Soil Enzyme Activities and Microbial Nutrient Limitation of Various Temperate Forest Types in Northeastern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Soil Sampling
2.3. Soil Analysis
2.3.1. Soil Chemical Analyses
2.3.2. Soil Enzymatic Activity Analyses
2.3.3. Soil Microbial Community Properties and the Fungi-to-Bacteria Ratio Assays
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Soil Properties
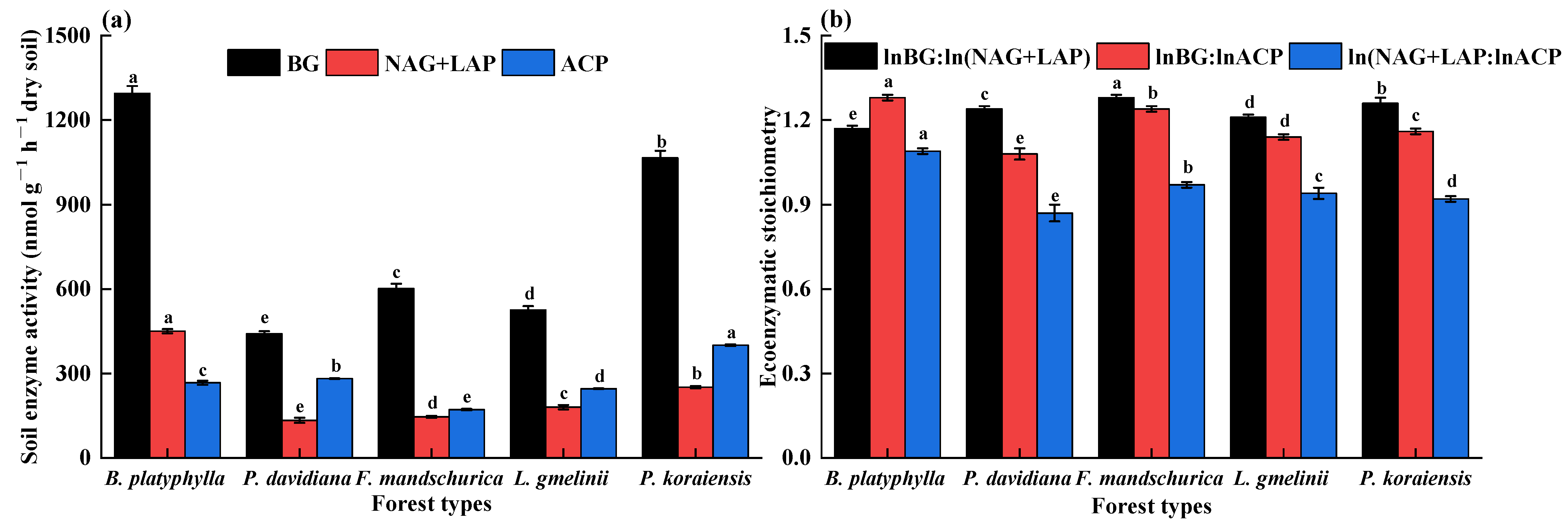
3.2. Soil Enzyme Activity and Stoichiometry
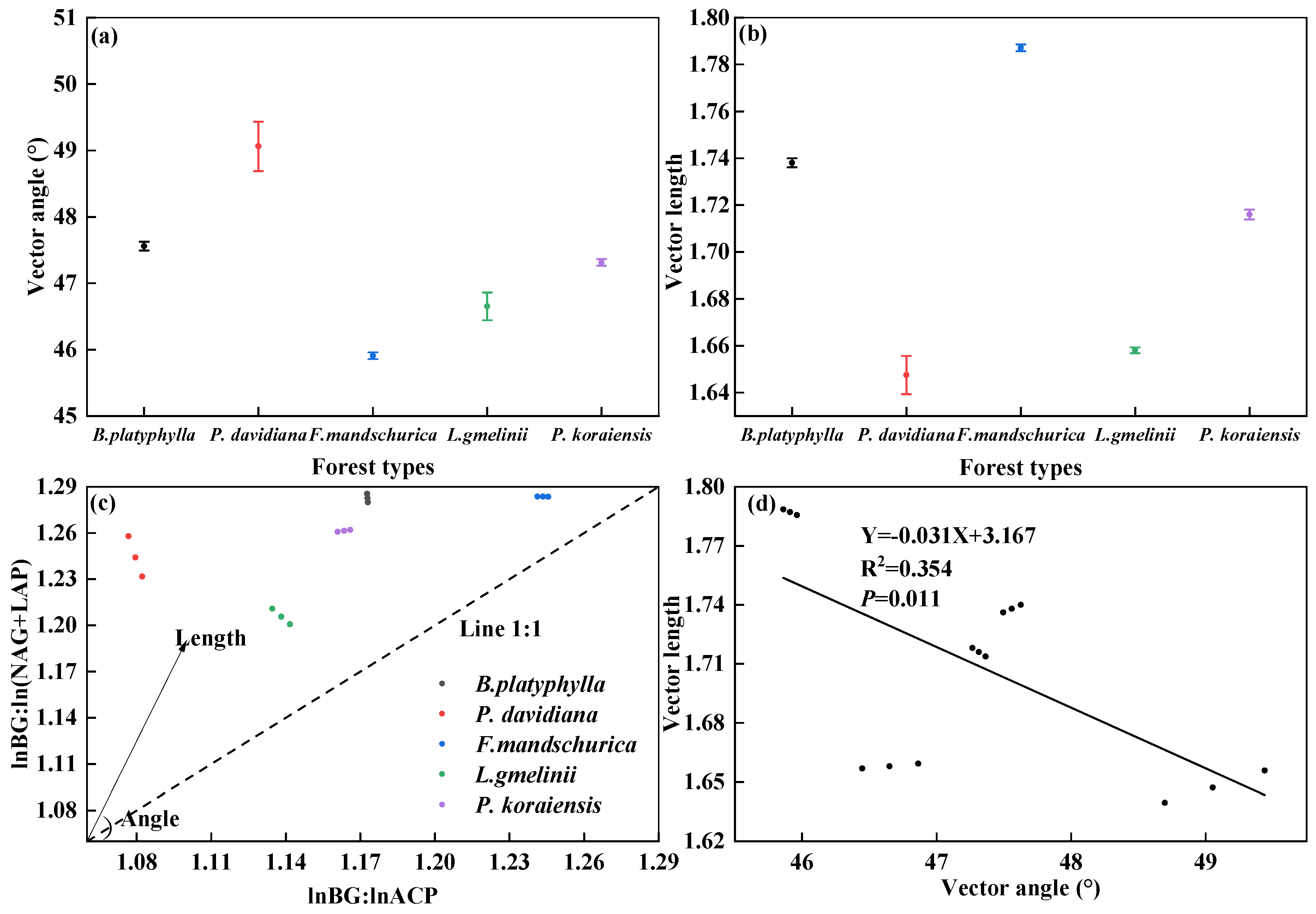
3.3. Microbial Nutrient Limitation
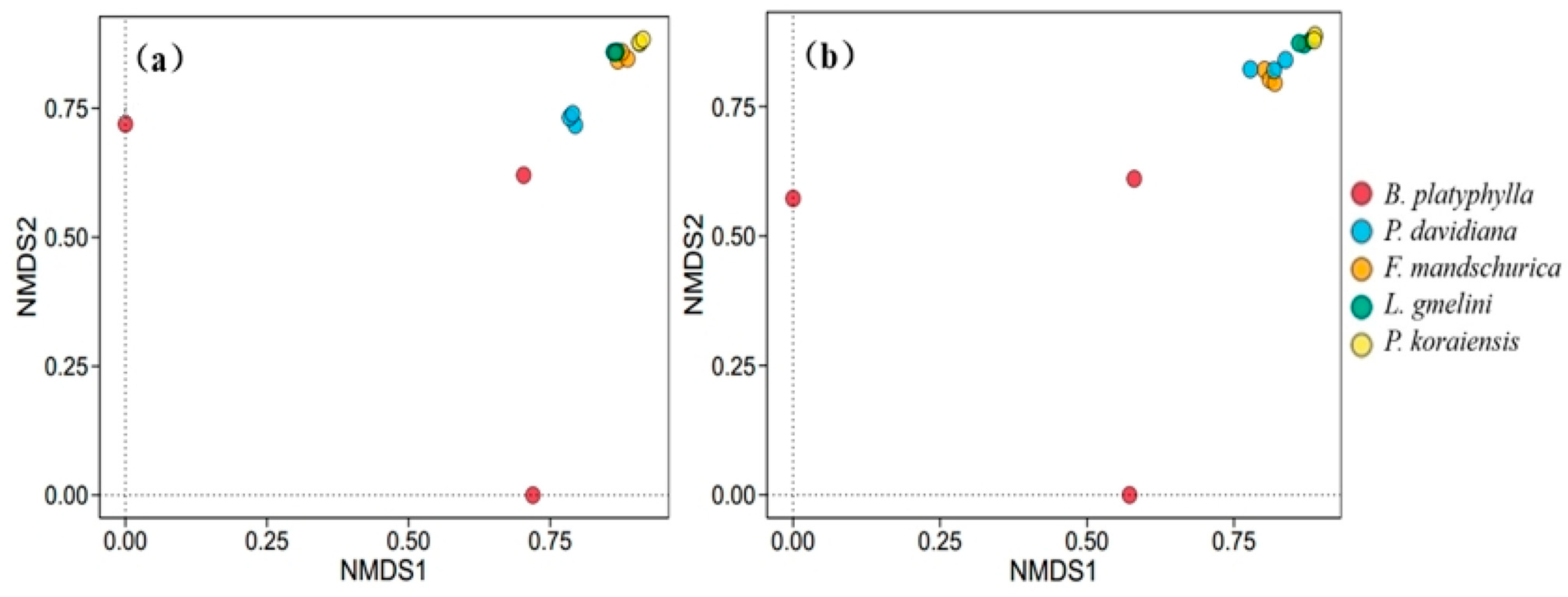
3.4. Soil Microbial Community Structure
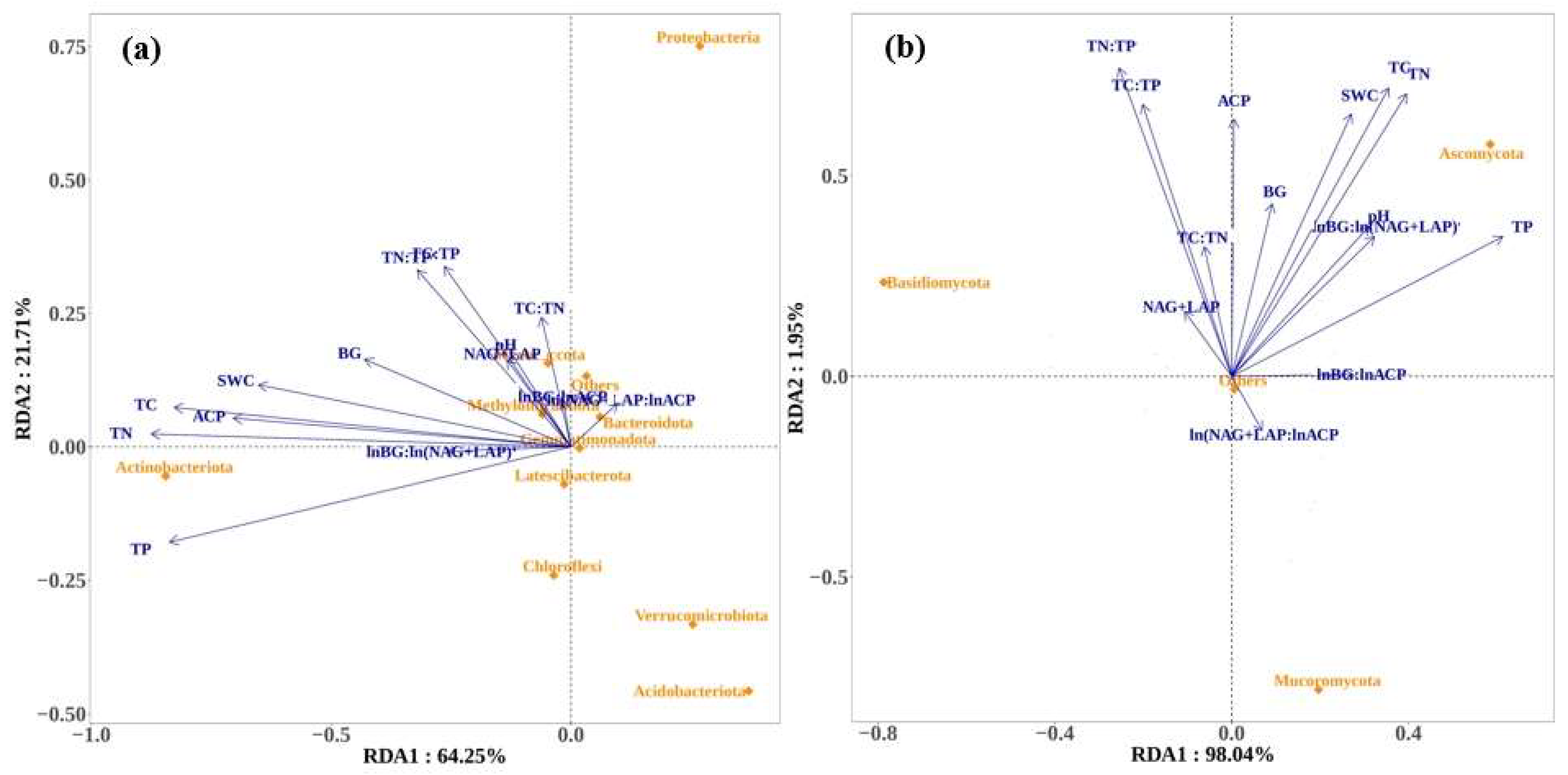
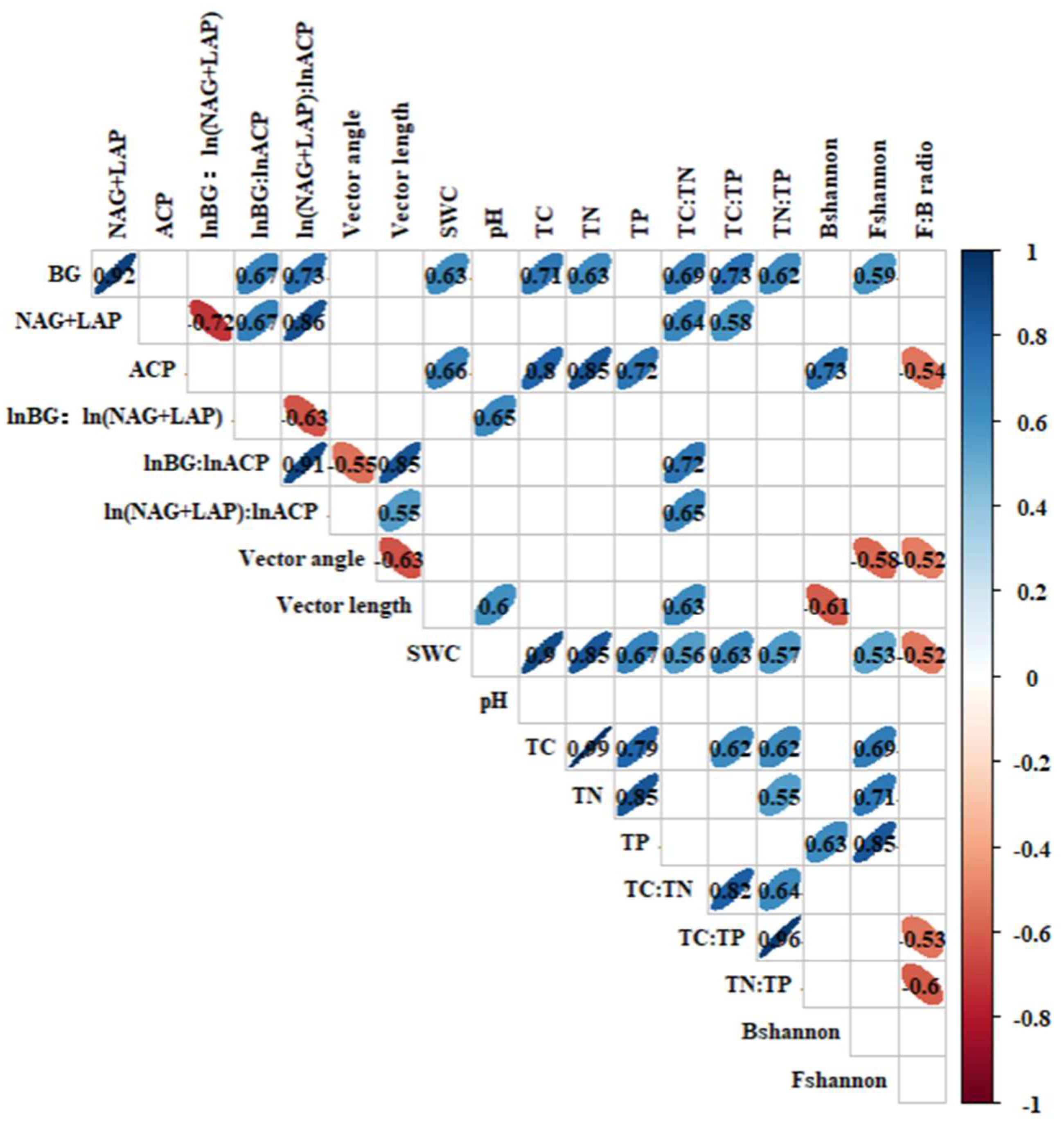
3.5. Relationship Among Soil Enzyme Activities, Microbial Nutrient Limitation, and Microbial Communities
4. Discussion
4.1. Impact of Forest Types on Soil Enzymatic Parameters and Microbial Nutrient Limitations
4.2. Impact of Forest Types on Soil Microbial Community
4.3. Relationships between Soil Enzymatic Parameters, Microbial Nutrient Limitation, and Microbial Community
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variables | SWC | pH | SOC | TN | TP | SOC/TN | SOC/TP | TN/TP |
|---|---|---|---|---|---|---|---|---|
| Bshannon | 0.332 | −0.471 * | 0.412 | 0.488 * | 0.631 * | −0.272 | −0.126 | −0.043 |
| Fshannon | 0.531 * | 0.017 | 0.693 ** | 0.712 ** | 0.850 ** | 0.150 | 0.092 | 0.015 |
| F:B ratio | −0.512 * | −0.209 | −0.423 | −0.398 | −0.101 | −0.245 | −0.529 * | −0.604 * |
| Microbial Community | SWC | pH | SOC | TN | TP | SOC/TN | SOC/TP | TN/TP | |
|---|---|---|---|---|---|---|---|---|---|
| Bacterial communities | Proteobacteria | −0.260 | 0.035 | −0.381 | −0.445 | −0.630 * | 0.137 | 0.172 | 0.164 |
| Acidobacteriota | −0.570 * | −0.274 | −0.678 ** | −0.709 ** | −0.576 * | −0.077 | −0.329 | −0.408 | |
| Actinobacteriota | 0.699 ** | 0.114 | 0.893 ** | 0.948 ** | 0.889 ** | 0.043 | 0.296 | 0.377 | |
| Verrucomicrobiota | −0.414 | −0.063 | −0.501 | −0.469 | −0.448 | −0.339 | −0.295 | −0.200 | |
| Chloroflexi | −0.148 | −0.280 | −0.074 | −0.018 | 0.345 | −0.289 | −0.511 | −0.574 * | |
| Myxococcota | 0.416 | 0.621 * | 0.383 | 0.342 | 0.153 | 0.364 | 0.401 | 0.376 | |
| Methylomirabilota | 0.525 * | 0.162 | 0.554 * | 0.519 * | 0.553 * | 0.400 | 0.263 | 0.131 | |
| Gemmatimonadota | −0.355 | −0.199 | −0.508 | −0.477 | −0.022 | −0.429 | −0.761 ** | −0.855 ** | |
| Bacteroidota | −0.294 | −0.006 | −0.388 | −0.506 | −0.593 * | 0.499 | 0.208 | 0.017 | |
| Latescibacterota | 0.013 | −0.136 | 0.164 | 0.153 | 0.254 | 0.161 | 0.006 | −0.100 | |
| Fungal communities | Ascomycota | 0.386 | 0.385 | 0.484 | 0.525 * | 0.704 ** | −0.018 | −0.114 | −0.156 |
| Basidiomycota | −0.259 | −0.316 | −0.347 | −0.391 | −0.640 * | 0.082 | 0.246 | 0.306 | |
| Mucoromycota | −0.084 | 0.095 | −0.042 | 0.004 | 0.395 | −0.227 | −0.544 * | −0.641 ** | |
References
- Zhang, S.; Yang, X.; Li, D.; Li, S.; Chen, Z.; Wu, J. A meta-analysis of understory plant removal impacts on soil properties in forest ecosystems. Geoderma 2022, 426, 116116. [Google Scholar] [CrossRef]
- Xu, M.; Li, W.; Wang, J.; Zhu, Y.; Feng, Y.; Yang, G.; Zhang, W.; Han, X. Soil ecoenzymatic stoichiometry reveals microbial phosphorus limitation after vegetation restoration on the loess plateau, china. Sci. Total Environ. 2022, 815, 152918. [Google Scholar] [CrossRef]
- Zheng, H.; Vesterdal, L.; Schmidt, I.K.; Rousk, J. Ecoenzymatic stoichiometry can reflect microbial resource limitation, substrate quality, or both in forest soils. Soil Biol. Biochem. 2022, 167, 108613. [Google Scholar] [CrossRef]
- Xu, H.; Qu, Q.; Li, G.; Liu, G.; Geissen, V.; Ritsema, C.J.; Xue, S. Impact of nitrogen addition on plant-soil-enzyme C–N–P stoichiometry and microbial nutrient limitation. Soil Biol. Biochem. 2022, 170, 108714. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Weedon, J.T.; Kowalchuk, G.A.; Aerts, R.; van Hal, J.R.; van Logtestijn, R.K.S.P.; Taş, N.; Roling, W.F.; van Bodegom, P.M. Summer warming accelerates sub-arctic peatland nitrogen cycling without changing enzyme pools or microbial community structure. Glob. Change Biol. 2012, 18, 138–150. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Y.; Li, J.; Mo, Q.; Tang, J.; Liu, W.; Batyrbek, M.; Liu, T.; Zhang, X.; Han, Q. Shaping the succession patterns of different soil nutrients, enzyme stoichiometry, and microbial communities through rotation systems. Catena 2024, 236, 107740. [Google Scholar] [CrossRef]
- Stone, M.M.; DeForest, J.L.; Plante, A.F. Changes in extracellular enzyme activity and microbial community structure with soil depth at the luquillo critical zone observatory. Soil Biol. Biochem. 2014, 75, 237–247. [Google Scholar] [CrossRef]
- Curtright, A.J.; Tiemann, L.K. Intercropping increases soil extracellular enzyme activity: A meta-analysis. Agric. Ecosyst. Environ. 2021, 319, 107489. [Google Scholar] [CrossRef]
- Wang, L.; Pang, X.; Li, N.; Qi, K.; Huang, J.; Yin, C. Effects of vegetation type, fine and coarse roots on soil microbial communities and enzyme activities in eastern Tibetan Plateau. Catena 2020, 194, 104694. [Google Scholar] [CrossRef]
- Pausch, J.; Kuzyakov, Y. Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Glob. Change Biol. 2017, 24, 1–12. [Google Scholar] [CrossRef]
- Ai, L.; Wu, F.; Fan, X.; Yang, Y.; Zhang, Y.; Zheng, X.; Zhu, J.; Ni, X. Different effects of litter and root inputs on soil enzyme activities in terrestrial ecosystems. Appl. Soil Ecol. 2023, 183, 104764. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, Y.; Zhang, J.; Chen, Y.; Yang, L.; Li, H.; Wang, L. Factors influencing soil enzyme activity in China’s forest ecosystems. Plant Ecol. 2017, 219, 31–44. [Google Scholar] [CrossRef]
- Brockett, B.F.T.; Prescott, C.E.; Grayston, S.J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Adamczyk, B.; Kilpeläinen, P.; Kitunen, V.; Smolander, A. Potential activities of enzymes involved in N, C, P and S cycling in boreal forest soil under different tree species. Pedobiologia 2014, 57, 97–102. [Google Scholar] [CrossRef]
- Avazpoor, Z.; Moradi, M.; Basiri, R.; Mirzaei, J.; Taghizadeh-Mehrjardi, R.; Kerry, R. Soil enzyme activity variations in riparian forests in relation to plant species and soil depth. Arab. J. Geosci. 2019, 12, 708. [Google Scholar] [CrossRef]
- Liu, G.; Wang, H.; Yan, G.; Wang, M.; Jiang, S.; Wang, X.; Xue, J.; Xu, M.; Xing, Y.; Wang, Q. Soil enzyme activities and microbial nutrient limitation during the secondary succession of boreal forests. Catena 2023, 230, 107268. [Google Scholar] [CrossRef]
- Waring, B.G.; Weintraub, S.R.; Sinsabaugh, R.L. Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 2014, 117, 101–113. [Google Scholar] [CrossRef]
- Qi, D.; Feng, F.; Lu, C.; Fu, Y. C:N:P stoichiometry of different soil components after the transition of temperate primary coniferous and broad-leaved mixed forests to secondary forests. Soil Tillage Res. 2022, 216, 105260. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Qiu, Q.; Zhou, Y.; You, W. Changes in soil properties and enzyme stoichiometry in three different forest types changed to tea plantations. Forests 2023, 14, 2043. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Gu, Y.; Guo, L.; Huang, Y.; Zhang, J.; Xu, Z.; Tan, B.; Zhang, L.; Chen, L.; et al. Ecoenzymatic stoichiometry and microbial nutrient limitations in rhizosphere soil along the hailuogou glacier forefield chronosequence. Sci. Total Environ. 2020, 704, 135413. [Google Scholar] [CrossRef]
- Xiao, H.; Rong, Y.; Li, P.; Liu, Y. Soil moisture drives the response of soil microbial nutrient limitation to N and P additions in an inner mongolian meadow steppe. Eur. J. Soil Biol. 2024, 120, 103601. [Google Scholar] [CrossRef]
- Shen, F.; Liu, N.; Shan, C.; Ji, L.; Wang, M.; Wang, Y.; Yang, L. Soil extracellular enzyme stoichiometry reveals the increased p limitation of microbial metabolism after the mixed cultivation of Korean pine and manchurian walnut in northeast china. Eur. J. Soil Biol. 2023, 118, 103539. [Google Scholar] [CrossRef]
- Cui, Y.; Bing, H.; Fang, L.; Jiang, M.; Shen, G.; Yu, J.; Wang, X.; Zhu, H.; Wu, Y.; Zhang, X. Extracellular enzyme stoichiometry reveals the carbon and phosphorus limitations of microbial metabolisms in the rhizosphere and bulk soils in alpine ecosystems. Plant Soil 2021, 458, 7–20. [Google Scholar] [CrossRef]
- He, Q.; Wu, Y.; Bing, H.; Zhou, J.; Wang, J. Vegetation type rather than climate modulates the variation in soil enzyme activities and stoichiometry in subalpine forests in the eastern tibetan plateau. Geoderma 2020, 374, 114424. [Google Scholar] [CrossRef]
- Yan, B.; Wang, X.; Sun, Y.; Fan, B.; Shi, L.; Liu, G. Vegetation rehabilitation increases soil enzyme activities in degraded land via carbon supply and nitrogen retention. Eur. J. Soil Biol. 2020, 98, 103186. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, B.; Zhu, Z.; Jia, B.; Xu, W.; Liu, M.; Gao, L.; Gregoire, T.G. The effects of crop tree thinning intensity on the ability of dominant tree species to sequester carbon in a temperate deciduous mixed forest, northeastern china. For. Ecol. Manag. 2022, 505, 119893. [Google Scholar] [CrossRef]
- Lin, S.; Fan, C.; Wang, J.; Zhang, C.; Zhao, X.; von Gadow, K. Chronic anthropogenic disturbance mediates the biodiversity-productivity relationship across stand ages in a large temperate forest region. J. Appl. Ecol. 2024, 61, 502–512. [Google Scholar] [CrossRef]
- Yuan, X.; Niu, D.; Gherardi, L.A.; Liu, Y.; Wang, Y.; Elser, J.J.; Fu, H. Linkages of stoichiometric imbalances to soil microbial respiration with increasing nitrogen addition: Evidence from a long-term grassland experiment. Soil Biol. Biochem. 2019, 138, 107580. [Google Scholar] [CrossRef]
- Zhong, Z.; Li, W.; Lu, X.; Gu, Y.; Wu, S.; Shen, Z.; Han, X.; Yang, G.; Ren, C. Adaptive pathways of soil microorganisms to stoichiometric imbalances regulate microbial respiration following afforestation in the loess plateau, China. Soil Biol. Biochem. 2020, 151, 108048. [Google Scholar] [CrossRef]
- Verchot, L.; Borelli, T. Application of -nitrophenol (NP) enzyme assays in degraded tropical soils. Soil Biol. Biochem. 2005, 37, 625–633. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhang, X.; Wang, X.; Fu, S.; Wu, S.; Lu, X.; Ren, C.; Han, X.; Yang, G. Soil bacteria and fungi respond differently to plant diversity and plant family composition during the secondary succession of abandoned farmland on the loess plateau, China. Plant Soil 2020, 448, 183–200. [Google Scholar] [CrossRef]
- Xiao, R.H.; Man, X.L.; Duan, B.X.; Cai, T.J.; Ge, Z.X.; Li, X.F.; Vesala, T. Changes in soil bacterial communities and nitrogen mineralization with understory vegetation in boreal larch forests. Soil Biol. Biochem. 2022, 166, 108572. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, J.; Li, J.; Zhang, C.; Liu, G.; Dong, Q. Ecoenzymatic stoichiometry reflects the regulation of microbial carbon and nitrogen limitation on soil nitrogen cycling potential in arid agriculture ecosystems. J. Soils Sediments 2022, 22, 1228–1241. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Xiao, K.; Wang, K.; Treseder, K. Soil microbial processes and resource limitation in karst and non-karst forests. Funct. Ecol. 2018, 32, 1400–1409. [Google Scholar] [CrossRef]
- Xie, X.; Pu, L.; Wang, Q.; Zhu, M.; Xu, Y.; Zhang, M. Response of soil physicochemical properties and enzyme activities to long-term reclamation of coastal saline soil, eastern China. Sci. Total Environ. 2017, 607–608, 1419–1427. [Google Scholar] [CrossRef]
- Sun, L.; Kominami, Y.; Yoshimura, K.; Kitayama, K. Root-exudate flux variations among four co-existing canopy species in a temperate forest, Japan. Ecol. Res. 2017, 32, 331–339. [Google Scholar] [CrossRef]
- Yang, L.; Gu, J.; Yang, Y.; Yang, Y.; Shan, C.; Shen, F. The effects of postfire regeneration patterns on soil microbial metabolic limitation based on eco-enzyme stoichiometry in the boreal forest of China. For. Ecol. Manag. 2024, 568, 122129. [Google Scholar] [CrossRef]
- Bradley, J.A.; Singarayer, J.S.; Anesio, A.M. Microbial community dynamics in the forefield of glaciers. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140882. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Yu, Z.; Shen, G.; Cheng, H.; Tao, S. Composition and diversity of soil microbial communities in the alpine wetland and alpine forest ecosystems on the Tibetan Plateau. Sci. Total Environ. 2020, 747, 141358. [Google Scholar] [CrossRef]
- Liu, T.; Wu, X.; Li, H.; Alharbi, H.; Wang, J.; Dang, P.; Chen, X.; Kuzyakov, Y.; Yan, W. Soil organic matter, nitrogen and pH driven change in bacterial community following forest conversion. For. Ecol. Manag. 2020, 477, 118473. [Google Scholar] [CrossRef]
- Zhang, P.; Guan, P.; Hao, C.; Yang, J.; Xie, Z.; Wu, D. Changes in assembly processes of soil microbial communities in forest-to-cropland conversion in changbai mountains, northeastern China. Sci. Total Environ. 2022, 818, 151738. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Zhang, C.; Wang, G.; Fang, L.; Cui, Y. Higher temporal turnover of soil fungi than bacteria during long-term secondary succession in a semiarid abandoned farmland. Soil Tillage Res. 2019, 194, 104305. [Google Scholar] [CrossRef]
- Ranjan, K.; Paula, F.S.; Mueller, R.C.; Jesus, E.d.C.; Cenciani, K.; Bohannan, B.J.M.; Nüsslein, K.; Rodrigues, J.L.M. Forest-to-pasture conversion increases the diversity of the phylum verrucomicrobia in amazon rainforest soils. Front. Microbiol. 2015, 6, 779. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, R.; Liu, Q.; Qiang, W.; Liang, J.; Hou, E.; Zhao, C.; Pang, X. Linking soil phosphorus fractions to abiotic factors and the microbial community during subalpine secondary succession: Implications for soil phosphorus availability. Catena 2023, 233, 107501. [Google Scholar] [CrossRef]
- Mason, L.M.; Eagar, A.; Patel, P.; Blackwood, C.B.; DeForest, J.L. Potential microbial bioindicators of phosphorus mining in a temperate deciduous forest. J. Appl. Microbiol. 2020, 130, 109–122. [Google Scholar] [CrossRef]
- Yang, B.; Qi, K.; Bhusal, D.R.; Huang, J.; Chen, W.; Wu, Q.; Hussain, A.; Pang, X. Soil microbial community and enzymatic activity in soil particle-size fractions of spruce plantation and secondary birch forest. Eur. J. Soil Biol. 2020, 99, 103196. [Google Scholar] [CrossRef]
- Duan, C.; Fang, L.; Yang, C.; Chen, W.; Cui, Y.; Li, S. Reveal the response of enzyme activities to heavy metals through in situ zymography. Ecotoxicol. Environ. Saf. 2018, 156, 106–115. [Google Scholar]
- Liu, C.; Zhou, Y.; Qin, H.; Liang, C.; Shao, S.; Fuhrmann, J.J.; Chen, J.; Xu, Q. Moso bamboo invasion has contrasting effects on soil bacterial and fungal abundances, co-occurrence networks and their associations with enzyme activities in three broadleaved forests across subtropical china. For. Ecol. Manag. 2021, 498, 119549. [Google Scholar]
- Fu, Z.; Chen, Q.; Lei, P.; Xiang, W.; Ouyang, S.; Chen, L. Soil fungal communities and enzyme activities along local tree species diversity gradient in subtropical evergreen forest. Forests 2021, 12, 1321. [Google Scholar] [CrossRef]
- Li, H.; Tian, H.; Wang, Z.; Liu, C.; Nurzhan, A.; Megharaj, M.; He, W. Potential effect of warming on soil microbial nutrient limitations as determined by enzymatic stoichiometry in the farmland from different climate zones. Sci. Total Environ. 2022, 802, 149657. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Y.; Duan, C.; Wang, X.; Zhang, X.; Ju, W.; Chen, H.; Yue, S.; Wang, Y.; Li, S.; et al. Ecoenzymatic stoichiometry reveals microbial phosphorus limitation decreases the nitrogen cycling potential of soils in semi-arid agricultural ecosystems. Soil Tillage Res. 2020, 197, 104463. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, S.; Wang, X.; Xu, X.; Ai, C.; Liang, G.; Zhu, P.; Zhou, W. Enzymatic stoichiometry reveals phosphorus limitation-induced changes in the soil bacterial communities and element cycling: Evidence from a long-term field experiment. Geoderma 2022, 426, 116124. [Google Scholar] [CrossRef]
- Wu, L.; Zou, B.; Wang, S.; Zhou, L.; Zheng, Y.; Huang, Z.; He, J.-Z. Effects of multispecies restoration on soil extracellular enzyme activity stoichiometry in pinus massoniana plantations of subtropical china. Soil Biol. Biochem. 2023, 178, 108967. [Google Scholar] [CrossRef]
- Feyissa, A.; Gurmesa, G.A.; Yang, F.; Long, C.; Zhang, Q.; Cheng, X. Soil enzyme activity and stoichiometry in secondary grasslands along a climatic gradient of subtropical China. Sci. Total Environ. 2022, 825, 154019. [Google Scholar] [CrossRef]


| Forest Types | Elevation (m) | Stand Age | DBH (cm) | Tree Height (m) | Canopy Density |
|---|---|---|---|---|---|
| B. platyphylla | 305 ± 5 | 53 | 15.30 ± 5.41 | 10.58 ± 0.53 | 0.75 |
| P. davidiana | 307 ± 3 | 55 | 12.78 ± 4.03 | 9.60 ± 1.04 | 0.85 |
| F. mandschurica | 305 ± 4 | 47 | 12.34 ± 3.2 | 9.97 ± 0.84 | 0.80 |
| L. gmelinii | 314 ± 4 | 50 | 14.48 ± 4.40 | 11.42 ± 1.05 | 0.85 |
| P. koraiensis | 307 ± 2 | 58 | 26.66 ± 4.75 | 10.80 ± 1.12 | 0.80 |
| Forest Types | Understory Vegetation Composition |
|---|---|
| B. platyphylla | Rhododendron simsii Planch Corylus mandshurica Maxim Spiraea salicifolia L. Euonymus verrucosus var. pauciflorus(Maxim.) Rege |
| P. davidiana | Rhododendron simsii Planch Adenophora capillaris subsp. Paniculate Rubus crataegifolius Bunge Eriophorum scheuchzeri Hoppe |
| F. mandschurica | Rhododendron simsii Planch Acer leptophyllum Fang Rhamnus davurica Pall. Eriophorum scheuchzeri Hoppe |
| L. gmelinii | Rhododendron simsii Planch Sorbaria sorbifolia (L.) A. Braun Rubus crataegifolius Bunge Chelidonium majus L. |
| P. koraiensis | Rhododendron simsii Planch Sorbaria sorbifolia (L.) A. Braun Phryma leptostachya subsp. asiatica (Hara) Kitamura Chelidonium majus L. |
| Enzyme | Substrate |
|---|---|
| β-1,4-glucosidase (BG) | ρNP-β-d-glucopyranoside |
| β-1,4-N-acetylglucosaminidase (NAG) | ρNP-N-acetyl-β-D-glucosaminide |
| leucine arylamidase (LAP) | Leucine ρ-nitroanilide |
| acid phosphatase (ACP) | ρNP-phosphate |
| Forest Types | SWC (%) | pH | TC (g·kg−1) | TN (g·kg−1) | TP (g·kg−1) |
|---|---|---|---|---|---|
| B. platyphylla | 28.94 ± 2.35 ab | 6.06 ± 0.10 b | 40.73 ± 3.58 b | 2.86 ± 0.15 b | 0.45 ± 0.02 c |
| P. davidiana | 24.94 ± 4.21 b | 6.10 ± 0.05 b | 23.77 ± 2.18 c | 1.97 ± 0.09 c | 0.33 ± 0.01 e |
| F. mandschurica | 26.32 ± 3.23 b | 6.35 ± 0.11 a | 29.15 ± 1.85 c | 2.21 ± 0.12 c | 0.40 ± 0.02 d |
| L. gmelinii | 24.44 ± 2.24 b | 6.00 ± 0.04 b | 24.28 ± 3.54 c | 2.16 ± 0.05 c | 0.60 ± 0.03 b |
| P. koraiensis | 34.84 ± 3.58 a | 6.20 ± 0.20 b | 68.35 ± 5.78 a | 5.29 ± 0.21 a | 0.80 ± 0.04 a |
| Forest Types | Bacterial Shannon Index | Fungal Shannon Index | F:B Ratio |
|---|---|---|---|
| B. platyphylla | 6.88 ± 0.04 b | 4.26 ± 0.07 a | 1.05 ± 0.08 b |
| P. davidiana | 6.79 ± 0.07 c | 3.28 ± 0.04 d | 0.99 ± 0.04 b |
| F. mandschurica | 6.63 ± 0.02 c | 3.89 ± 0.13 c | 1.14 ± 0.02 a |
| L. gmelinii | 7.05 ± 0.03 a | 4.37 ± 0.04 a | 1.19 ± 0.03 a |
| P. koraiensis | 6.86 ± 0.05 b | 4.12 ± 0.03 b | 0.99 ± 0.01 b |
| Soil Properties | Bacteria | Fungi | ||||||
|---|---|---|---|---|---|---|---|---|
| RDA1 | RDA2 | r2 | p | RDA1 | RDA2 | r2 | p | |
| SWC | −0.984 | 0.176 | 0.503 | 0.017 * | 0.382 | 0.924 | 0.575 | 0.006 * |
| pH | −0.578 | 0.816 | 0.055 | 0.694 | 0.639 | 0.769 | 0.271 | 0.155 |
| TC | −0.996 | 0.089 | 0.792 | 0.003 ** | 0.443 | 0.897 | 0.738 | 0.001 ** |
| TN | −1.000 | 0.028 | 0.880 | 0.002 ** | 0.490 | 0.872 | 0.749 | 0.001 ** |
| TP | −0.978 | −0.209 | 0.841 | 0.001 ** | 0.870 | 0.494 | 0.571 | 0.008 ** |
| TC/TN | −0.246 | 0.969 | 0.072 | 0.669 | −0.188 | 0.982 | 0.124 | 0.446 |
| TC/AP | −0.615 | 0.788 | 0.211 | 0.239 | −0.285 | 0.959 | 0.574 | 0.005 ** |
| TN/TP | −0.695 | 0.719 | 0.244 | 0.167 | −0.315 | 0.949 | 0.753 | 0.003 ** |
| BG | −0.934 | 0.356 | 0.244 | 0.183 | 0.208 | 0.978 | 0.222 | 0.204 |
| NAG + LAP | −0.634 | 0.774 | 0.051 | 0.760 | −0.550 | 0.835 | 0.043 | 0.777 |
| ACP | −0.997 | 0.076 | 0.572 | 0.007 ** | 0.008 | 1.000 | 0.472 | 0.020 * |
| lnBG:ln(NAG + LAP) | −0.999 | −0.033 | 0.076 | 0.619 | 0.680 | 0.734 | 0.259 | 0.184 |
| lnBG:lnACP | −0.180 | 0.984 | 0.009 | 0.946 | 1.000 | 0.018 | 0.086 | 0.558 |
| ln(NAG + LAP):lnACP | 0.765 | 0.644 | 0.019 | 0.903 | 0.453 | −0.892 | 0.026 | 0.841 |
| Microbial Communities | BG | NAG + LAP | ACP | lnBG:ln(NAG + LAP) | lnBG:lnACP | ln(NAG + LAP):lnACP | Vector Angle | Vector Length | |
|---|---|---|---|---|---|---|---|---|---|
| Bacterial communities | Proteobacteria | −0.112 | 0.053 | −0.249 | −0.156 | −0.046 | 0.040 | 0.477 | −0.116 |
| Acidobacteriota | −0.280 | −0.005 | −0.599 * | −0.368 | 0.079 | 0.220 | 0.005 | −0.120 | |
| Actinobacteriota | 0.440 | 0.119 | 0.807 ** | 0.287 | −0.055 | −0.167 | −0.108 | 0.096 | |
| Verrucomicrobiota | −0.620 * | −0.527 * | −0.216 | 0.189 | −0.522 * | −0.501 | 0.343 | −0.407 | |
| Chloroflexi | −0.022 | 0.017 | −0.175 | −0.191 | 0.169 | 0.205 | −0.596 * | 0.047 | |
| Myxococcota | 0.190 | −0.019 | 0.052 | 0.454 | 0.286 | 0.034 | −0.281 | 0.526 * | |
| Methylomirabilota | 0.620 * | 0.502 | 0.190 | −0.159 | 0.577 * | 0.528 * | −0.529 * | 0.472 | |
| Gemmatimonadota | −0.371 | −0.157 | −0.429 | −0.366 | −0.100 | 0.064 | −0.334 | −0.309 | |
| Bacteroidota | 0.282 | 0.509 | −0.659 ** | −0.456 | 0.739 ** | 0.792 ** | −0.211 | 0.484 | |
| Latescibacterota | 0.427 | 0.414 | −0.163 | −0.214 | 0.640 * | 0.601 * | −0.601 * | 0.504 | |
| Fungal communities | Ascomycota | 0.161 | −0.089 | 0.101 | 0.394 | 0.291 | 0.052 | −0.808 ** | 0.483 |
| Basidiomycota | −0.078 | 0.122 | 0.025 | −0.331 | −0.294 | −0.080 | 0.872 ** | −0.450 | |
| Mucoromycota | −0.140 | −0.185 | −0.327 | 0.118 | 0.250 | 0.133 | −0.898 ** | 0.290 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, R.; Duan, B.; Dai, C.; Wu, Y. Soil Enzyme Activities and Microbial Nutrient Limitation of Various Temperate Forest Types in Northeastern China. Forests 2024, 15, 1815. https://doi.org/10.3390/f15101815
Xiao R, Duan B, Dai C, Wu Y. Soil Enzyme Activities and Microbial Nutrient Limitation of Various Temperate Forest Types in Northeastern China. Forests. 2024; 15(10):1815. https://doi.org/10.3390/f15101815
Chicago/Turabian StyleXiao, Ruihan, Beixing Duan, Changlei Dai, and Yu Wu. 2024. "Soil Enzyme Activities and Microbial Nutrient Limitation of Various Temperate Forest Types in Northeastern China" Forests 15, no. 10: 1815. https://doi.org/10.3390/f15101815
APA StyleXiao, R., Duan, B., Dai, C., & Wu, Y. (2024). Soil Enzyme Activities and Microbial Nutrient Limitation of Various Temperate Forest Types in Northeastern China. Forests, 15(10), 1815. https://doi.org/10.3390/f15101815





