Assessment of Climate Impact of Sustainable Forestry Based on Landscape Structure
Abstract
1. Introduction
2. Methods
2.1. Qualitative Overview
2.2. Analytical Approach
3. Results: Application to Swedish Forestry
Swedish Total Green House Gas (GHG) Emissions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malcolm, J.R.; Holtsmark, B.; Piascik, P.W. Forest harvesting and the carbon debt in boreal east-central Canada. Clim. Change 2020, 161, 433–449. [Google Scholar] [CrossRef]
- Mitchell, S.R.; Harmon, M.E.; O’Connell, K.E.B. Carbon debt and carbon sequestration parity in forest bioenergy production. Glob. Change Biol. Bioenergy 2012, 4, 818–827. [Google Scholar] [CrossRef]
- Schulze, E.-D.; Körner, C.; Law, B.E.; Haberl, H.; Luyssaert, S. Large-scale bioenergy from additional harvest of forest biomass is neither sustainable nor greenhouse gas neutral. Glob. Change Biol. Bioenergy 2012, 4, 611–616. [Google Scholar] [CrossRef]
- Zanchi, G.; Pena, N.; Bird, N. Is woody bioenergy carbon neutral? A comparative assessment of emissions from consumption of woody bioenergy and fossil fuel. Glob. Change Biol. Bioenergy 2012, 4, 761–772. [Google Scholar] [CrossRef]
- Repo, A.; Tuovinen, J.-P.; Liski, J. Can we produce carbon and climate neutral forest bioenergy? Glob. Change Biol. Bioenergy 2015, 7, 253–262. [Google Scholar] [CrossRef]
- Dean, C.; Kirkpatrick, J.B.; Friedland, A.J. Conventional intensive logging promotes loss of organic carbon from the mineral soil. Glob. Change Biol. 2017, 23, 1–11. [Google Scholar] [CrossRef]
- Stokland, J.N. Volume increment and carbon dynamics in boreal forest when extending the rotation length towards biologically old stands. For. Ecol. Manag. 2021, 488, 119017. [Google Scholar] [CrossRef]
- Bentsen, N.S. Carbon debt and payback time—Lost in the forest? Renew. Sustain. Energy Rev. 2017, 73, 1211–1217. [Google Scholar] [CrossRef]
- Soimakallio, S.; Kalliokoski, T.; Lehtonen, A.; Salminen, O. On the trade-offs and synergies between forest carbon sequestration and substitution. Mitig. Adapt. Strateg. Glob. Change 2021, 26, 4. [Google Scholar] [CrossRef]
- Wang, M.Q.; Han, J.; Haq, Z.; Tyner, W.E.; Wu, M.; Elgowainy, A. Energy and greenhouse gas emission effects of corn and cellulosic ethanol with technology improvements and land use changes. Biomass Bioenergy 2011, 35, 1885–1896. [Google Scholar] [CrossRef]
- Leturcq, P. GHG displacement factors of harvested wood products: The myth of substitution. Sci. Rep. 2020, 10, 20752. [Google Scholar] [CrossRef] [PubMed]
- Daioglou, V.; Doelman, J.C.; Stehfest, E.; Müller, C.; Wicke, B.; Faaij, A.; van Vuuren, D.P. Greenhouse gas emission curves for advanced biofuel supply chains. Nat. Clim. Change 2017, 7, 920–924. [Google Scholar] [CrossRef]
- Searchinger, T.D.; Wirsenius, S.; Beringer, T.; Dumas, P. Assessing the efficiency of changes in land use for mitigating climate change. Nature 2018, 564, 249–253. [Google Scholar] [CrossRef]
- Harmon, M.E. Have product substitution carbon benefits been overestimated? A sensitivity analysis of key assumptions. Environ. Res. Lett. 2019, 14, 065008. [Google Scholar] [CrossRef]
- Merfort, L.; Bauer, N.; Humpenöder, F.; Klein, D.; Strefler, J.; Popp, A.; Luderer, G.; Kriegler, E. Bioenergy-induced land-use-change emissions with sectorally fragmented policies. Nat. Clim. Change 2023, 13, 685–692. [Google Scholar] [CrossRef]
- Haberl, H.; Sprinz, D.; Bonazountas, M.; Cocco, P.; Desaubies, Y.; Henze, M.; Hertel, O.; Johnson, R.K.; Kastrup, U.; Laconte, P.; et al. Correcting a fundamental error in greenhouse gas accounting related to bioenergy. Energy Policy 2012, 45, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Babiker, M.; Berndes, G.; Blok, K.; Cohen, B.; Cowie, A.; Geden, O.; Ginzburg, V.; Leip, A.; Smith, P.; Sugiyama, M.; et al. Cross-Sectoral Perspectives—Supplementary Material (Chapter 12). In Climate Change 2022—Mitigation of Climate Change—Working Group III Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- DeCicco, J.M.; Schlesinger, W.H. Reconsidering bioenergy given the urgency of climate protection. Proc. Natl. Acad. Sci. USA 2018, 115, 9642–9645. [Google Scholar] [CrossRef]
- Shukla, P.R.; Skea, J.; Reisinger, A.; Slade, R.; Fradera, R.; Pathak, M.; Al Khourdajie, A.; Belkacemi, M.; van Diemen, R.; Hasija, A.; et al. Climate Change 2022—Mitigation of Climate Change—Working Group III Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Lewis, S.L.; Wheeler, C.E.; Mitchard, E.T.A.; Koch, A. Restoring natural forests is the best way to remove atmospheric carbon. Nature 2019, 568, 25–28. [Google Scholar] [CrossRef]
- Rogers, B.M.; Mackey, B.; Shestakova, T.A.; Keith, H.; Young, V.; Kormos, C.F.; DellaSala, D.A.; Dean, J.; Birdsey, R.; Bush, G.; et al. Using ecosystem integrity to maximize climate mitigation and minimize risk in international forest policy. Front. For. Glob. Change 2022, 5, 929281. [Google Scholar] [CrossRef]
- Erb, K.-H.; Kastner, T.; Plutzar, C.; Bais, A.L.S.; Carvalhais, N.; Fetzel, T.; Gingrich, S.; Haberl, H.; Lauk, C.; Niedertscheider, M.; et al. Unexpectedly large impact of forest management and grazing on global vegetation biomass. Nature 2018, 553, 73–76. [Google Scholar] [CrossRef]
- Skytt, T.; Englund, G.; Jonsson, B.-G. Climate mitigation forestry—Temporal trade-offs. Environ. Res. Lett. 2021, 16, 114037. [Google Scholar] [CrossRef]
- Seppälä, J.; Heinonen, T.; Pukkala, T.; Kilpeläinen, A.; Mattila, T.; Myllyviita, T.; Asikainen, A.; Peltola, H. Effect of increased wood harvesting and utilization on required greenhouse gas displacement factors of wood-based products and fuels. J. Environ. Manag. 2019, 247, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Hammar, T.; Levihn, F. Time-dependent climate impact of biomass use in a fourth generation district heating system, including BECCS. Biomass Bioenergy 2020, 138, 105606. [Google Scholar] [CrossRef]
- Thiffault, E.; Gianvenuti, A.; Zuzhang, X.; Walter, S. The Role of Wood Residues in the Transition to Sustainable Bioenergy; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Cowie, A.L.; Berndes, G.; Bentsen, N.S.; Brandão, M.; Cherubini, F.; Egnell, G.; George, B.; Gustavsson, L.; Hanewinkel, M.; Harris, Z.M.; et al. Applying a science-based systems perspective to dispel misconceptions about climate effects of forest bioenergy. Glob. Chang. Biol. Bioenergy 2021, 13, 1210–1231. [Google Scholar] [CrossRef]
- Gustafsson, L.; Bauhus, J.; Asbeck, T.; Augustynczik, A.L.D.; Basile, M.; Frey, J.; Gutzat, F.; Hanewinkel, M.; Helbach, J.; Jonker, M.; et al. Retention as an integrated biodiversity conservation approach for continuous-cover forestry in Europe. Ambio 2020, 49, 85–97. [Google Scholar] [CrossRef]
- Deahl, C.; Walloe, L.; Norton, M. EASAC Open Letter to IEA-Bioenergy. Available online: https://easac.eu/news/details/iea-bioenergy-critique-of-easac-publications-on-forest-bioenergy/ (accessed on 11 February 2023).
- United Nations Climate Change. Reporting and Accounting of LULUCF Activities Under the Kyoto Protocol. 2008. Available online: https://unfccc.int/topics/land-use/workstreams/lulucf-under-the-kyoto-protocol/reporting-and-accounting-of-lulucf-activities-under-the-kyoto-protocol (accessed on 19 February 2023).
- European Commission. Taxonomy Regulation. Available online: https://ec.europa.eu/finance/docs/level-2-measures/taxonomy-regulation-delegated-act-2021-2800-annex-1_en.pdf (accessed on 28 October 2024).
- Luyssaert, S.; Ciais, P.; Piao, S.L.; Schulze, E.; Jung, M.; Zaehle, S.; Schelhaas, M.J.; Reichstein, M.; Churkina, G.; Papale, D.; et al. The European carbon balance. Part 3: Forests. Glob. Change Biol. 2010, 16, 1429–1450. [Google Scholar] [CrossRef]
- Le Quéré, C.; Andrew, R.M.; Friedlingstein, P.; Sitch, S.; Hauck, J.; Pongratz, J.; Pickers, P.A.; Korsbakken, J.I.; Peters, G.P.; Canadell, J.G.; et al. Global Carbon Budget 2018. Earth Syst. Sci. Data 2018, 10, 2141–2194. [Google Scholar] [CrossRef]
- Högberg, P.; Ceder, L.A.; Astrup, R.; Binkley, D.; Bright, R.; Dalsgaard, L.; Egnell, G.; Filipchuk, A.; Genet, H.; Ilintsev, A.; et al. Sustainable Boreal Forest Management—Challenges and Opportunities for Climate Change Mitigation. 2021. Available online: https://www.skogsstyrelsen.se/globalassets/om-oss/rapporter/rapporter-20222021202020192018/rapport-2021-11-sustainable-boreal-forest-management-challenges-and-opportunities-for-climate-change-mitigation-002.pdf (accessed on 20 February 2023).
- Huang, Y.; Chen, Y.; Castro-Izaguirre, N.; Baruffol, M.; Brezzi, M.; Lang, A.; Li, Y.; Härdtle, W.; von Oheimb, G.; Yang, X.; et al. Impacts of species richness on productivity in a large-scale subtropical forest experiment. Science 2018, 362, 80–83. [Google Scholar] [CrossRef]
- Merino, A.; Real, C.; Álvarez-González, J.G.; Rodríguez-Guitián, M.A. Forest structure and C stocks in natural Fagus sylvatica forest in southern Europe: The effects of past management. Ecol. Manag. 2007, 250, 206–214. [Google Scholar] [CrossRef]
- Keith, H.; Mackey, B.G.; Lindenmayer, D.B. Re-evaluation of forest biomass carbon stocks and lessons from the world’s most carbon-dense forests. Proc. Natl. Acad. Sci. USA 2009, 106, 11635–11640. [Google Scholar] [CrossRef]
- Pelkman, L. IEA Bioenergy Countries’ Report—Update 2021. IEA Bioenergy. Available online: https://www.ieabioenergy.com/wp-content/uploads/2021/11/CountriesReport2021_final.pdf (accessed on 28 October 2024).
- Swedish Forest Agency. Forestry Production. Available online: https://www.skogsstyrelsen.se/en/statistics/ (accessed on 28 October 2024).
- Statistics Sweden. Skogsdata 2022. Umeå, 2022. Available online: https://www.slu.se/globalassets/ew/org/centrb/rt/dokument/skogsdata/skogsdata_2022_webb.pdf (accessed on 13 May 2023).
- Swedish Environmental Protection Agency. UN GHG National Inventiry Report Sweden. Available online: https://unfccc.int/documents/461776 (accessed on 11 February 2023).
- Crippa, M.; Guizzardi, D.; Solazzo, E.; Muntean, M. European Commission, JRC Report: GHG Emissions of All World Countries. 2021. Available online: https://data.europa.eu/doi/10.2760/173513 (accessed on 11 February 2023).
- Wikström, P.; Edenius, L.; Elfving, B.; Eriksson, L.O.; Lämås, T.; Sonesson, J.; Öhman, K.; Wallerman, J.; Waller, C.; Klintebäck, F. The Heureka Forestry Decision Support System: An Overview. Math. Comput. For. Nat.-Resour. Sci. 2011, 3, 87–94. [Google Scholar]
- Peng, L.; Searchinger, T.D.; Zionts, J.; Waite, R. The carbon costs of global wood harvests. Nature 2023, 620, 110–115. [Google Scholar] [CrossRef]
- Department of Forest Resource Management, Swedish University of Agricultural Sciences. Skogsdata (Forest Data) 2023. Available online: https://www.slu.se/globalassets/ew/org/centrb/rt/dokument/skogsdata/skogsdata_2023_webb.pdf (accessed on 28 October 2024).
- IRENA. Bioenergy from Boreal Forests: Swedish Approach to Sustainable Wood Use. Abu Dhabi. 2019. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2019/Mar/IRENA_Swedish_forest_bioenergy_2019.pdf (accessed on 25 February 2023).
- Keith, H.; Lindenmayer, D.B.; Mackey, B.G.; Blair, D.; Carter, L.; McBurney, L.; Okada, S.; Konishi-Nagano, T. Accounting for Biomass Carbon Stock Change Due to Wildfire in Temperate Forest Landscapes in Australia. PLoS ONE 2014, 9, e107126. [Google Scholar] [CrossRef]
- Harmon, M.E.; Hanson, C.T.; DellaSala, D.A. Combustion of Aboveground Wood from Live Trees in Megafires, CA, USA. Forests 2022, 13, 391. [Google Scholar] [CrossRef]
- Meigs, G.W.; Donato, D.C.; Campbell, J.L.; Martin, J.G.; Law, B.E. Forest Fire Impacts on Carbon Uptake, Storage, and Emission: The Role of Burn Severity in the Eastern Cascades, Oregon. Ecosystems 2009, 12, 1246–1267. [Google Scholar] [CrossRef]
- Campbell, J.; Donato, D.; Azuma, D.; Law, B. Pyrogenic carbon emission from a large wildfire in Oregon, United States. J. Geophys. Res. Biogeosci. 2007, 112, G04014. [Google Scholar] [CrossRef]
- Lindroth, A. Clarifying the carbon balance recovery time after clear-cutting. Glob. Change Biol. 2023, 29, 4178–4179. [Google Scholar] [CrossRef] [PubMed]
- Linder, P.; Östlund, L. Structural changes in three mid-boreal Swedish forest landscapes, 1885–1996. Biol. Conserv. 1998, 85, 9–19. [Google Scholar] [CrossRef]
- Davis, E.C.; Sohngen, B.; Lewis, D.J. The effect of carbon fertilization on naturally regenerated and planted US forests. Nat. Commun. 2022, 13, 5490. [Google Scholar] [CrossRef]
- Dinerstein, E.; Vynne, C.; Sala, E.; Joshi, A.R.; Fernando, S.; Lovejoy, T.E.; Mayorga, J.; Olson, D.; Asner, G.P.; Baillie, J.E.M.; et al. A Global Deal for Nature: Guiding principles, milestones, and targets. Sci. Adv. 2019, 5, eaaw2869. [Google Scholar] [CrossRef]
- European Parliament. Deforestation Regulation. Available online: https://www.europarl.europa.eu/doceo/document/TA-9-2022-0311_EN.pdf (accessed on 28 October 2024).
- Lindahl, K.B.; Sténs, A.; Sandström, C.; Johansson, J.; Lidskog, R.; Ranius, T.; Roberge, J.-M. The Swedish forestry model: More of everything? Policy Econ. 2017, 77, 44–55. [Google Scholar] [CrossRef]
- Angelstam, P.; Manton, M.; Green, M.; Jonsson, B.-G.; Mikusiński, G.; Svensson, J.; Sabatini, F.M. Sweden does not meet agreed national and international forest biodiversity targets: A call for adaptive landscape planning. Landsc. Urban Plan. 2020, 202, 103838. [Google Scholar] [CrossRef]

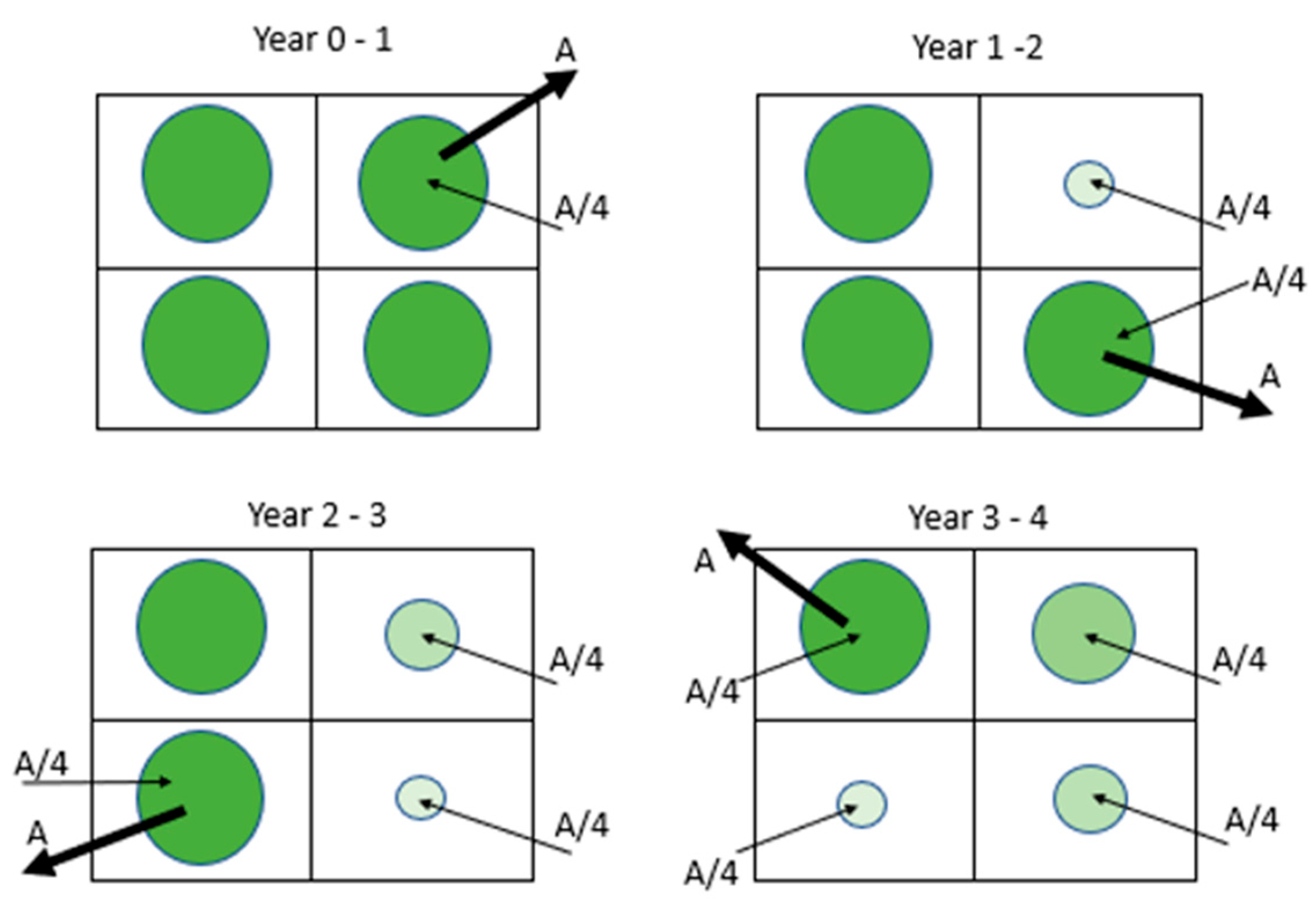
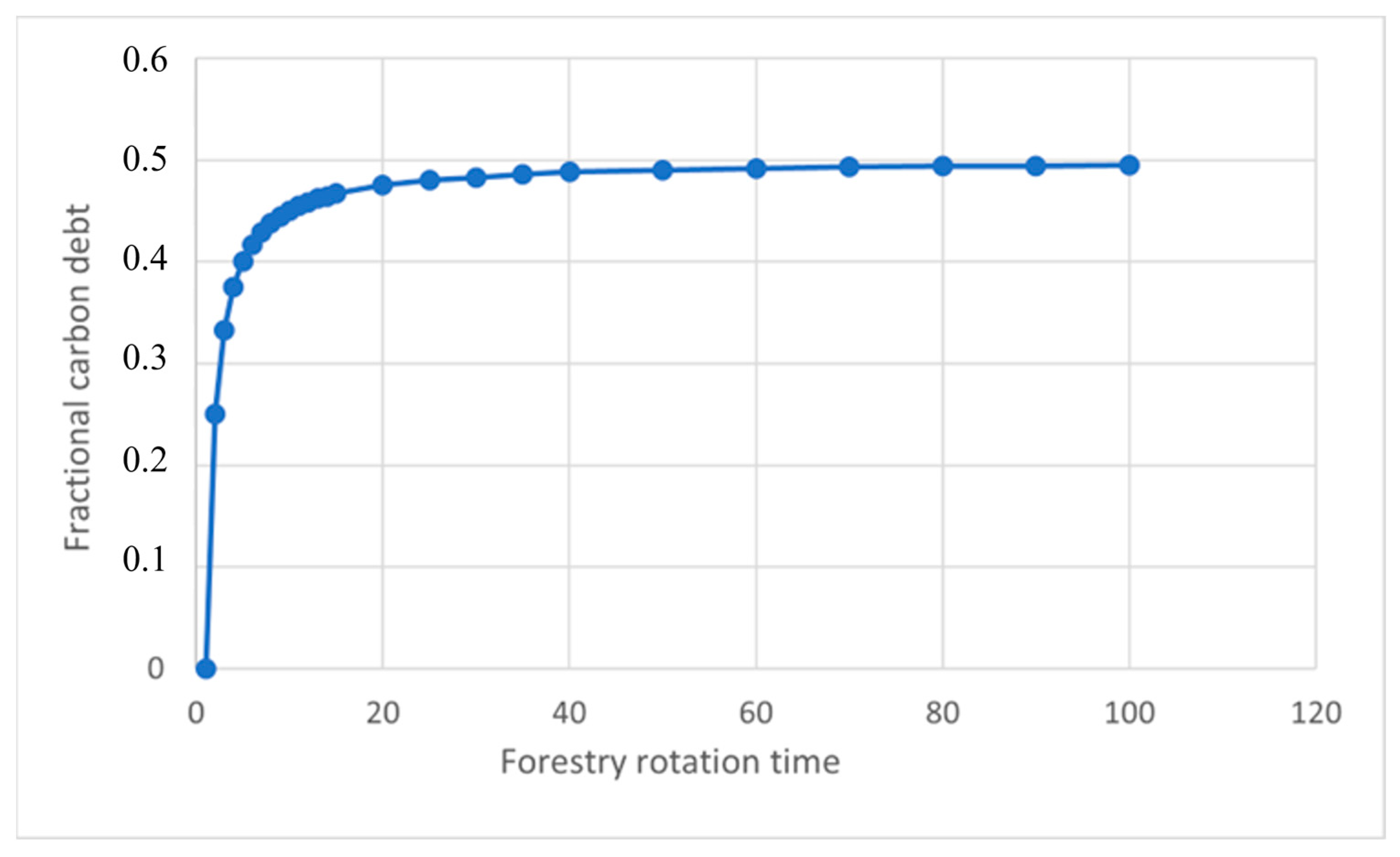
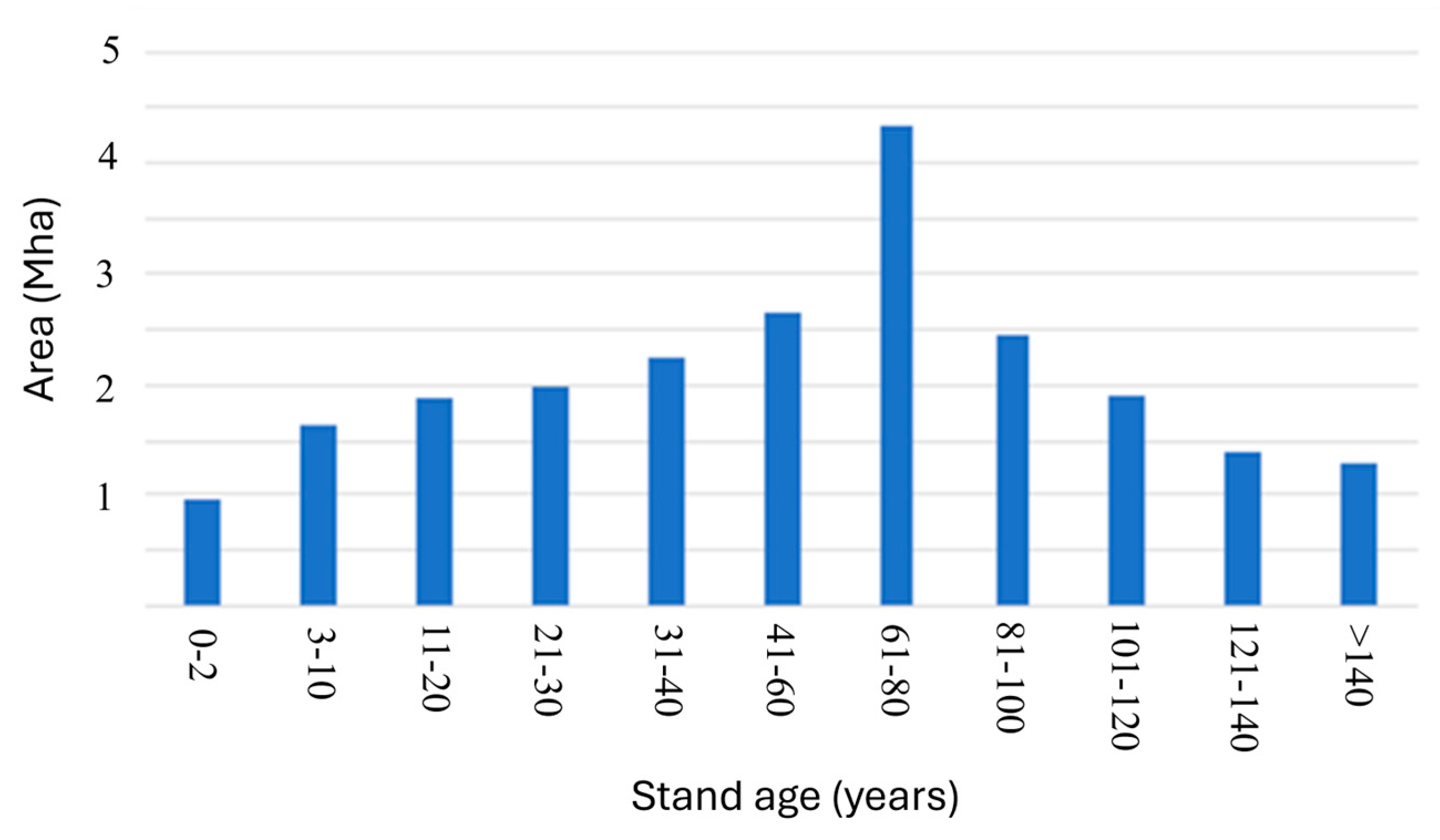
| Numbers of Years Since Start of Forestry | Fractional Carbon Paid Back of the Grid | Fractional Carbon Paid Back of the Full Forestry Area |
|---|---|---|
| 100 | 100% | 1 · 0.01 · X |
| 99 | 99% | 0.99 · 0.01 · X |
| 98 | 98% | 0.98 · 0.01 · X |
| 97 | 97% | 0.97 · 0.01 · X |
| Fossil | Biogenic | Total | |
|---|---|---|---|
| Sweden | 5 | 12 | 17 |
| USA | 16 | 2 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prytz, K.; van der Spoel, D. Assessment of Climate Impact of Sustainable Forestry Based on Landscape Structure. Forests 2024, 15, 1955. https://doi.org/10.3390/f15111955
Prytz K, van der Spoel D. Assessment of Climate Impact of Sustainable Forestry Based on Landscape Structure. Forests. 2024; 15(11):1955. https://doi.org/10.3390/f15111955
Chicago/Turabian StylePrytz, Kjell, and David van der Spoel. 2024. "Assessment of Climate Impact of Sustainable Forestry Based on Landscape Structure" Forests 15, no. 11: 1955. https://doi.org/10.3390/f15111955
APA StylePrytz, K., & van der Spoel, D. (2024). Assessment of Climate Impact of Sustainable Forestry Based on Landscape Structure. Forests, 15(11), 1955. https://doi.org/10.3390/f15111955






