Dynamic Changes in Flavonoids’ Accumulation Pattern in Tilia miqueliana Flowers at Different Developmental Stages Based on Widely Targeted Metabolomic Analysis
Abstract
1. Introduction
2. Materials and Methods
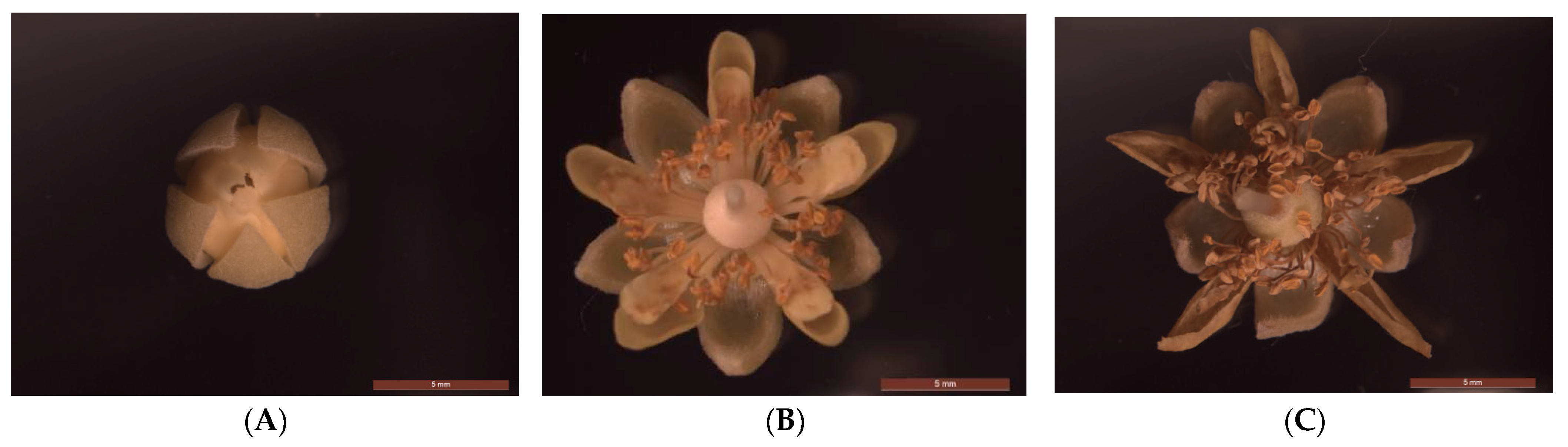
2.1. Plant Materials and Reagents
2.2. Sample Preparation
2.3. Sample Detection and Analysis
2.4. Qualitative and Quantitative Analysis of Metabolites
2.5. Statistical Analysis
3. Results
3.1. Sample Quality Evaluation and Multivariate Statistical Analysis
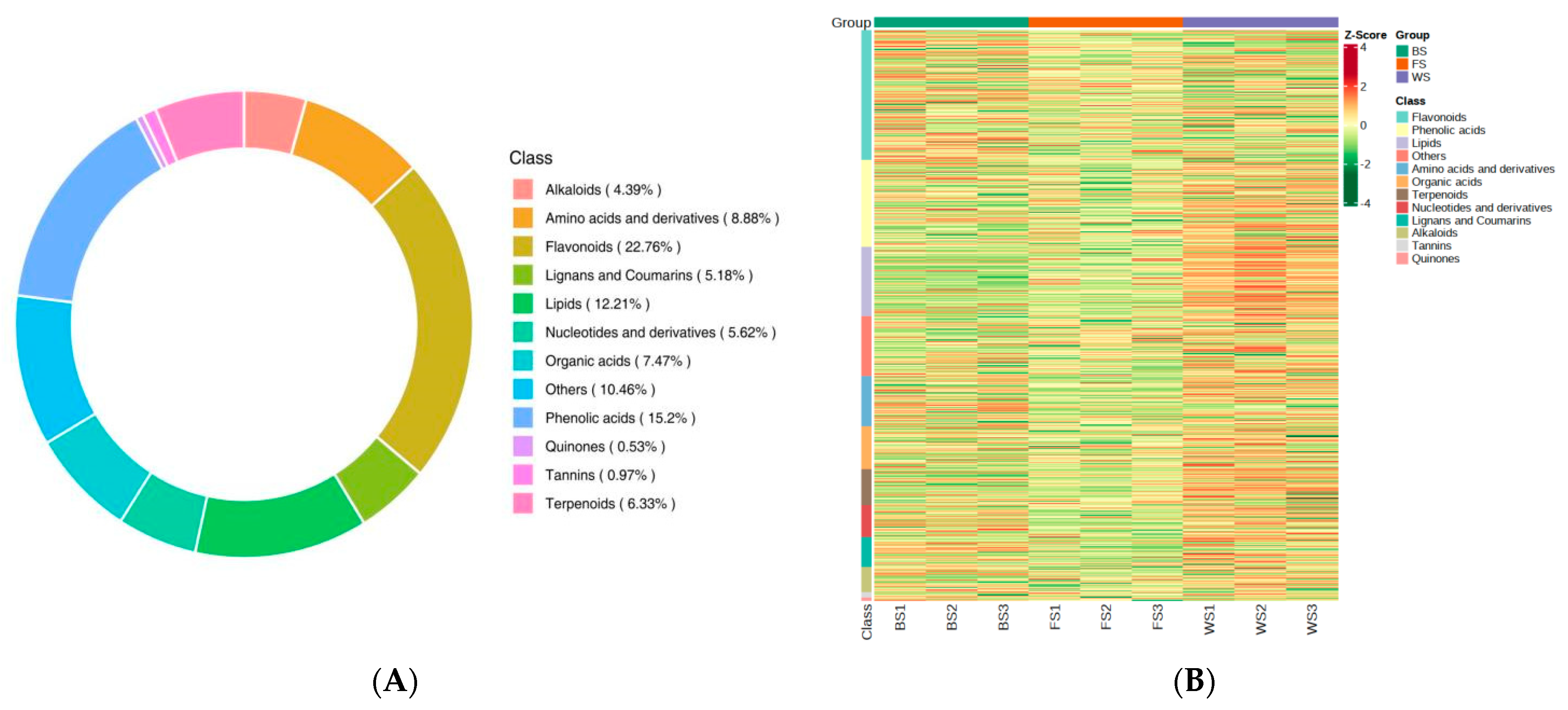
3.2. Metabolites Profiling Analysis of T. miqueliana Flowers
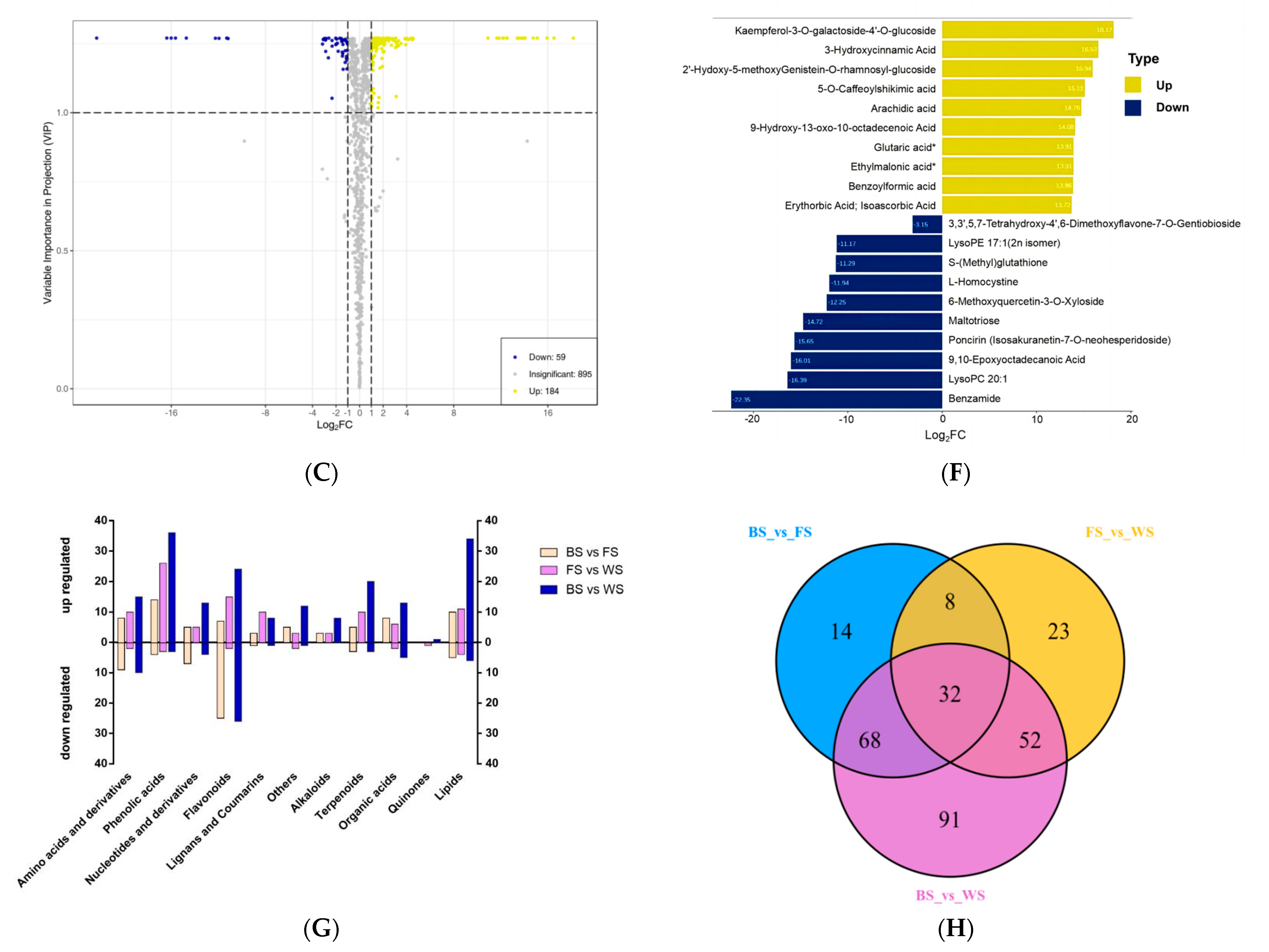
3.3. Identification and Characteristics Analysis of DAMs
3.4. Variation Trends Analysis of DAMs
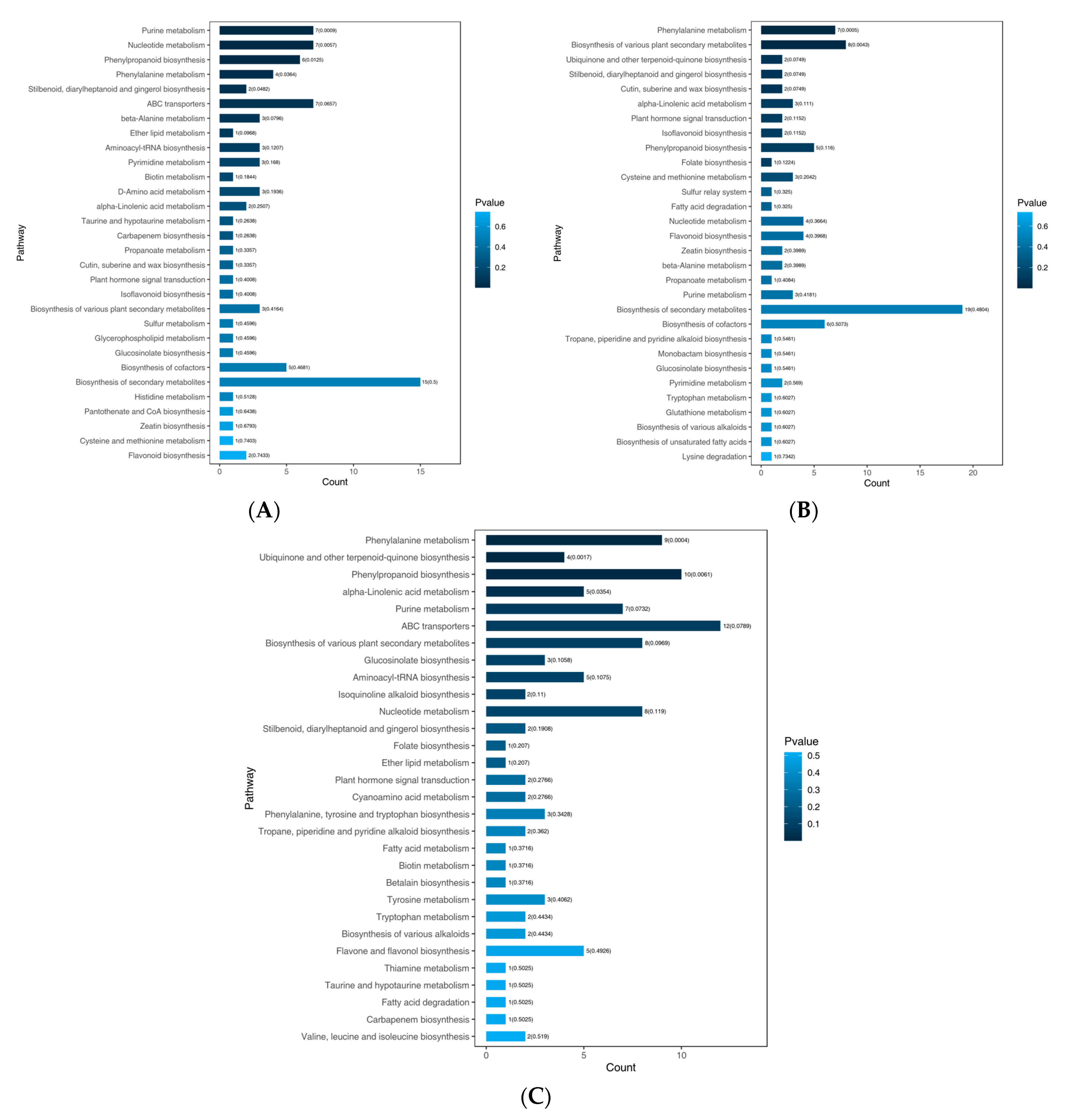
3.5. KEGG Enrichment and Biosynthesis Pathway Analysis for DAMs
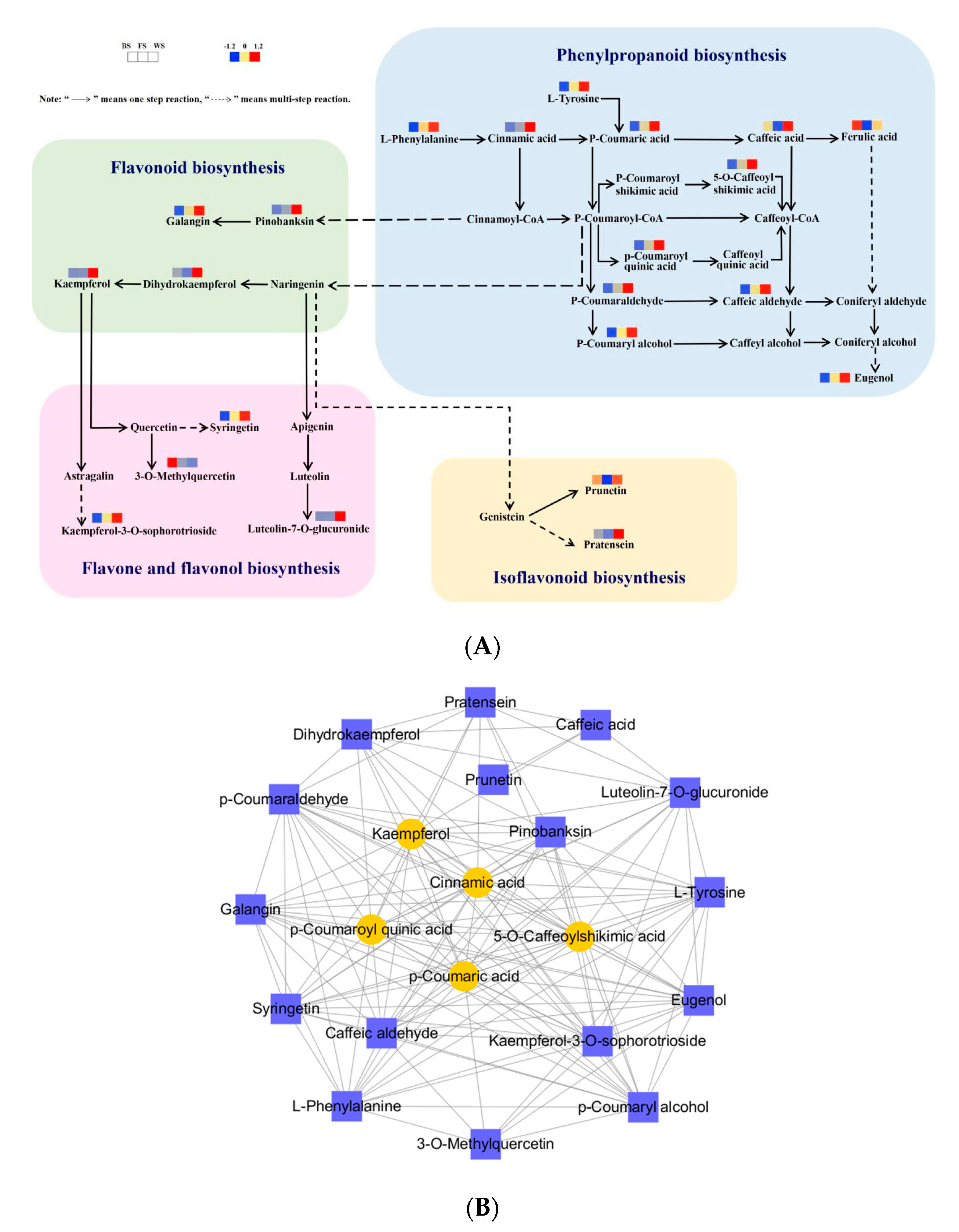
3.6. Analysis of DAMs in Flavonoid Biosynthesis-Related Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Radoglou, K.; Dobrowolska, D.; Spyroglou, G.; Nicolescu, V.N. A Review on the Ecology and Silviculture of Limes (Tilia cordata Mill., Tilia platyphyllos Scop. and Tilia tomentosa Moench.) in Europe. Romania. 2008. 29p. Available online: http://www.valbro.uni-freiburg.de/ (accessed on 15 July 2022).
- Karioti, A.; Chiarabini, L.; Alachkar, A.; Chehna, M.F.; Vincieri, F.; Bilia, A. HPLC-DAD and HPLC-ESI-MS analyses of Tiliae flos and its preparations. J. Pharm. Biomed. Anal. 2014, 100, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Hernández, E.; González-Trujano, M.E.; Martínez, A.L.; Moreno, J.; Kite, G.; Terrazas, T.; Soto-Hernández, M. HPLC/MS analysis and anxiolytic-like effect of quercetin and kaempferol flavonoids from Tilia americana var. mexicana. J. Ethnopharmacol. 2010, 127, 91–97. [Google Scholar] [CrossRef]
- Kosakowska, O.K.; Bączek, K.; Przybył, J.L.; Ejdys, M.; Kuźma, P.; Obiedziński, M.; Węglarz, Z. Intraspecific variability in the content of phenolic compounds, essential oil and mucilage of small-leaved lime (Tilia cordata Mill.) from Poland. Ind. Crop. Prod. 2015, 78, 58–65. [Google Scholar] [CrossRef]
- Szucs, Z.; Cziaky, Z.; Kiss-Szikszai, A.; Sinka, L.; Vasas, G.; Gonda, S. Comparative metabolomics of Tilia platyphyllos Scop. bracts during phenological development. Phytochemistry 2019, 167, 112084. [Google Scholar] [CrossRef] [PubMed]
- Kruk, A.; Granica, S.; Popowski, D.; Malinowska, N.; Piwowarski, J.P. Tiliae flos metabolites and their beneficial influence on human gut microbiota biodiversity ex vivo. J. Ethnopharmacol. 2022, 294, 115355. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Khalid, M.; Rahman, S.U.; Bilal, M.; Huang, D. Role of flavonoids in plant interactions with the environment and against human pathogens—A review. J. Integr. Agric. 2019, 18, 211–230. [Google Scholar] [CrossRef]
- Griesbach, R.J. Biochemistry and genetics of flower color. Plant Breed. Rev. 2005, 25, 89–114. [Google Scholar] [CrossRef]
- Sundaravarathan, S.; Kannaiyan, S. Role of plant flavonoids as signal molecules to Rhizobium. Biol. Environ. Sci. 2022, 144–164. [Google Scholar]
- Guven, H.; Arici, A.; Simsek, O. Simsek, Flavonoids in Our Foods: A Short Review. J. Basic Clin. Health Sci. 2019, 3, 96–106. [Google Scholar] [CrossRef]
- Carradori, S.; Gidaro, M.C.; Petzer, A.; Costa, G.; Guglielmi, P.; Chimenti, P.; Alcaro, S.; Petzer, J.P. Inhibition of Human Monoamine Oxidase: Biological and Molecular Modeling Studies on Selected Natural Flavonoids. J. Agric. Food Chem. 2016, 64, 9004–9011. [Google Scholar] [CrossRef] [PubMed]
- Gentile, D.; Fornai, M.; Pellegrini, C.; Colucci, R.; Blandizzi, C.; Antonioli, L. Dietary flavonoids as a potential intervention to improve redox balance in obesity and related co-morbidities: A review. Nutr. Res. Rev. 2018, 31, 239–247. [Google Scholar] [CrossRef]
- Delnavazi, M.R.; \Shahabi, M.; Yassa, N. Flavonoids from the leaves of Iranian Linden; Tilia rubra subsp. caucasica. Res. J. Pharmacogn. 2015, 2, 17–22. [Google Scholar]
- Kang, Y.-M.; Lee, N.-H. A New Isoflavone Glycoside from the Stems of Tilia taquetii Schneider. Bull. Korean Chem. Soc. 2011, 32, 1048–1050. [Google Scholar] [CrossRef][Green Version]
- Matsuda, H.; Ninomiya, K.; Shimoda, H.; Yoshikawa, M. Hepatoprotective principles from the flowers of Tilia argentea (Linden): Structure requirements of tiliroside and mechanisms of action. Bioorg. Med. Chem. 2002, 10, 707–712. [Google Scholar] [CrossRef]
- Yan, L.; Huang, X.; Yue, Y.; Tang, S.; Wang, H. Analyses on diversity and variation of phenotypic traits of natural populations of Tilia miqueliana. J. Plant Resour. Environ. 2021, 30, 29–37. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, W.H.; Peng, C.Y.; Shen, Y.B.; Visscher, A.M.; Pritchard, H.W.; Gao, Q.; Sun, X.R.; Wang, M.Z.; Deng, Z. Effects of H2SO4, GA3, and cold stratification on the water content, coat composition, and dormancy release of Tilia miqueliana seeds. Front. Plant Sci. 2023, 14, 1240028. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Qin, X.; Liu, Y.; Zhang, Y.; Bao, H.; Hu, Y.; Shen, X. Analysis of flavonoid metabolism during the process of petal discoloration in three Malus Crabapple Cultivars. ACS Omega 2022, 7, 37304–37314. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Liao, D.; Chen, Y.; Zhang, C.; An, R.; Zeng, Q.; Li, X. A Widely Metabolomic Analysis Revealed Metabolic Alterations of Epimedium Pubescens Leaves at Different Growth Stages. Molecules 2020, 25, 137. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Yang, Z.; Song, X.; Ma, Y.; Zhao, J.; Wang, X.; Liu, H.; Fan, L. Widely target metabolomics analysis of the differences in metabolites of licorice under drought stress. Ind. Crop. Prod. 2023, 202, 117071. [Google Scholar] [CrossRef]
- Chen, Z.; Hao, S.; He, Z.; Liu, J.; Zhao, J.; Chen, C.; Jia, G.; Chen, H. Widely targeted metabolomics analysis reveals the major metabolites in the hemp seeds from the longevity village of Bama, China. Ind. Crop. Prod. 2023, 206, 117661. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Gu, C.; He, S.; Zhu, D.; Huang, Y.; Zhou, Q. Widely targeted metabolomics analysis reveals new biomarkers and mechanistic insights on chestnut (Castanea mollissima Bl.) calcification process. Food Res Int. 2021, 141, 110128. [Google Scholar] [CrossRef]
- Fraga, C.G.; Clowers, B.H.; Moore, R.J.; Zink, E.M. Signature-discovery approach for sample matching of a nerve-agent precursor using liquid chromatography-mass spectrometry, XCMS, and chemometrics. Anal. Chem. 2010, 82, 4165–4173. [Google Scholar] [CrossRef]
- Qian, G.; Li, X.; Zhang, H.; Zhang, H.; Zhou, J.; Ma, X.; Sun, W.; Yang, W.; He, R.; Wahab, A.-T.; et al. Metabolomics analysis reveals the accumulation patterns of flavonoids and phenolic acids in quinoa (Chenopodium quinoa Willd.) grains of different colors. Food Chem. X 2023, 17, 100594. [Google Scholar] [CrossRef]
- Chai, Z.; Zhang, M.; Li, L.; Zhang, C.; Duan, Y. Non-Targeted Metabolomics Analysis of the Petals of Osmanthus fragrans ‘Yanzhi Hong’ in Different Developmental Phases. J. Northwest. Univ. 2022, 37, 107–113. [Google Scholar] [CrossRef]
- Chen, H.; Yu, T.; Ma, Y.; Yang, W.; HUAng, H.; Xu, W.; Wei, H.; Gong, J.; Ni, S. Overview of Pharmacological Research on Tilia L. J. Anhui Agric. Sci 2014, 36, 12912–12914. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.-J. Multifaceted regulations of gateway enzyme phenylalanine ammonia-lyase in the biosynthesis of phenylpropanoids. Mol. Plant 2015, 8, 17–27. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Mo, Y.; Nagel, C.; Taylor, L.P. Biochemical complementation of chalcone synthase mutants defines a role for flavonols in functional pollen. Proc. Natl. Acad. Sci. USA 1992, 89, 7213–7217. [Google Scholar] [CrossRef]
- Fattahi, M.; Bonfill, M.; Fattahi, B.; Torras-Claveria, L.; Sefidkon, F.; Cusido, R.M.; Palazon, J. Secondary metabolites profiling of Dracocephalum kotschyi Boiss at three phenological stages using uni- and multivariate methods. J. Appl. Res. Med. Aromat. Plants 2016, 3, 177–185. [Google Scholar] [CrossRef]
- Zhai, R.; Liu, X.-T.; Feng, W.-T.; Chen, S.-S.; Xu, L.-F.; Wang, Z.-G.; Zhang, J.-L.; Li, P.-M.; Ma, F.-W. Different biosynthesis patterns among flavonoid 3-glycosides with distinct effects on accumulation of other flavonoid metabolites in pears (Pyrus bretschneideri Rehd.). PLoS ONE 2014, 9, e91945. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ye, G.-Y.; Liu, H.-L.; Wang, Z.-H. New insights on Abelmoschus manihot flower development: Dynamic changes of flavonoids based on a metabolomic approach. J. Plant Biochem. Biotechnol. 2021, 31, 351–360. [Google Scholar] [CrossRef]
- Yang, N.; Zhao, K.; Li, X.; Zhao, R.; Aslam, M.Z.; Yu, L.; Chen, L. Comprehensive analysis of wintersweet flower reveals key structural genes involved in flavonoid biosynthetic pathway. Gene 2018, 676, 279–289. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.-J.; Song, A.; Wang, Y.; Geng, Z.; Zhao, K.; Jiang, J.; Chen, S.; Chen, F. Comparative transcriptome analysis and flavonoid profiling of floral mutants reveals CmMYB11 regulating flavonoid biosynthesis in chrysanthemum. Plant Sci. 2023, 336, 111837. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.-Y.; Liu, H.; Zhang, X.-S.; Ni, R.; Wang, P.-Y.; Gao, S.; Lou, H.-X.; Cheng, A.-X. Functional characterization of a liverworts bHLH transcription factor involved in the regulation of bisbibenzyls and flavonoids biosynthesis. BMC Plant Biol. 2019, 19, 497. [Google Scholar] [CrossRef]
- Grunewald, W.; de Smet, I.; Lewis, D.R.; Löfke, C.; Jansen, L.; Goeminne, G.; Vanden Bossche, R.; Karimi, M.; de Rybel, B.; Vanholme, B.; et al. Transcription factor WRKY23 assists auxin distribution patterns during Arabidopsis root development through local control on flavonol biosynthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 1554–1559. [Google Scholar] [CrossRef]
- Hua, H. Functional Characterization of One CsWD40 Gene Involved in Flavonoid Biosynthesis in Tea Plants. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2018. [Google Scholar]
- Le Roy, J.; Huss, B.; Creach, A.; Hawkins, S.; Neutelings, G. Neutelings, Glycosylation Is a Major Regulator of Phenylpropanoid Availability and Biological Activity in Plants. Front. Plant Sci. 2016, 7, 735. [Google Scholar] [CrossRef]
- Schmitzer, V.; Mikulic-Petkovsek, M.; Stampar, F. Sepal phenolic profile during Helleborus niger flower development. J. Plant Physiol. 2013, 170, 1407–1415. [Google Scholar] [CrossRef]
- Wan, H.; Yu, C.; Han, Y.; Guo, X.; Ahmad, S.; Tang, A.; Wang, J.; Cheng, T.; Pan, H.; Zhang, Q. Flavonols and Carotenoids in Yellow Petals of Rose Cultivar (Rosa ‘Sun City’): A Possible Rich Source of Bioactive Compounds. J. Agric. Food Chem. 2018, 66, 4171–4181. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Veitch, N.C.; Grayer, R.J. Flavonoids and their glycosides, including anthocyanins. Nat. Prod. Rep. 2011, 28, 1626–1695. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Muzashvili, T.S.; Georgiev, M.I. Advances in the biotechnological glycosylation of valuable flavonoids. Biotechnol. Adv. 2014, 32, 1145–1156. [Google Scholar] [CrossRef]
- Negri, G.; Santi, D.; Tabach, R. Flavonol glycosides found in hydroethanolic extracts from Tilia cordata, a species utilized as anxiolytics. Rev. Bras. Plantas Med. 2013, 15, 217–224. [Google Scholar] [CrossRef]
- Pietta, P.; Mauri, P.; Bruno, A.; Zini, L. High-performance liquid chromatography and micellar electrokinetic chromatography of flavonol glycosides from Tilia. J. Chromatogr. A 1993, 638, 357–361. [Google Scholar] [CrossRef]
- Ziaja, M.; Pawłowska, K.A.; Józefczyk, K.; Pruś, A.; Stefańska, J.; Granica, S. UHPLC-DAD-MS/MS analysis of extracts from linden flowers (Tiliae flos): Differences in the chemical composition between five Tilia species growing in Europe. Ind. Crop. Prod. 2020, 154, 112691. [Google Scholar] [CrossRef]
- Plaza, M.; Pozzo, T.; Liu, J.; Ara, K.Z.G.; Turner, C.; Karlsson, E.N. Substituent Effects on in Vitro Antioxidizing Properties, Stability, and Solubility in Flavonoids. J. Agric. Food Chem. 2014, 62, 3321–3333. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Li, X.; Zhang, H.; Zhou, D.; Wang, W.; Li, W.; Zhang, X.; Li, X.; Hou, Y.; et al. Bioactive Phenols as Potential Neuroinflammation Inhibitors from the Leaves of Xanthoceras Sorbifolia Bunge. Bioorganic Med. Chem. Lett. 2016, 26, 5018–5023. [Google Scholar] [CrossRef]
- Kashchenko, N.I.; Olennikov, D.N.; Chirikova, N.K. Metabolites of Geum aleppicum and Sibbaldianthe bifurca: Diversity and α-Glucosidase Inhibitory Potential. Metabolites 2023, 13, 689. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, W.; Shen, Y.; Verdonk, J.C. Dynamic Changes in Flavonoids’ Accumulation Pattern in Tilia miqueliana Flowers at Different Developmental Stages Based on Widely Targeted Metabolomic Analysis. Forests 2024, 15, 1795. https://doi.org/10.3390/f15101795
Bao W, Shen Y, Verdonk JC. Dynamic Changes in Flavonoids’ Accumulation Pattern in Tilia miqueliana Flowers at Different Developmental Stages Based on Widely Targeted Metabolomic Analysis. Forests. 2024; 15(10):1795. https://doi.org/10.3390/f15101795
Chicago/Turabian StyleBao, Wenqin, Yongbao Shen, and Julian C. Verdonk. 2024. "Dynamic Changes in Flavonoids’ Accumulation Pattern in Tilia miqueliana Flowers at Different Developmental Stages Based on Widely Targeted Metabolomic Analysis" Forests 15, no. 10: 1795. https://doi.org/10.3390/f15101795
APA StyleBao, W., Shen, Y., & Verdonk, J. C. (2024). Dynamic Changes in Flavonoids’ Accumulation Pattern in Tilia miqueliana Flowers at Different Developmental Stages Based on Widely Targeted Metabolomic Analysis. Forests, 15(10), 1795. https://doi.org/10.3390/f15101795





