Altitudinal Influences on Soil Microbial Diversity and Community Assembly in Topsoil and Subsoil Layers: Insights from the Jinsha River Basin, Southwest China
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Field Survey and Soil Sampling
2.3. Soil Physical and Chemical Property
2.4. DNA Extraction and Sequencing
2.5. Data Analysis
3. Results
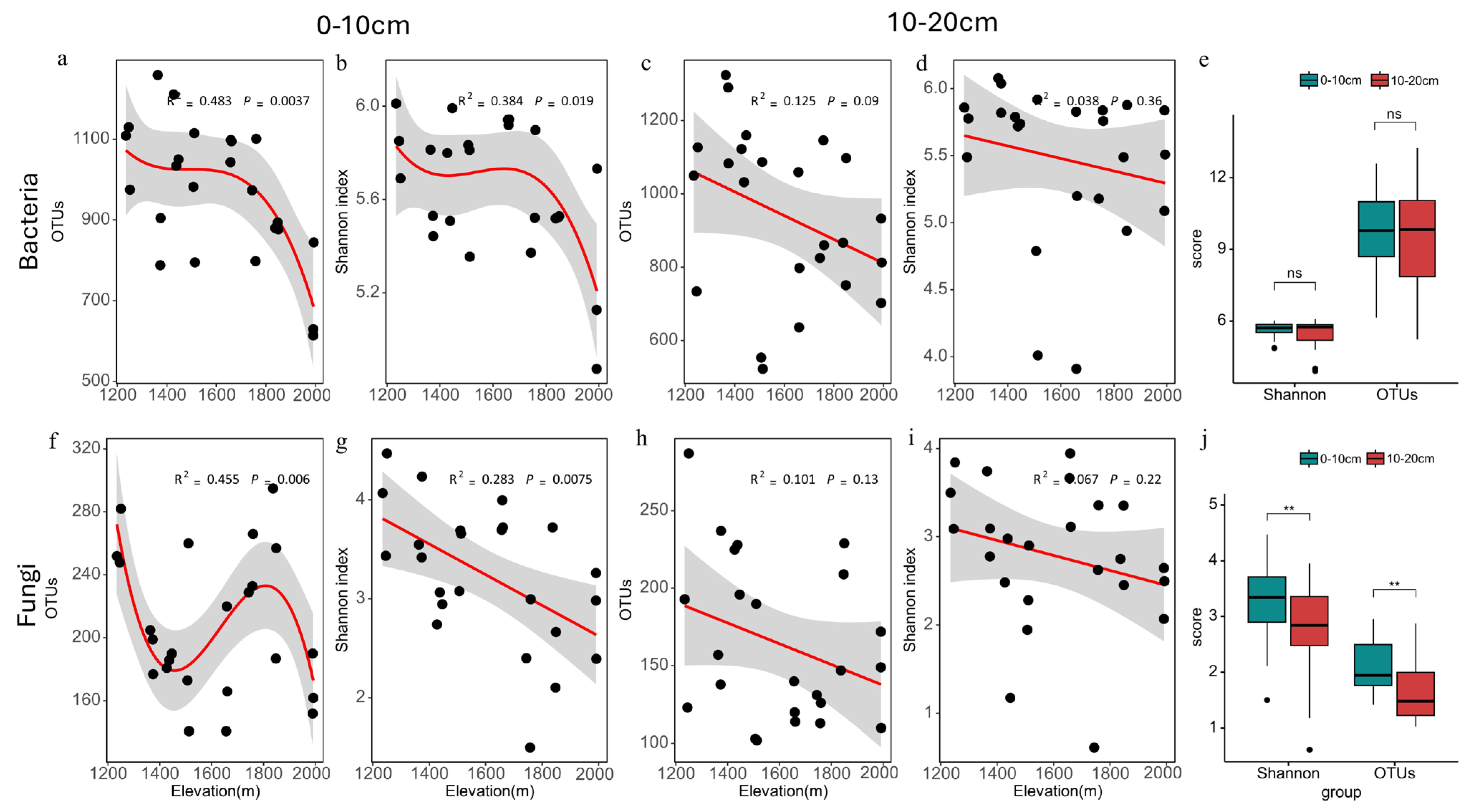
3.1. Changes in Soil Microbial α-Diversity along an Altitudinal Gradient
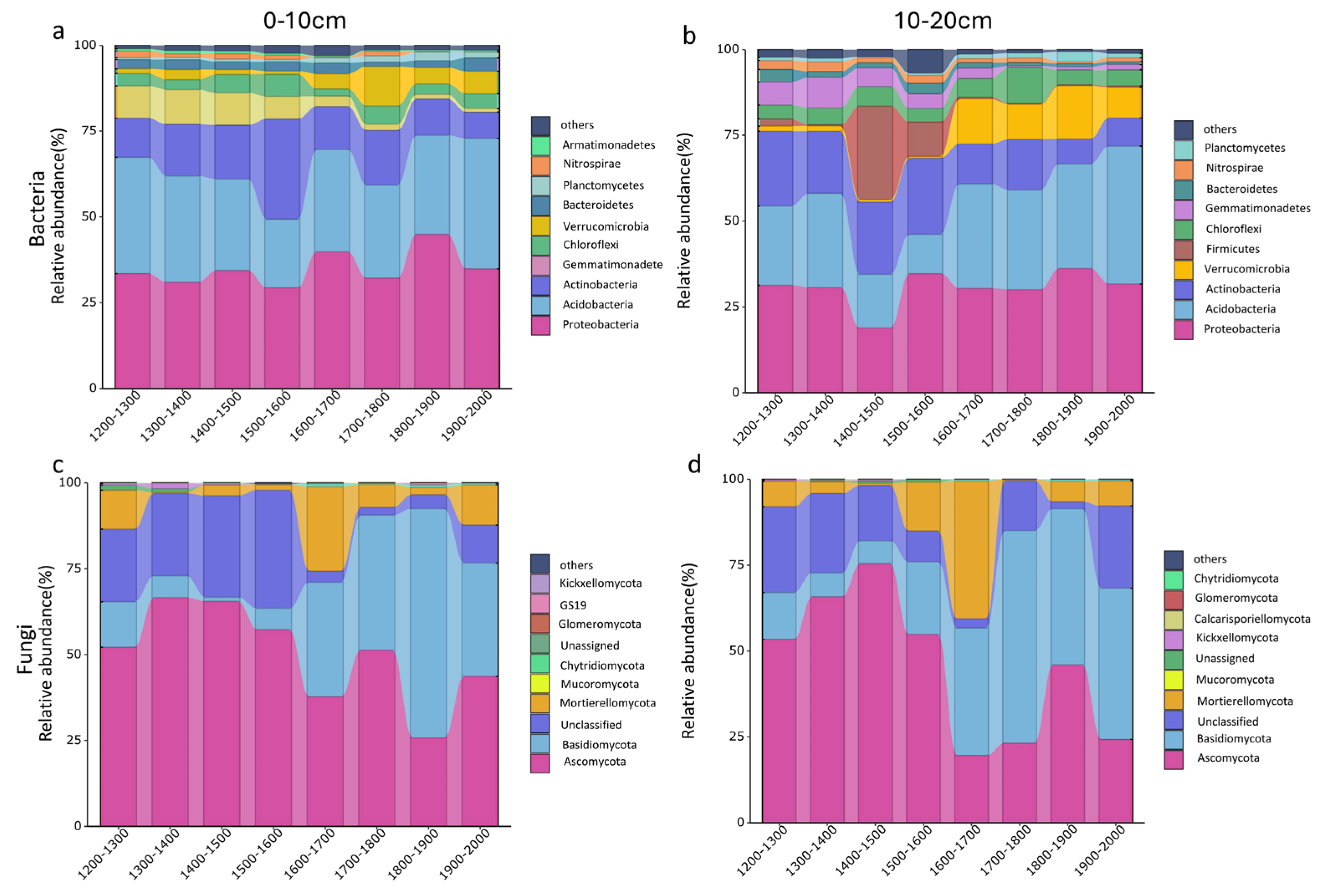
3.2. Changes in Soil Microbial Community Composition along an Altitudinal Gradient
3.3. Changes in Soil Main Microbial Phylum along an Altitudinal Gradient
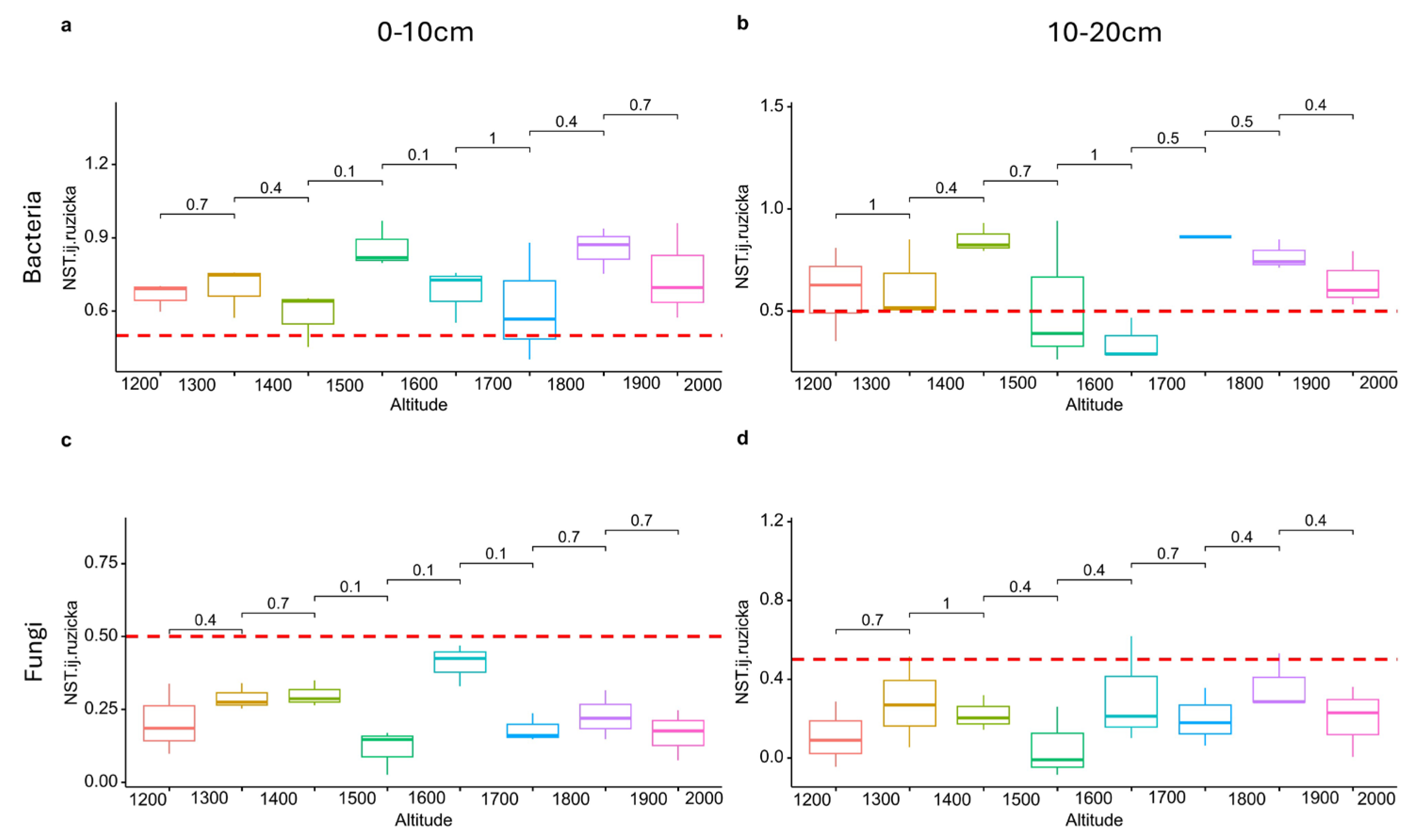
3.4. Changes in Soil Community Assembly along the Altitudinal Gradient
3.5. Changes in Soil Microbial Network Complexity along an Altitudinal Gradient
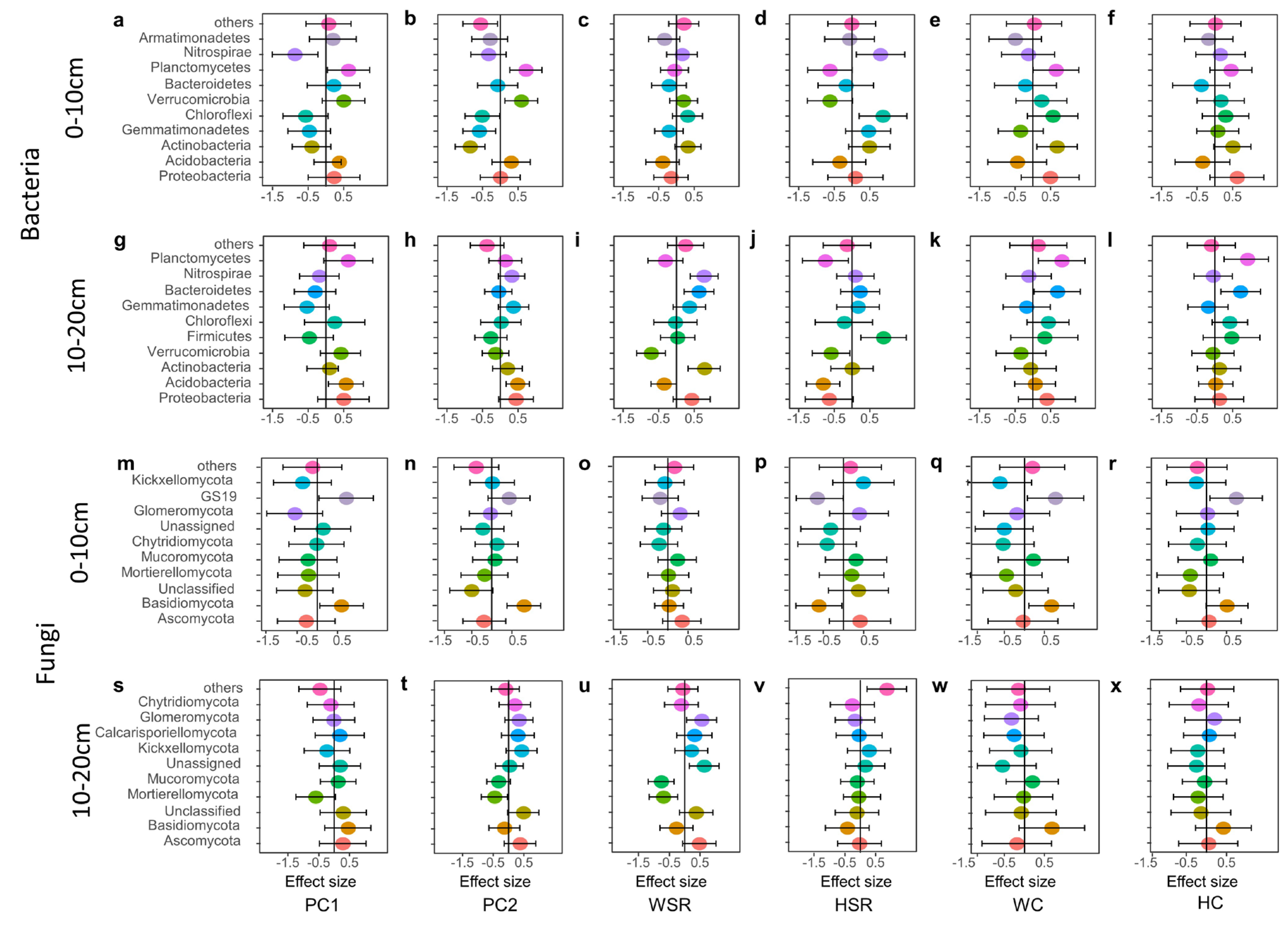
3.6. Factors Affecting the Relative Abundance of Soil Microbial Main Phylum
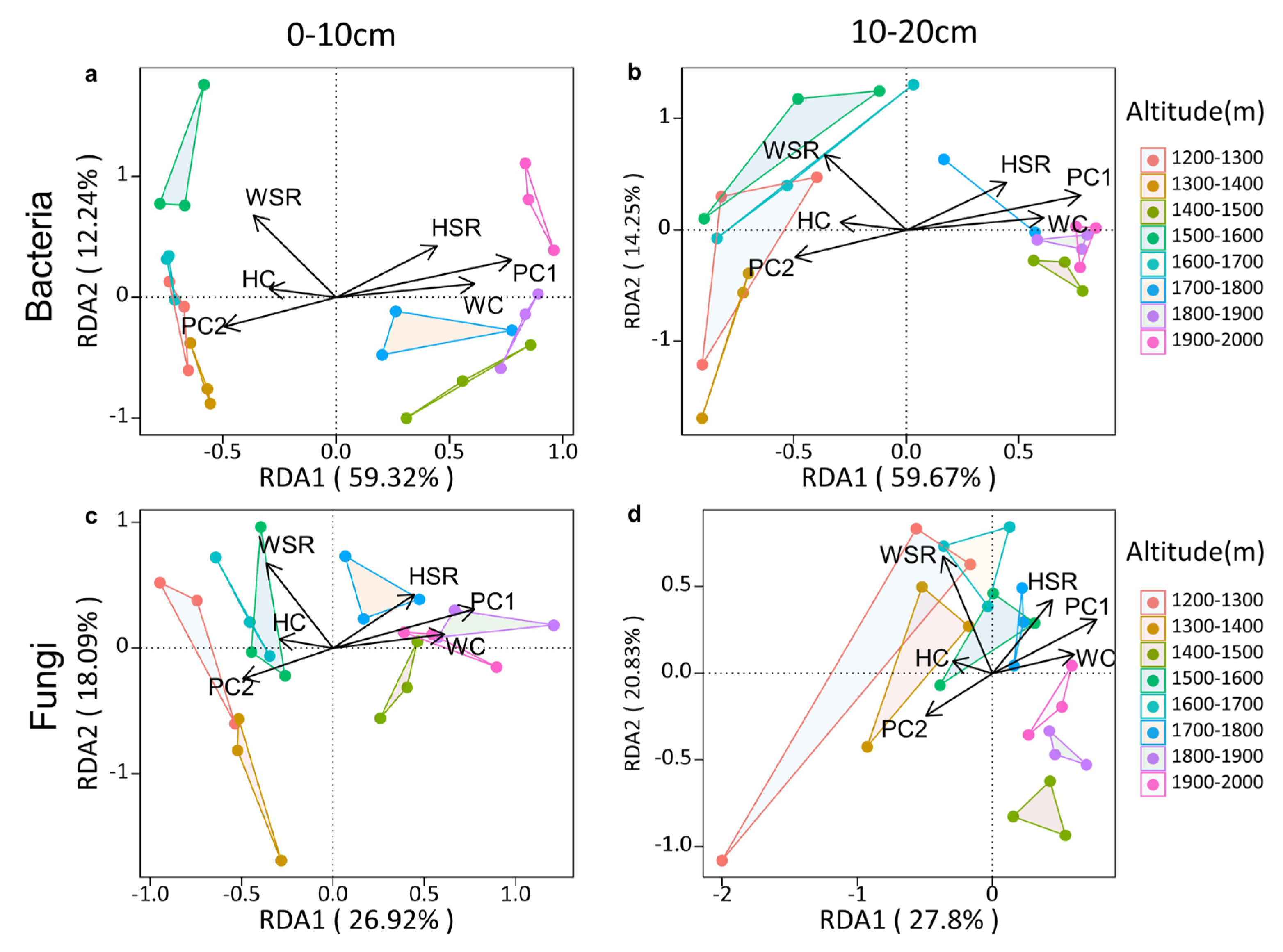
3.7. Factors Affecting Soil Microbial Community Composition
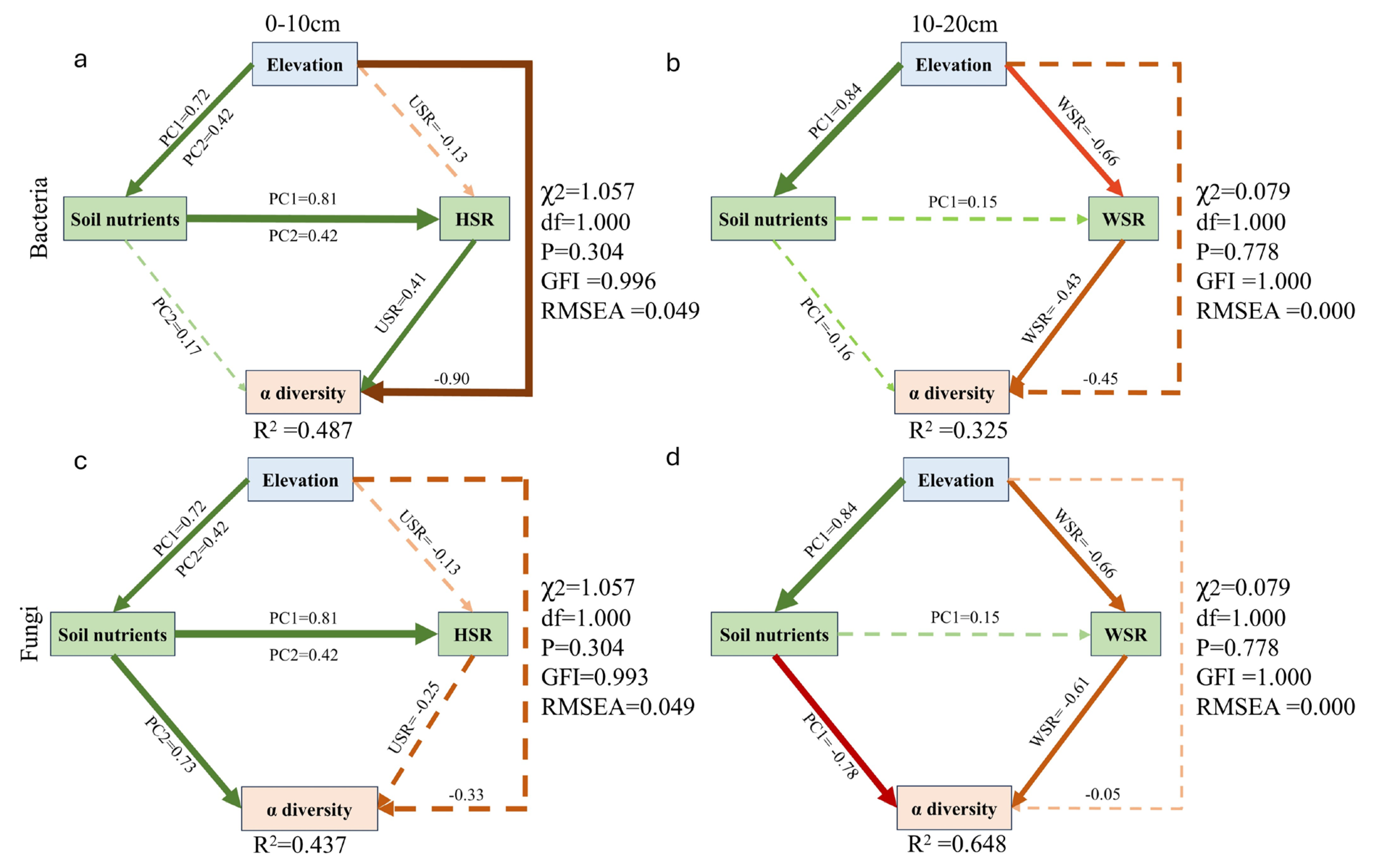
3.8. Combined Effects of Soil Nutrients and Plant Diversity on Soil Microbial Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aqeel, M.; Khalid, N.; Noman, A.; Ran, J.Z.; Manan, A.; Hou, Q.Q.; Dong, L.W.; Sun, Y.; Deng, Y.; Lee, S.S. Interplay between edaphic and climatic factors unravels plant and microbial diversity along an altitudinal gradient. Environ. Res. 2024, 242, 117711. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.Q.; Gelwick, K.; Willett, S.D.; Shen, X.W.; Albouy, C.; Luo, A.; Wang, Z.H.; Zimmermann, N.E.; Pellissier, L. Phytodiversity is associated with habitat heterogeneity from Eurasia to the Hengduan Mountains. New Phytol. 2023, 240, 1647–1658. [Google Scholar] [CrossRef] [PubMed]
- Dani, R.S.; Divakar, P.K.; Baniya, C.B. Diversity and composition of plants species along elevational gradient: Research trends. Biol. Conserv. 2023, 32, 2961–2980. [Google Scholar] [CrossRef]
- Song, X.; Cao, M.; Li, J.; Kitching, R.L.; Nakamura, A.; Laidlaw, M.J.; Tang, Y.; Sun, Z.; Zhang, W.; Yang, J. Different environmental factors drive tree species diversity along elevation gradients in three climatic zones in Yunnan, southern China. Plant Divers. 2021, 43, 433–443. [Google Scholar] [CrossRef]
- Gan, D.; Zeng, H.; Zhu, B. The rhizosphere effect on soil gross nitrogen mineralization: A meta-analysis. Soil Ecol. Lett. 2022, 4, 144–154. [Google Scholar] [CrossRef]
- Fu, F.W.; Li, J.R.; Li, S.F.; Chen, W.S.; Ding, H.H.; Xiao, S.Y.; Li, Y.Y. Elevational distribution patterns and drivers of soil microbial diversity in the Sygera Mountains, southeastern Tibet, China. Catena 2023, 221, 106738. [Google Scholar] [CrossRef]
- Bryant, J.A.; Lamanna, C.; Morlon, H.; Kerkhoff, A.J.; Enquist, B.J.; Green, J.L. Microbes on mountainsides: Contrasting elevational patterns of bacterial and plant diversity. Proc. Natl. Acad. Sci. USA 2008, 105, 11505–11511. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Wu, H.T.; Zhang, Y.F.; Wu, Q.; Guan, Q.; Lu, K.L.; Lin, Y.L. Differential distribution patterns and assembly processes of soil microbial communities under contrasting vegetation types at distinctive altitudes in the Changbai Mountain. Front. Microbiol. 2023, 14, 1152818. [Google Scholar] [CrossRef]
- Meng, H.; Li, K.; Nie, M.; Wan, J.R.; Quan, Z.X.; Fang, C.M.; Chen, J.K.; Gu, J.D.; Li, B. Responses of bacterial and fungal communities to an elevation gradient in a subtropical montane forest of China. Appl. Microbiol. Biotechnol. 2013, 97, 2219–2230. [Google Scholar] [CrossRef]
- Anslan, S.; Bahram, M.; Tedersoo, L. Seasonal and annual variation in fungal communities associated with epigeic springtails (Collembola spp.) in boreal forests. Soil Biol. Biochem. 2018, 116, 245–252. [Google Scholar] [CrossRef]
- Wang, J.J.; Hu, A.; Meng, F.F.; Zhao, W.Q.; Yang, Y.F.; Soininen, J.; Shen, J.; Zhou, J.Z. Embracing mountain microbiome and ecosystem functions under global change. New Phytol. 2022, 234, 1987–2002. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.M.; Chen, Y.; Li, Y.P.; Zhang, C.C.; Huang, Z.P.; Xiao, W. Taxonomic and phylogenetic perspectives reveal the community assembly of different forest strata along an altitudinal gradient. Ecol. Res. 2024, 39, 72–83. [Google Scholar] [CrossRef]
- Zhou, J.; Ning, D. Stochastic community assembly: Does it matter in microbial ecology? Microbiol. Mol. Biol. Rev. 2017, 81, e00002-17. [Google Scholar] [CrossRef]
- Zhang, X.; Johnston, E.R.; Liu, W.; Li, L.; Han, X. Environmental changes affect the assembly of soil bacterial community primarily by mediating stochastic processes. Glob. Change Biol. 2016, 22, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Sheng, S.; Shen, F.Y.; Yang, L.L.; Wen, S.Z.; He, G.X.; Wang, N.; Wang, X.; Yang, L.X. Stochastic processes dominated the soil bacterial community assemblages along an altitudinal gradient in boreal forests. Catena 2024, 237, 107816. [Google Scholar] [CrossRef]
- Lin, Y.M.; Cui, P.; Ge, Y.G.; Chen, C.; Wang, D.J.; Wu, C.Z.; Li, J.; Yu, W.; Zhang, G.S.; Lin, H. The succession characteristics of soil erosion during different vegetation succession stages in dry-hot river valley of Jinsha River, upper reaches of Yangtze River. Ecol. Eng. 2014, 62, 13–26. [Google Scholar] [CrossRef]
- Schimel, J.P. Life in dry soils: Effects of drought on soil microbial communities and processes. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 409–432. [Google Scholar] [CrossRef]
- Zhang, R.; Bai, X.P.; Tian, X.; Chen, Z.J.; Zhang, H.Y.; Liu, H.T. Rapid warming exacerbates winter drought stress in trees at high-altitude areas in northeast China. Forests 2024, 15, 565. [Google Scholar] [CrossRef]
- Agler, M.T.; Ruhe, J.; Kroll, S.; Morhenn, C.; Kim, S.T.; Weigel, D.; Kemen, E.M. Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol. 2016, 14, e1002352. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, Q.C.; Xu, D.L.; Li, Z.; Chao, L.M.; Li, X.Y.; Liu, H.J.; Wang, P.F.; Zheng, Y.X.; Liu, X.Y. Soil microbial community composition and co-occurrence network responses to mild and severe disturbances in volcanic areas. Sci. Total Environ. 2023, 901, 165889. [Google Scholar] [CrossRef]
- Chen, W.Q.; Wang, J.Y.; Chen, X.; Meng, Z.X.; Xu, R.; Duoji, D.; Zhang, J.H.; He, J.M.; Wang, Z.G.; Chen, J.; et al. Soil microbial network complexity predicts ecosystem function along elevation gradients on the Tibetan Plateau. Soil Biol. Biochem. 2022, 172, 108766. [Google Scholar] [CrossRef]
- Wang, C.W.; Ma, L.N.; Zuo, X.A.; Ye, X.H.; Wang, R.Z.; Huang, Z.Y.; Liu, G.F.; Cornelissen, J.H.C. Plant diversity has stronger linkage with soil fungal diversity than with bacterial diversity across grasslands of northern China. Glob. Ecol. Biogeogr. 2022, 31, 886–900. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef]
- Clark, M.; Schoenbohm, L.; Royden, L.; Whipple, K.; Burchfiel, B.; Zhang, X.; Tang, W.; Wang, E.; Chen, L. Surface uplift, tectonics, and erosion of eastern Tibet from large-scale drainage patterns. Tectonics 2004, 23. [Google Scholar] [CrossRef]
- Li, S.; Huang, X.; Tang, R.; Li, J.; Zhu, B.; Su, J. Soil microbial diversity and network complexity sustain ecosystem multifunctionality following afforestation in a dry-hot valley savanna. Catena 2023, 231, 107329. [Google Scholar] [CrossRef]
- Li, S.; Huang, X.; Lang, X.; Shen, J.; Xu, F.; Su, J. Cumulative effects of multiple biodiversity attributes and abiotic factors on ecosystem multifunctionality in the Jinsha River valley of southwestern China. For. Ecol. Manag. 2020, 472, 118281. [Google Scholar] [CrossRef]
- Zhu, H.; Tan, Y.H.; Yan, L.C.; Liu, F.Y. Flora of the savanna-like vegetation in hot dry valleys, southwestern China with implications to their origin and evolution. Bot. Rev. 2020, 86, 281–297. [Google Scholar] [CrossRef]
- Wu, H.; Xiong, D.H.; Xiao, L.; Zhang, S.; Yuan, Y.; Su, Z.A.; Zhang, B.J.; Yang, D. Effects of vegetation coverage and seasonal change on soil microbial biomass and community structure in the dry-hot valley region. J. Mt. Sci. 2018, 15, 1546–1558. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Jing, X.; Sanders, N.J.; Shi, Y.; Chu, H.Y.; Classen, A.T.; Zhao, K.; Chen, L.T.; Shi, Y.; Jiang, Y.X.; He, J.S. The links between ecosystem multifunctionality and above-and belowground biodiversity are mediated by climate. Nat. Commun. 2015, 6, 8159. [Google Scholar] [CrossRef]
- Ji, L.; Yang, Y.C.; Yang, L.X. Seasonal variations in soil fungal communities and co-occurrence networks along an altitudinal gradient in the cold temperate zone of China: A case study on Oakley Mountain. Catena 2021, 204, 105448. [Google Scholar] [CrossRef]
- Ning, D.L.; Deng, Y.; Tiedje, J.M.; Zhou, J.Z. A general framework for quantitatively assessing ecological stochasticity. Proc. Natl. Acad. Sci. USA 2019, 116, 16892–16898. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.Q.W.; Sorensen, P.O.; Zhu, G.Y.; Jia, X.Y.; Liu, J.; Shangguan, Z.P.; Wang, R.W.; Yan, W.M. Differential microbial assembly processes and co-occurrence networks in the soil-root continuum along an environmental gradient. iMeta 2022, 1, e18. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Zhao, C.; Kirkby, C.A.; Coggins, S.; Zhao, S.; Bissett, A.; van der Heijden, M.G.; Kirkegaard, J.A.; Richardson, A.E. Microbial interkingdom associations across soil depths reveal network connectivity and keystone taxa linked to soil fine-fraction carbon content. Agric. Ecosyst. Environ. 2021, 320, 107559. [Google Scholar] [CrossRef]
- Yuan, M.M.; Guo, X.; Wu, L.W.; Zhang, Y.; Xiao, N.J.; Ning, D.L.; Shi, Z.; Zhou, X.S.; Wu, L.Y.; Yang, Y.F. Climate warming enhances microbial network complexity and stability. Nat. Clim. Change 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Jiao, S.; Peng, Z.H.; Qi, J.J.; Gao, J.M.; Wei, G.H. Linking bacterial-fungal relationships to microbial diversity and soil nutrient cycling. Msystems 2021, 6, e01052-20. [Google Scholar] [CrossRef]
- Grace, J.B. Structural equation modeling for observational studies. J. Wildl. Manag. 2008, 72, 14–22. [Google Scholar] [CrossRef]
- Yang, N.; Li, X.X.; Liu, D.; Zhang, Y.; Chen, Y.H.; Wang, B.; Hua, J.N.; Zhang, J.B.; Peng, S.L.; Ge, Z.W. Diversity patterns and drivers of soil bacterial and fungal communities along elevational gradients in the Southern Himalayas, China. Appl. Soil Ecol. 2022, 178, 104563. [Google Scholar] [CrossRef]
- Ma, L.W.; Liu, L.; Lu, Y.S.; Chen, L.; Zhang, Z.C.; Zhang, H.W.; Wang, X.R.; Shu, L.; Yang, Q.P.; Song, Q.N. When microclimates meet soil microbes: Temperature controls soil microbial diversity along an elevational gradient in subtropical forests. Soil Biol. Biochem. 2022, 166, 108566. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Winterfeldt, S.; Brangarí, A.C.; Hicks, L.C.; Rousk, J. Higher resistance and resilience of bacterial growth to drought in grasslands with historically lower precipitation. Soil Biol. Biochem. 2023, 177, 108889. [Google Scholar] [CrossRef]
- Cordero, I.; Leizeaga, A.; Hicks, L.C.; Rousk, J.; Bardgett, R.D. High intensity perturbations induce an abrupt shift in soil microbial state. ISME J. 2023, 17, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Yang, W.; Cai, Y.P.; Yi, Y.J.; Li, X.X. Relationship between vegetation and environment in an arid-hot valley in southwestern China. Sustainability 2018, 10, 4774. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, D.M.; Zang, L.P.; Zhang, G.Q.; Liu, Q.F.; He, Y.J.; Ding, F.J.; Wang, S.S.; Zhou, C.J.; Yang, Y.S. Natural restoration of degraded karst vegetation shifts soil microbial phosphorus acquisition strategies. Plant Soil. 2023, 490, 201–215. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Lundell, T.K.; Mäkelä, M.R.; Hildén, K. Lignin-modifying enzymes in filamentous basidiomycetes–ecological, functional and phylogenetic review. J. Basic Microbiol. 2010, 50, 5–20. [Google Scholar] [CrossRef]
- Osono, T.; Takeda, H. Fungal decomposition of Abies needle and Betula leaf litter. Mycologia 2006, 98, 172–179. [Google Scholar] [CrossRef]
- Naylor, D.; Coleman-Derr, D. Drought stress and root-associated bacterial communities. Front. Plant Sci. 2018, 8, 303756. [Google Scholar] [CrossRef]
- He, H.R.; Xu, M.Z.; Li, W.T.; Chen, L.; Chen, Y.N.; Moorhead, D.L.; Brangarí, A.C.; Liu, J.; Cui, Y.X.; Zeng, Y. Linking soil depth to aridity effects on soil microbial community composition, diversity and resource limitation. Catena 2023, 232, 107393. [Google Scholar] [CrossRef]
- Zhang, Y.; Biswas, A.; Adamchuk, V.I. Implementation of a sigmoid depth function to describe change of soil pH with depth. Geoderma 2017, 289, 1–10. [Google Scholar] [CrossRef]
- Yao, X.D.; Zhang, N.L.; Zeng, H.; Wang, W. Effects of soil depth and plant–soil interaction on microbial community in temperate grasslands of northern China. Sci. Total Environ. 2018, 630, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Aaron Hogan, J.; Crowther, T.W.; Xu, S.J.; Zhao, R.S.; Song, P.F.; Cui, M.F.; Song, X.Y.; Cao, M.; Yang, J. Drivers and mechanisms that contribute to microbial β-diversity patterns and range sizes in mountains across a climatic variability gradient. Ecography 2024, 3, e07049. [Google Scholar] [CrossRef]
- Boer, W.D.; Folman, L.B.; Summerbell, R.C.; Boddy, L. Living in a fungal world: Impact of fungi on soil bacterial niche development. FEMS Microbiol. Rev. 2005, 29, 795–811. [Google Scholar] [CrossRef] [PubMed]
- Hale, L.; Feng, W.T.; Yin, H.Q.; Guo, X.; Zhou, X.S.; Bracho, R.; Pegoraro, E.; Penton, C.R.; Wu, L.Y.; Cole, J. Tundra microbial community taxa and traits predict decomposition parameters of stable, old soil organic carbon. ISME J. 2019, 13, 2901–2915. [Google Scholar] [CrossRef]
- De Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef]
- Muneer, M.A.; Hou, W.; Li, J.; Huang, X.; Ur Rehman Kayani, M.; Cai, Y.; Yang, W.; Wu, L.; Ji, B.; Zheng, C. Soil pH: A key edaphic factor regulating distribution and functions of bacterial community along vertical soil profiles in red soil of pomelo orchard. BMC Microbiol. 2022, 22, 38. [Google Scholar] [CrossRef]
- Fierer, N.; McCain, C.M.; Meir, P.; Zimmermann, M.; Rapp, J.M.; Silman, M.R.; Knight, R. Microbes do not follow the elevational diversity patterns of plants and animals. Ecology 2011, 92, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Wei, Y.Q.; Lian, L.; Wei, B.; Bi, Y.X.; Liu, N.; Yang, G.W.; Zhang, Y.J. Macrofungi promote SOC decomposition and weaken sequestration by modulating soil microbial function in temperate steppe. Sci. Total Environ. 2023, 899, 165556. [Google Scholar] [CrossRef]
- Aguirre-von-Wobeser, E.; Rocha-Estrada, J.; Shapiro, L.R.; de la Torre, M. Enrichment of Verrucomicrobia, Actinobacteria and Burkholderiales drives selection of bacterial community from soil by maize roots in a traditional milpa agroecosystem. PLoS ONE 2018, 13, e0208852. [Google Scholar] [CrossRef]
- Kivlin, S.N.; Winston, G.C.; Goulden, M.L.; Treseder, K.K. Environmental filtering affects soil fungal community composition more than dispersal limitation at regional scales. Fungal Ecol. 2014, 12, 14–25. [Google Scholar] [CrossRef]
- Yang, T.; Sun, H.B.; Shen, C.C.; Chu, H.Y. Fungal assemblages in different habitats in an Erman’s birch forest. Front. Microbiol. 2016, 7, 1368. [Google Scholar] [CrossRef] [PubMed]
- Stegen, J.; Lin, X.; Konopka, A.; Fredrickson, J.K. Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J. 2012, 6, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Chu, H.Y.; Zhang, B.G.; Wei, X.R.; Chen, W.M.; Wei, G.H. Linking soil fungi to bacterial community assembly in arid ecosystems. iMeta 2022, 1, e2. [Google Scholar] [CrossRef]
- Fan, Q.P.; Liu, K.F.; Wang, Z.L.; Liu, D.; Li, T.; Hou, H.Y.; Zhang, Z.J.; Chen, D.H.; Zhang, S.; Yu, A.L. Soil microbial subcommunity assembly mechanisms are highly variable and intimately linked to their ecological and functional traits. Mol. Ecol. 2024, 33, e17302. [Google Scholar] [CrossRef]
- He, L.B.; Sun, X.Y.; Li, S.Y.; Zhou, W.Z.; Yu, J.T.; Zhao, G.Y.; Chen, Z.; Bai, X.T.; Zhang, J.S. Depth effects on bacterial community altitudinal patterns and assembly processes in the warm-temperate montane forests of China. Sci. Total Environ. 2024, 914, 169905. [Google Scholar] [CrossRef]
- ZHAO, C.M.; CHEN, W.L.; TIAN, Z.Q.; XIE, Z.Q. Altitudinal pattern of plant species diversity in Shennongjia Mountains, central China. J. Integr. Plant Biol. 2005, 47, 1431–1449. [Google Scholar] [CrossRef]
- Seabloom, E.W.; Caldeira, M.C.; Davies, K.F.; Kinkel, L.; Knops, J.M.; Komatsu, K.J.; MacDougall, A.S.; May, G.; Millican, M.; Moore, J.L.; et al. Globally consistent response of plant microbiome diversity across hosts and continents to soil nutrients and herbivores. Nat. Commun. 2023, 14, 3516. [Google Scholar] [CrossRef]
- Djukic, I.; Zehetner, F.; Mentler, A.; Gerzabek, M.H. Microbial community composition and activity in different alpine vegetation zones. Soil Biol. Biochem. 2010, 42, 155–161. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.Y.; Niu, J.F.; Dang, K.K.; Zhang, S.K.; Wang, S.Q.; Wang, Z.Z. Changes in physicochemical properties, enzymatic activities, and the microbial community of soil significantly influence the continuous cropping of Panax quinquefolius L. (American ginseng). Plant Soil. 2021, 463, 427–446. [Google Scholar] [CrossRef]
- Pan, J.W.; Guo, Q.Q.; Li, H.E.; Luo, S.Q.; Zhang, Y.Q.; Yao, S.; Fan, X.; Sun, X.G.; Qi, Y.J. Dynamics of soil nutrients, microbial community structure, enzymatic activity, and their relationships along a chronosequence of Pinus massoniana plantations. Forests 2021, 12, 376. [Google Scholar] [CrossRef]
- Zhang, B.; Xue, K.; Zhou, S.; Che, R.; Du, J.; Tang, L.; Pang, Z.; Wang, F.; Wang, D.; Cui, X.; et al. Phosphorus mediates soil prokaryote distribution pattern along a small-scale elevation gradient in Noijin Kangsang Peak, Tibetan Plateau. FEMS Microbiol. Ecol. 2019, 95, fiz076. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.L.; Li, W.T.; Pang, X.Y.; Liu, Q.H.; Yin, C.Y. Soil properties and root traits are important factors driving rhizosphere soil bacterial and fungal community variations in alpine Rhododendron nitidulum shrub ecosystems along an altitudinal gradient. Sci. Total Environ. 2023, 864, 161048. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Huang, X.; Wang, T.; Su, J.; Li, S. Altitudinal Influences on Soil Microbial Diversity and Community Assembly in Topsoil and Subsoil Layers: Insights from the Jinsha River Basin, Southwest China. Forests 2024, 15, 1746. https://doi.org/10.3390/f15101746
Guo Z, Huang X, Wang T, Su J, Li S. Altitudinal Influences on Soil Microbial Diversity and Community Assembly in Topsoil and Subsoil Layers: Insights from the Jinsha River Basin, Southwest China. Forests. 2024; 15(10):1746. https://doi.org/10.3390/f15101746
Chicago/Turabian StyleGuo, Zhihong, Xiaobo Huang, Tongli Wang, Jianrong Su, and Shuaifeng Li. 2024. "Altitudinal Influences on Soil Microbial Diversity and Community Assembly in Topsoil and Subsoil Layers: Insights from the Jinsha River Basin, Southwest China" Forests 15, no. 10: 1746. https://doi.org/10.3390/f15101746








