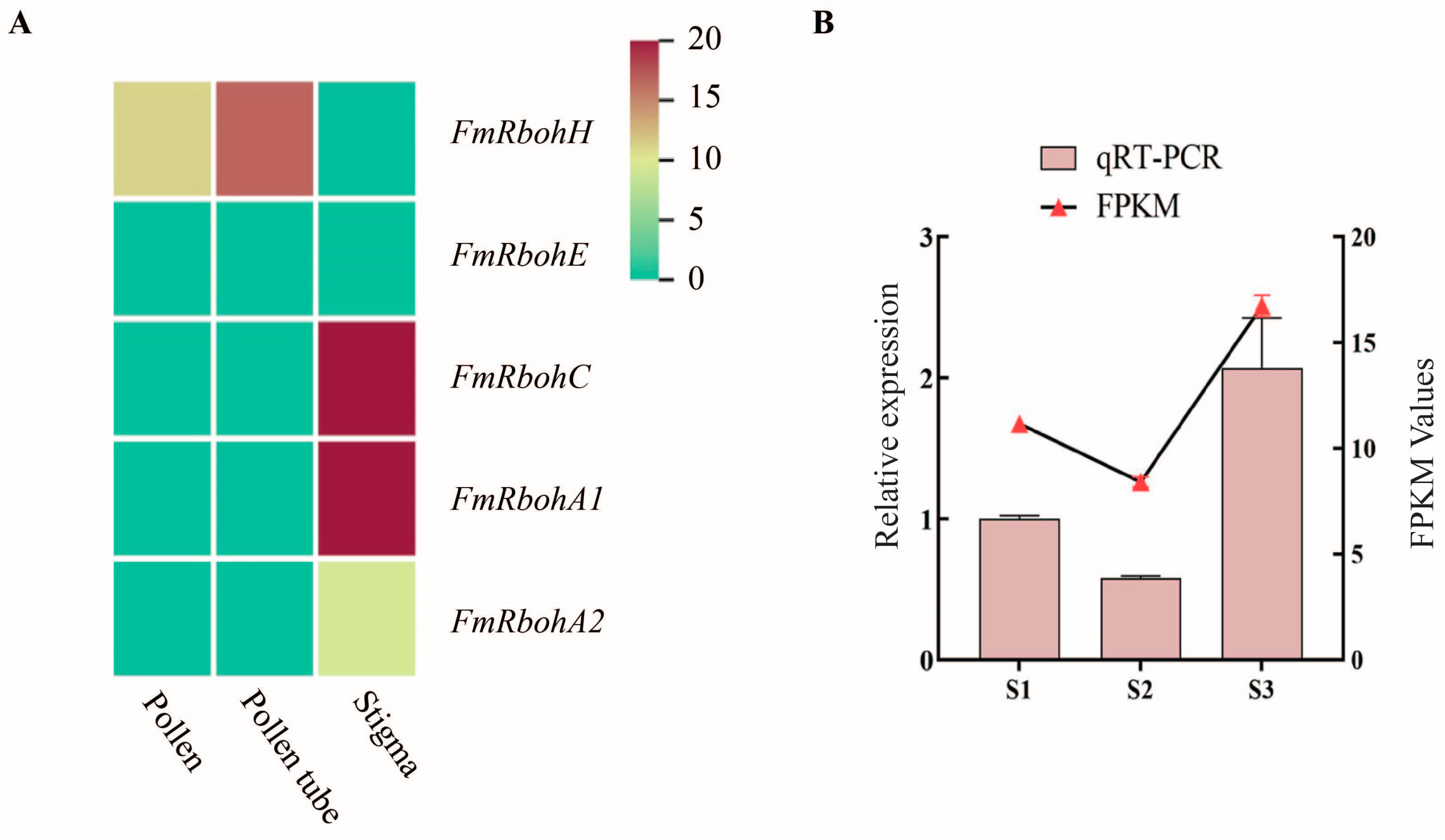
To understand the expression pattern of FmRboh genes in tissues of F. mandshurica, a heatmap of FmRbohA1, FmRbohC, FmRbohH, FmRbohA2, and FmRbohE was generated using the RNA-seq data. The RNA-seq data showed that FmRboh genes were expressed in the pollen, pollen tube, and stigma tissues of F. mandshurica, with FmRbohH having higher expression in the pollen and pollen tube (Figure 6A). Also, qPCR was performed to confirm the expression profile of FmRbohH in the S1, S2, and S3 stages of the pollen tube, wherein the qPCR data showed high consistency with the transcriptome data (Figure 6B). This result suggests that FmRbohH may be a crucial regulator of pollen germination proliferation in F. mandshurica.
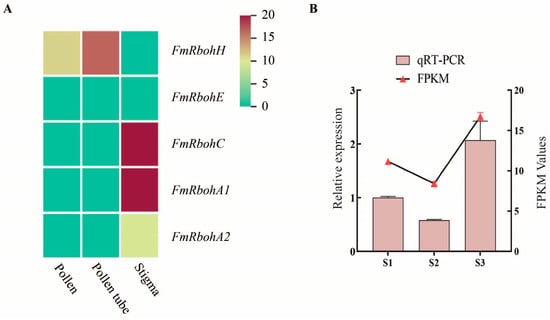
Figure 6.
Expression profile of FmRboh genes in F. mandshurica. (A) Heatmap expression profile of FmRboh genes in the pollen, pollen tube, and stigma of F. mandshurica. (B) qPCR expression data and FPKM expression data of FmRbohH in the S1, S2, and S3 stages of pollen tube growth in F. mandshurica.
3.7. FmRbohH Controls the Growth of the Pollen Tube in F. mandshurica
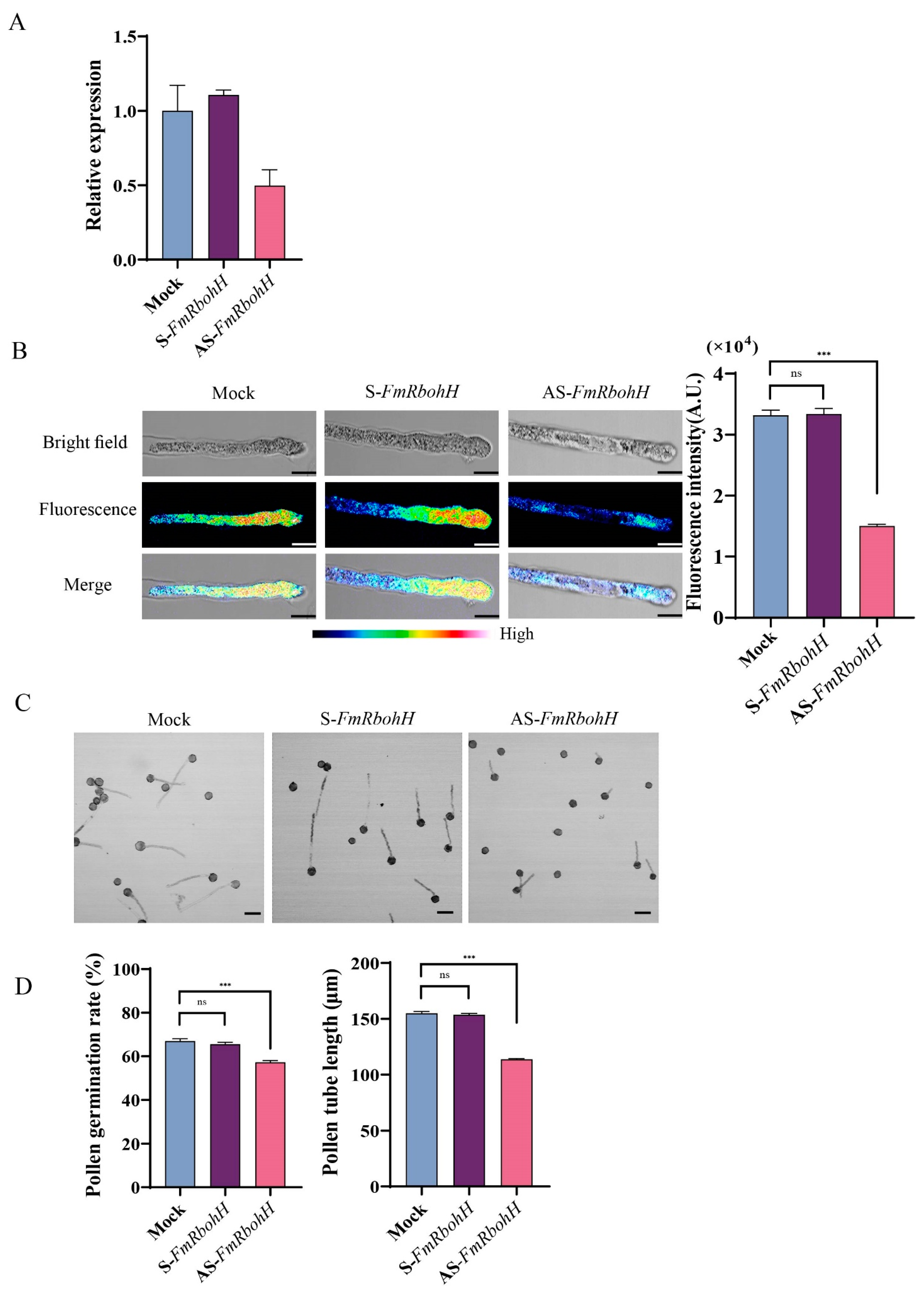
To show how FmRbohH functions in the pollen tube growth of F. mandshurica, the expression of FmRbohH was knocked down using AS-FmRbohH after germination on the media with cytofectin. After the knockdown of FmRbohH, the expression of FmRbohH was observed to be low (Figure 7A). Also, the pollen tube ROS fluorescence of the S-FmRbohH and the mock were at the same level (3.3) compared to the AS-FmRbohH, which was significantly less. Similarly, the pollen tube germination rate was significantly higher in the mock and S-FmRbohH 64% compared to the AS-FmRbohH, which was 57%. The pollen tube length was significantly low in the AS-FmRbohH 105 µm compared to the mock and the AS-FmRbohH with 151 µm (Figure 7B–D). This finding demonstrated the critical role of FmRbohH in F. mandshurica pollen tube growth.
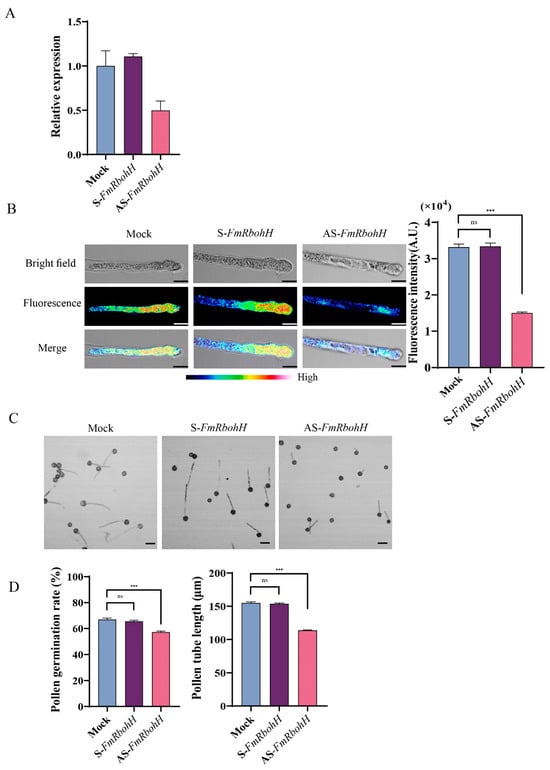
Figure 7.
FmRbohH enhances pollen tube growth in F. mandshurica. (A) Expression levels decreased after AS-FmRbohH and S-FmRbohH treatment. (B) ROS signal intensity from pollen tube after treatment with AS-FmRbohH and S-FmRbohH; scale bar = 10 μm. (C) Phenotypes of pollen tubes after AS-FmRbohH and S-FmRbohH assay; scale bar = 50 µm. (D) Pollen tube length in µm and germination rate as a percentage (%). The ns denotes p-value > 0.05, and the *** denotes p-value < 0.001 by Student’s t-test.
4. Discussion
The effect of temperature and rainfall significantly influence the development of female flowers, pollen formation, and pollen tube growth, leading to low fertilization efficiency and thereby reducing the population of F. mandshurica [31]. Development, germination, and proliferation of pollen are essential for effective fertilization during sexual reproduction in higher plants [3]. More and more transcriptome studies have revealed the mechanism of pollination in plants [39,40]. Few studies have been conducted on both male and female flower development in F. mandshurica [31,32], but studies on the pollen tube growth mechanism in F. mandshurica is limited. In this study, our data revealed 14296 DEGs across the pollen tube growth stages (S1 vs. S2, S2 vs. S3, and S1 vs. S3 libraries). KEGG analysis showed the association of the DEGs with the oxidative phosphorylation signaling pathway. Oxidative phosphorylation occurs in the prokaryotic cytoplasm and the inner membrane of eukaryotic mitochondria (Figure 1A–D). This reaction is part of the respiratory chain, which harnesses energy from chemical oxidation to produce ATP [41]. This process generates a significant amount of ROS as byproducts. Therefore, we hypothesized that the ROS concentration changes depending on the pollen tube growth stage, so we conducted a comprehensive analysis of this pathway.
The process of oxidative phosphorylation takes place in mitochondria. The systems responsible for this process are arranged in complexes on the mitochondrial inner membrane, forming a respiratory chain or electron transport chain (ETC) [42]. The ETC uses oxygen to transfer electrons and H+ and produce ATP and H2O [43]. We analyzed the transcript of the oxidative phosphorylation pathway and screened 92 DEGs. The mitochondrial oxidative phosphorylation ROS byproducts are mainly produced by complexes I, II, and III [41,44]. Complex I derives electrons from NADH used in mitochondrial ATP synthesis [45]. In complex I, all the DEGs were highly expressed in stage S1, except for 3 Ndufa, 2 Ndufv, and 2 ND, which were highly expressed in stage S3. Complex II, on the other hand, is mainly involved in the oxidation of FADH2 to reduce ubiquinone (UQ) and ensure electron flow to complex III [38,46]. In complex II, all DEGs were highly expressed in stages S1 and S2, except for SDHD, which was highly expressed in stage S3. Complex III was reported to be an important site for ROS generation; it comprises three conserved subunits, the cytochrome c oxidoreductase (CYC), iron-sulfur protein (ISP), and ubiquinol-cytochrome c oxidoreductase (QCR) [46,47]. All the genes in complex III were highly expressed in stages S1 and S2. Complex IV is comprised of cytochrome c oxidase genes (COX); this complex is the last step of mitochondrial ETC, and it reduces O2 to H2O [48]. On the other hand, complex V enables the ATP synthase proteins to phosphorylate ADP [49]. However, in complexes IV and V, the genes showed different expression patterns at the S1, S2, and S3 stages. Differential gene expression in complexes I, II, and III followed a consistent trend, suggesting fluctuating ROS levels during pollen tube growth (Figure 2A,B). Glycolysis and the TCA cycle are closely related to oxidative phosphorylation [45]. At the same time, we found that almost all the DEGs enriched in glycolysis and TCA cycle were highly expressed in stages S1 or S2, which could be due to the large amount of energy required for pollen tube growth (Figure S1). This trend is the same as the DEG trend in complexes I, II, and III.
ROS can be generated in nearly every subcellular compartment, and each has its unique clearance system [50,51,52]. There are four main types of ROS in plants: H2O2 (hydrogen peroxide), 1O2 (singlet oxygen), O2•− (superoxide), and HO• (hydroxyl radical) [53]. The ROS pathway regulates important processes in plants, whether it be stress response or developmental processes [54,55,56,57]. Many studies have revealed how ROS regulates plants’ reproductive processes [17,18,58,59]. Our data revealed changes in gene expression related to ROS generation across different cellular compartments during pollen tube growth (Figure 3A). In chloroplasts, the auto-oxidation of ferredoxin (Fd) is accompanied by the generation of O2•−, and superoxide dismutase (SOD) dismutates the O2•− generated by Fd to form H2O2. The generated H2O2 is decomposed by ascorbate peroxidase (APX), through ascorbate (ASA) as a reducing agent, and monodehydroascorbate reductase (MDHAR) generates ASA [51]. Cluster analysis revealed that Fd, APX, and MDHAR were highly expressed in stage S1 and decreased in stage S3, indicating the function of Fd in maintaining the ROS equilibrium in stages S1 and S3. Peroxiredoxins (Prdx) and thioredoxins (TRX) are also systems involved in chloroplastic ROS clearance. Prdx is a thiol-based peroxidase that scavenges on H2O2 through TRX [60]. The high expression of Prdx, TRX, and SOD in stage S3 also suggests ROS homeostasis in stage S3. In apoplasts, only one polyamine oxidase (PAO) is highly expressed in stage S1, and one NADPH oxidase (Rboh) and one PAO are highly expressed in stage S3, whereas peroxidases (PRX) are highly expressed at all stages. NADPH oxidase and PAO regulate the accumulation of H2O2 and O2•− in plant tissues [61,62]. Our findings indicated that PAO and Rboh might be crucial for ROS generation during stage S3. In the cytoplasm, only one QSOX that generates ROS was identified and is highly expressed in stage S1, and it was significantly decreased in stage S3; the rest were genes related to ROS scavenging. In mitochondria, most of the DEGs in the complex were highly expressed in stage S1, and genes such as TRX, APX, MDHAR, and SOD were highly expressed in stage S1. Previous studies have shown that TRX, APX, MDHAR, and SOD are crucial in the ROS scavenging system [51,63,64,65]. In peroxisomes, Acyl-CoA oxidase (ACOX) and urate oxidase (UOX) were highly expressed in stage S1, MDHAR showed higher expression in the S1 stage, and catalase (CAT) showed higher expression in the S3 stage. The enzyme acyl-CoA oxidase mediates the formation of trans-2-enyl-CoA from acyl-CoA in the β-oxidation pathway and generates H2O2 as a byproduct [66]; urate oxidase can oxidize uric acid to produce hydrogen peroxide as a byproduct [44]; and mutation in the peroxisomal H2O2 scavengers, the catalases (CATs), leads to severe growth abnormalities [67]. We clustered the differentially expressed genes related to ROS and found that only RbohH and PAO2 in the exoplasm showed higher expression in the S3 stage, while the other genes that generate ROS showed higher expression in the S1 stage. The genes that scavenge ROS had different expressions in each period (Figure 3B and Figure 4). This indicates that there are changes in ROS content during pollen tube growth in plants, and ROS in pollen tubes are mainly produced by Fd, QSOX, UOX, and ACOX in the S1 stage. In the S3 stage, the ROS in pollen tubes are by PAO and Rboh.
ROS accumulation in the pollen, pollen tube, and pollen tube tips significantly affect the pollen tube growth process [17,68,69]. In the present study, H2DCFDA treatment on the pollen and growing pollen tubes revealed that ROS was highly accumulated in the pollen and pollen tubes of F. mandshurica, suggesting its involvement in pollen tube growth (Figure 5A). Moreover, CuCl2 and DPI treatment reduced the ROS fluorescence in pollen tubes (Figure 5B,E). CuCl2 and DPI scavenge on the ROS from stigmas and pollen [14,70]. Moreover, the germination rate and the length of pollen tubes were greatly reduced after treatment with CuCl2 and DPI. In Olea europaea L., it was found that treating pollen tubes with DPI significantly lowered ROS in the pollen tube, and the germination and pollen tube length were affected [11]. The same phenomenon was found in pears but without the pollen tube tips swelling [14].
Rboh genes, among other ROS-related genes, are essential for both stress responses and reproductive development. A myriad of pieces of evidence proved that Rboh genes are expressed in various tissues of plants, including male and female reproductive tissues [71]. Many Rboh genes were reported to promote pistil development in plants [72,73]. In this study, Rboh genes showed expression in various tissues of F. mandshurica, suggesting their different roles during growth and development. Among the FmRboh genes, only FmRbohH showed a higher expression level in the pollen and pollen tubes (Figure 6A,B). In the closely related species Olea europaea L., only RbohH was found to express in the pollen, but RbohH and RbohJ in Arabidopsis showed pollen-specific expression [11]. It was also reported in another study that AtRbohH and AtRbohJ regulate pollen tube development in Arabidopsis [74]. Also, RbohJ in olive plants regulates pollen tube growth [9,11]. Arabidopsis RbohH and RbohJ mutants showed severe fertility defects as a result of a reduction in pollen tube growth [74]. In our study, the knockdown of FmRbohH using the antisense oligonucleotide (AS-ODN) reduced pollen tube growth in F. mandshurica. Also, the ROS fluorescence intensity was highly diminished (Figure 7). These findings suggest that FmRbohH is crucial for ROS generation to enhance pollen tube growth in F. mandshurica.
5. Conclusions
This study used transcriptome data to investigate ROS-related genes that regulate pollen tube growth in F. mandshurica. Our findings revealed the role of cellular compartments in ROS generation during pollen tube growth. The H2DCFDA stain showed ROS accumulation during pollen growth, and treatment with ROS scavengers CuCl2 and DPI decreased pollen tube germination and ROS fluorescence. Similarly, knocking down FmRbohH significantly reduced ROS fluorescence and impaired pollen tube growth.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/f15101735/s1. Supplementary Table S1. qRT-PCR primer sequences Supplementary Table S2. Oligonucleotide primer sequences (AS-ODN and S-ODN) Supplementary Table S3. Raw and clean reads of 9 RNA samples. Supplemental Figure S1. TCA pathway in F. mandshurica during pollen tube germination.
Author Contributions
Conceptualization and design: X.L., S.W. and B.H.J. Data curation: S.W., B.H.J., Z.H., Y.W., S.Y. and Y.L. Manuscript writing: B.H.J. and S.W. Data analysis and manuscript revision: B.H.J., S.W. and X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China (2021YFD220030301) and Heilongjiang Touyan Innovation Team Program (Tree Genetics and Breeding Innovation Team).
Data Availability Statement
The transcriptome data can be accessed through https://www.ncbi.nlm.nih.gov using the BioSample accessions SAMN42883688, SAMN42883689, and SAMN42883690.
Acknowledgments
We thank the members of Lan Xingguo for their support in this project.
Conflicts of Interest
The authors declare no known conflicting interests.
References
- Adhikari, P.B.; Liu, X.; Wu, X.; Zhu, S.; Kasahara, R.D. Fertilization in flowering plants: An odyssey of sperm cell delivery. Plant Mol. Biol. 2020, 103, 9–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, Y.; Yi, R.; Shen, J.; Huang, S. Actin cytoskeleton in the control of vesicle transport, cytoplasmic organization, and pollen tube tip growth. Plant Physiol. 2023, 193, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Franklin-Tong, V.E. Signaling and the modulation of pollen tube growth. Plant Cell 1999, 11, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Grebnev, G.; Cvitkovic, M.; Fritz, C.; Cai, G.; Smith, A.-S.; Kost, B. Quantitative structural organization of bulk apical membrane traffic in pollen tubes. Plant Physiol. 2020, 183, 1559–1585. [Google Scholar] [CrossRef]
- Ruan, H.; Li, J.; Wang, T.; Ren, H. Secretory vesicles targeted to plasma membrane during pollen germination and tube growth. Front. Cell Dev. Biol. 2021, 8, 615447. [Google Scholar] [CrossRef]
- Hou, S.; Shi, J.; Hao, L.; Wang, Z.; Liao, Y.; Gu, H.; Dong, J.; Dresselhaus, T.; Zhong, S.; Qu, L.-J. VPS18-regulated vesicle trafficking controls the secretion of pectin and its modifying enzyme during pollen tube growth in Arabidopsis. Plant Cell 2021, 33, 3042–3056. [Google Scholar] [CrossRef]
- Miyazaki, S.; Murata, T.; Sakurai-Ozato, N.; Kubo, M.; Demura, T.; Fukuda, H.; Hasebe, M. ANXUR1 and 2, sister genes to FERONIA/SIRENE, are male factors for coordinated fertilization. Curr. Biol. 2009, 19, 1327–1331. [Google Scholar] [CrossRef]
- Ge, Z.; Bergonci, T.; Zhao, Y.; Zou, Y.; Du, S.; Liu, M.-C.; Luo, X.; Ruan, H.; García-Valencia, L.E.; Zhong, S. Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science 2017, 358, 1596–1600. [Google Scholar] [CrossRef]
- Kaya, H.; Nakajima, R.; Iwano, M.; Kanaoka, M.M.; Kimura, S.; Takeda, S.; Kawarazaki, T.; Senzaki, E.; Hamamura, Y.; Higashiyama, T. Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 2014, 26, 1069–1080. [Google Scholar] [CrossRef]
- Maksimov, N.; Evmenyeva, A.; Breygina, M.; Yermakov, I. The role of reactive oxygen species in pollen germination in Picea pungens (blue spruce). Plant Reprod. 2018, 31, 357–365. [Google Scholar] [CrossRef]
- Jimenez-Quesada, M.J.; Traverso, J.A.; Potocký, M.; Žárský, V.; Alché, J.d.D. Generation of superoxide by OeRbohH, a NADPH oxidase activity during olive (Olea europaea L.) pollen development and germination. Front. Plant Sci. 2019, 10, 455319. [Google Scholar] [CrossRef] [PubMed]
- Sumimoto, H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008, 275, 3249–3277. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, S.; Ju, Y.; Kessler, S.A. Reactive oxygen species as mediators of gametophyte development and double fertilization in flowering plants. Front. Plant Sci. 2020, 11, 568949. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Tang, C.; Lv, S.; Zhang, S.; Wu, J.; Wang, P. PbRbohH/J mediates ROS generation to regulate the growth of pollen tube in pear. Plant Physiol. Biochem. 2024, 207, 108342. [Google Scholar] [CrossRef]
- Zhang, M.J.; Zhang, X.S.; Gao, X.-Q. ROS in the male–female interactions during pollination: Function and regulation. Front. Plant Sci. 2020, 11, 514851. [Google Scholar] [CrossRef] [PubMed]
- Speranza, A.; Crinelli, R.; Scoccianti, V.; Geitmann, A. Reactive oxygen species are involved in pollen tube initiation in kiwifruit. Plant Biol. 2012, 14, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Potocký, M.; Jones, M.A.; Bezvoda, R.; Smirnoff, N.; Žárský, V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol. 2007, 174, 742–751. [Google Scholar] [CrossRef]
- Do, T.H.T.; Choi, H.; Palmgren, M.; Martinoia, E.; Hwang, J.-U.; Lee, Y. Arabidopsis ABCG28 is required for the apical accumulation of reactive oxygen species in growing pollen tubes. Proc. Natl. Acad. Sci. USA 2019, 116, 12540–12549. [Google Scholar] [CrossRef]
- Oda, T.; Hashimoto, H.; Kuwabara, N.; Akashi, S.; Hayashi, K.; Kojima, C.; Wong, H.L.; Kawasaki, T.; Shimamoto, K.; Sato, M. Structure of the N-terminal regulatory domain of a plant NADPH oxidase and its functional implications. J. Biol. Chem. 2010, 285, 1435–1445. [Google Scholar] [CrossRef]
- Torres, M.A.; Dangl, J.L. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 2005, 8, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-F.; Li, W.-Q.; Li, W.-Y.; Wu, G.-L.; Zhou, C.-Y.; Chen, K.-M. Characterization of rice NADPH oxidase genes and their expression under various environmental conditions. Int. J. Mol. Sci. 2013, 14, 9440–9458. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, D.J.; Boettcher, A.; Little, A.; Shirley, N.; Able, A.J. Identification and characterisation of barley (Hordeum vulgare) respiratory burst oxidase homologue family members. Funct. Plant Biol. 2008, 35, 347–359. [Google Scholar] [CrossRef]
- Cheng, C.; Xu, X.; Gao, M.; Li, J.; Guo, C.; Song, J.; Wang, X. Genome-wide analysis of respiratory burst oxidase homologs in grape (Vitis vinifera L.). Int. J. Mol. Sci. 2013, 14, 24169–24186. [Google Scholar] [CrossRef]
- Cepauskas, D.; Miliute, I.; Staniene, G.; Gelvonauskiene, D.; Stanys, V.; Jesaitis, A.J.; Baniulis, D. Characterization of apple NADPH oxidase genes and their expression associated with oxidative stress in shoot culture in vitro. Plant Cell Tissue Organ Cult. (PCTOC) 2016, 124, 621–633. [Google Scholar] [CrossRef]
- Marino, D.; Andrio, E.; Danchin, E.G.; Oger, E.; Gucciardo, S.; Lambert, A.; Puppo, A.; Pauly, N. A Medicago truncatula NADPH oxidase is involved in symbiotic nodule functioning. New Phytol. 2011, 189, 580–592. [Google Scholar] [CrossRef]
- Sagi, M.; Fluhr, R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006, 141, 336–340. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Luo, L.; Lu, C.; Kong, W.; Cheng, L.; Xu, X.; Liu, J. Genome wide identification of respiratory burst oxidase homolog (Rboh) genes in Citrus sinensis and functional analysis of CsRbohD in cold tolerance. Int. J. Mol. Sci. 2022, 23, 648. [Google Scholar] [CrossRef]
- Zhu, Y.; Su, H.; Liu, X.-X.; Sun, J.-F.; Xiang, L.; Liu, Y.-J.; Hu, Z.-W.; Xiong, X.-Y.; Yang, X.-M.; Bhutto, S.H. Identification of NADPH Oxidase Genes Crucial for Rice Multiple Disease Resistance and Yield Traits. Rice 2024, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Su, W.; Zhang, H.; Zhan, Y.; Zeng, F. Fraxinus mandshurica 4-coumarate-CoA ligase 2 enhances drought and osmotic stress tolerance of tobacco by increasing coniferyl alcohol content. Plant Physiol. Biochem. 2020, 155, 697–708. [Google Scholar] [CrossRef]
- Wallander, E. Systematics of Fraxinus (Oleaceae) and evolution of dioecy. Plant Syst. Evol. 2008, 273, 25–49. [Google Scholar] [CrossRef]
- Zhu, Z.; Qi, F.; Yan, C.; Zhan, Y. Sexually different morphological, physiological and molecular responses of Fraxinus mandshurica flowers to floral development and chilling stress. Plant Physiol. Biochem. 2016, 99, 97–107. [Google Scholar] [CrossRef]
- Wang, S.; Yang, S.; Jakada, B.H.; Qin, H.; Zhan, Y.; Lan, X. Transcriptomics reveal the involvement of reactive oxygen species production and sequestration during stigma development and pollination in Fraxinus mandshurica. For. Res. 2024, 4, e014. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Zhou, H.; Zhang, L.; Jiang, X.; Liu, Y. Ca2+ participates in programmed cell death by modulating ROS during pollen cryopreservation. Plant Cell Rep. 2022, 41, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.X.; Shi, Y.; Di, W.; Jiang, X.R.; Xu, J.; Liu, Y. ROS-induced oxidative stress is closely related to pollen deterioration following cryopreservation. Vitr. Cell. Dev. Biol.-Plant 2017, 53, 433–439. [Google Scholar] [CrossRef]
- Chen, J.; Wang, P.; de Graaf, B.H.J.; Zhang, H.; Jiao, H.; Tang, C.; Zhang, S.; Wu, J. Phosphatidic Acid Counteracts S-RNase Signaling in Pollen by Stabilizing the Actin Cytoskeleton. Plant Cell 2018, 30, 1023–1039. [Google Scholar] [CrossRef]
- Huang, S.; Braun, H.P.; Gawryluk, R.M.; Millar, A.H. Mitochondrial complex II of plants: Subunit composition, assembly, and function in respiration and signaling. Plant J. 2019, 98, 405–417. [Google Scholar] [CrossRef]
- Honys, D.; Twell, D. Comparative analysis of the Arabidopsis pollen transcriptome. Plant Physiol. 2003, 132, 640–652. [Google Scholar] [CrossRef]
- Kim, E.-J.; Hong, W.-J.; Kim, Y.-J.; Jung, K.-H. Transcriptome analysis of triple mutant for OsMADS62, OsMADS63, and OsMADS68 reveals the downstream regulatory mechanism for pollen germination in rice (Oryza sativa). Int. J. Mol. Sci. 2021, 23, 239. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxidative Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Kadenbach, B. Introduction to mitochondrial oxidative phosphorylation. In Mitochondrial Oxidative Phosphorylation: Nuclear-Encoded Genes, Enzyme Regulation, and Pathophysiology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–11. [Google Scholar]
- Bazil, J.N.; Beard, D.A.; Vinnakota, K.C. Catalytic coupling of oxidative phosphorylation, ATP demand, and reactive oxygen species generation. Biophys. J. 2016, 110, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Biol. 2004, 7, 254–261. [Google Scholar] [CrossRef]
- Okoye, C.N.; Koren, S.A.; Wojtovich, A.P. Mitochondrial complex I ROS production and redox signaling in hypoxia. Redox Biol. 2023, 67, 102926. [Google Scholar] [CrossRef]
- Maldonado, M.; Guo, F.; Letts, J.A. Atomic structures of respiratory complex III(2), complex IV, and supercomplex III(2)-IV from vascular plants. Elife 2021, 10, e62047. [Google Scholar] [CrossRef]
- Mansilla, N.; Racca, S.; Gras, D.E.; Gonzalez, D.H.; Welchen, E. The Complexity of Mitochondrial Complex IV: An Update of Cytochrome c Oxidase Biogenesis in Plants. Int. J. Mol. Sci. 2018, 19, 662. [Google Scholar] [CrossRef] [PubMed]
- Petersen, G.; Shyama Prasad Rao, R.; Anderson, B.; Zervas, A.; Seberg, O.; Rasmusson, A.G.; Max Møller, I. Genes from oxidative phosphorylation complexes II-V and two dual-function subunits of complex I are transcribed in Viscum album despite absence of the entire mitochondrial holo-complex I. Mitochondrion 2022, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Corpas, F.J.; Gupta, D.K.; Palma, J.M. Production sites of reactive oxygen species (ROS) in organelles from plant cells. In Reactive Oxygen Species and Oxidative Damage in Plants Under Stress; Springer: Cham, Switzerland, 2015; pp. 1–22. [Google Scholar]
- de Almeida, A.; de Oliveira, J.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways. Oxid. Med. Cell Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and oxidative burst: Roots in plant development. Plant Divers. 2020, 42, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.-J.; Zhou, Y.-H.; Shi, K.; Zhou, J.; Foyer, C.H.; Yu, J.-Q. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 2015, 66, 2839–2856. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Bae, H. ROS interplay between plant growth and stress biology: Challenges and future perspectives. Plant Physiol. Biochem. 2023, 203, 108032. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, M.H.; Hong, W.J.; Moon, S.; Kim, S.T.; Park, S.K.; Jung, K.H. OsMTD2-mediated reactive oxygen species (ROS) balance is essential for intact pollen-tube elongation in rice. Plant J. 2021, 107, 1131–1147. [Google Scholar] [CrossRef]
- Duan, Q.; Kita, D.; Johnson, E.A.; Aggarwal, M.; Gates, L.; Wu, H.-M.; Cheung, A.Y. Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat. Commun. 2014, 5, 3129. [Google Scholar] [CrossRef]
- Puerto-Galán, L.; Pérez-Ruiz, J.M.; Ferrández, J.; Cano, B.; Naranjo, B.; Nájera, V.A.; González, M.; Lindahl, A.M.; Cejudo, F.J. Overoxidation of chloroplast 2-Cys peroxiredoxins: Balancing toxic and signaling activities of hydrogen peroxide. Front. Plant Sci. 2013, 4, 310. [Google Scholar] [CrossRef]
- Benkő, P.; Gémes, K.; Fehér, A. Polyamine Oxidase-Generated Reactive Oxygen Species in Plant Development and Adaptation: The Polyamine Oxidase—NADPH Oxidase Nexus. Antioxidants 2022, 11, 2488. [Google Scholar] [CrossRef] [PubMed]
- Gémes, K.; Kim, Y.J.; Park, K.Y.; Moschou, P.N.; Andronis, E.; Valassaki, C.; Roussis, A.; Roubelakis-Angelakis, K.A. An NADPH-oxidase/polyamine oxidase feedback loop controls oxidative burst under salinity. Plant Physiol. 2016, 172, 1418–1431. [Google Scholar] [CrossRef]
- Schürmann, P.; Jacquot, J.-P. Plant thioredoxin systems revisited. Annu. Rev. Plant Biol. 2000, 51, 371–400. [Google Scholar] [CrossRef] [PubMed]
- Maruta, T.; Tanouchi, A.; Tamoi, M.; Yabuta, Y.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. Arabidopsis chloroplastic ascorbate peroxidase isoenzymes play a dual role in photoprotection and gene regulation under photooxidative stress. Plant Cell Physiol. 2010, 51, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Serrano, M.; Romero-Puertas, M.C.; Pastori, G.M.; Corpas, F.J.; Sandalio, L.M.; del Río, L.A.; Palma, J.M. Peroxisomal membrane manganese superoxide dismutase: Characterization of the isozyme from watermelon (Citrullus lanatus Schrad.) cotyledons. J. Exp. Bot. 2007, 58, 2417–2427. [Google Scholar] [CrossRef] [PubMed]
- Arent, S.; Pye, V.E.; Henriksen, A. Structure and function of plant acyl-CoA oxidases. Plant Physiol. Biochem. 2008, 46, 292–301. [Google Scholar] [CrossRef]
- Queval, G.; Issakidis-Bourguet, E.; Hoeberichts, F.A.; Vandorpe, M.; Gakière, B.; Vanacker, H.; Miginiac-Maslow, M.; Van Breusegem, F.; Noctor, G. Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J. 2007, 52, 640–657. [Google Scholar]
- Muhlemann, J.K.; Younts, T.L.; Muday, G.K. Flavonols control pollen tube growth and integrity by regulating ROS homeostasis during high-temperature stress. Proc. Natl. Acad. Sci. USA 2018, 115, E11188–E11197. [Google Scholar] [CrossRef]
- Mangano, S.; Juárez, S.P.D.; Estevez, J.M. ROS regulation of polar growth in plant cells. Plant Physiol. 2016, 171, 1593–1605. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, J.; Su, S.; Wei, X.; Yang, L.; Zhao, H.; Yu, J.; Wang, J.; Hui, J.; Hao, S. FERONIA receptor kinase-regulated reactive oxygen species mediate self-incompatibility in Brassica rapa. Curr. Biol. 2021, 31, 3004–3016.e3004. [Google Scholar] [CrossRef]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory burst oxidases: The engines of ROS signaling. Curr. Opin. Plant Biol. 2011, 14, 691–699. [Google Scholar] [CrossRef]
- Yadegari, R.; Drews, G.N. Female Gametophyte Development. Plant Cell 2004, 16 (Suppl. S1), S133–S141. [Google Scholar] [CrossRef]
- Zafra, A.; Rejón, J.D.; Hiscock, S.J.; Alché, J.d.D. Patterns of ROS accumulation in the stigmas of angiosperms and visions into their multi-functionality in plant reproduction. Front. Plant Sci. 2016, 7, 186512. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.; Iwano, M.; Takeda, S.; Kanaoka, M.M.; Kimura, S.; Abe, M.; Kuchitsu, K. Apoplastic ROS production upon pollination by RbohH and RbohJ in Arabidopsis. Plant Signal. Behav. 2015, 10, e989050. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).