Characterization of Indonesian Sugar Palm Bunch (Arenga longipes Mogea) Properties for Various Utilization Purposes
Abstract
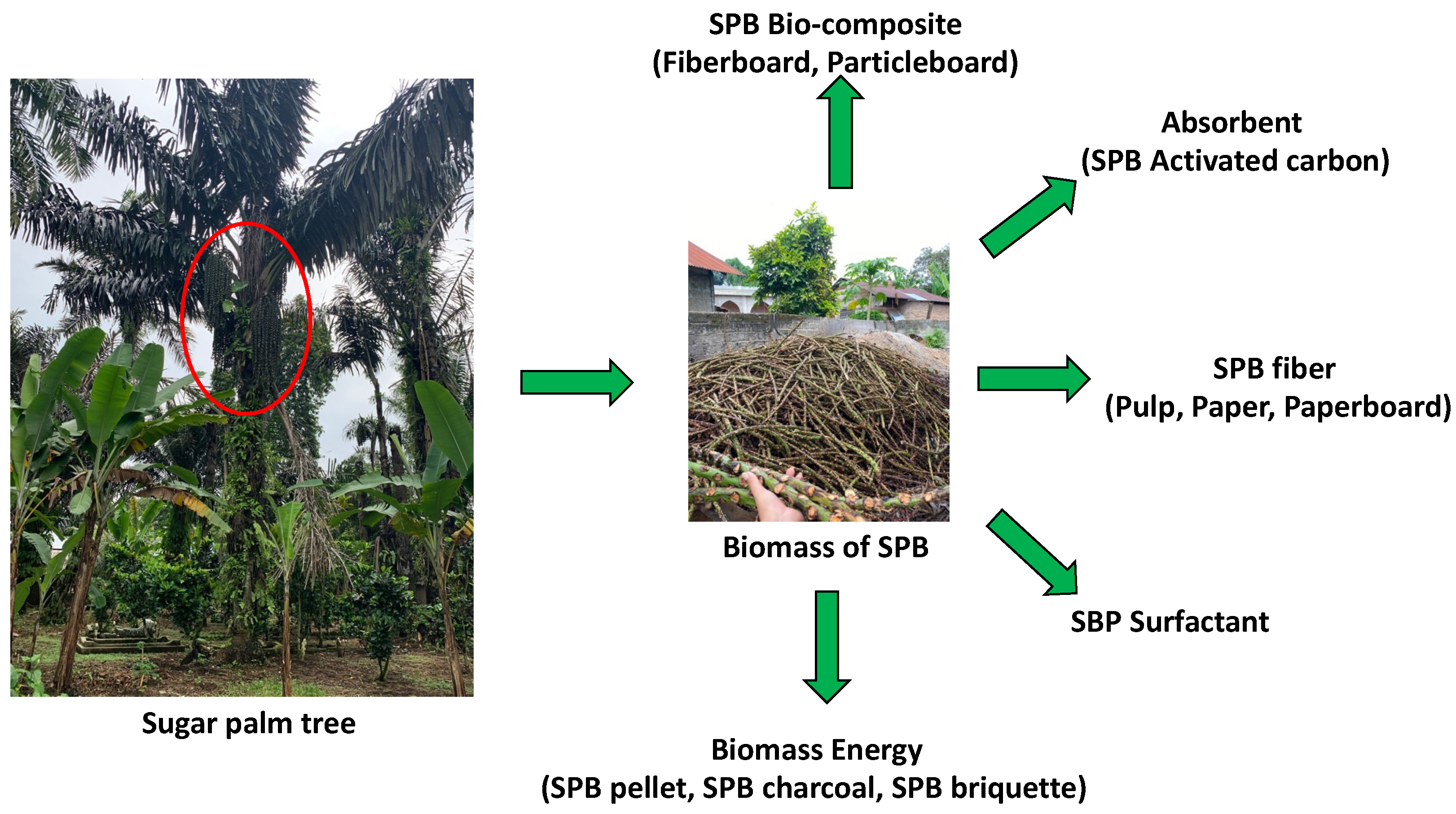
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Observation of Anatomical Properties
2.3. Physical and Mechanical Properties Measurement of FVB
2.4. Ultrastructure of SPB via Scanning Electron Microscopy
2.5. Chemical Subtituents Analysis
2.6. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
2.7. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.8. Thermogravimetry Analysis (TGA)
2.9. Index Crystallinity of SPB via X-ray Diffraction (XRD)
3. Results
3.1. Anatomical Properties
3.2. Fiber Morphology and Fiber Derivates
3.3. Ultrastructure of FVB via Scanning Electron Microscopy (SEM)
3.4. Chemical Properties
3.5. Physical and Mechanical Properties of FVB
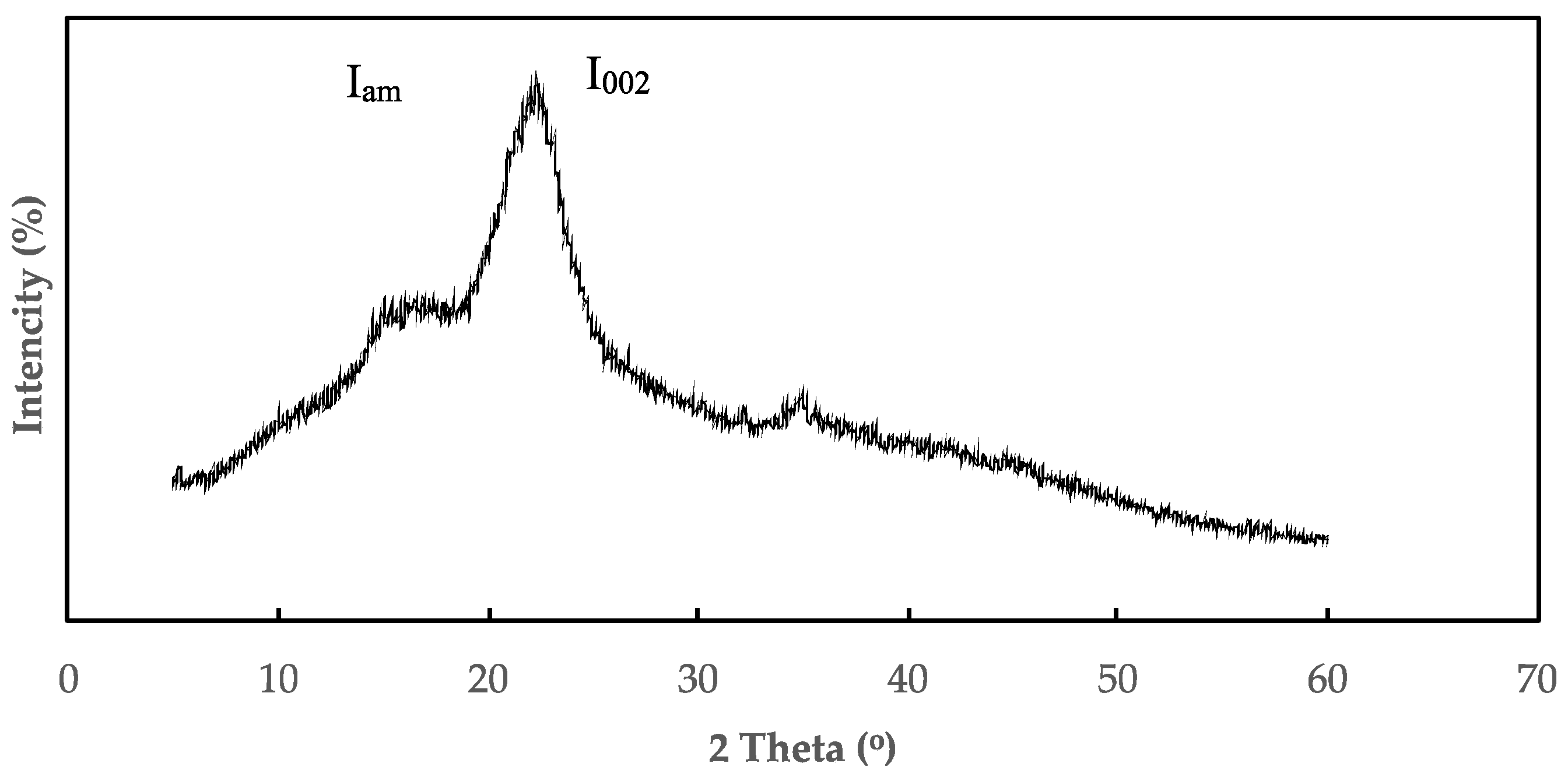
3.6. XRD (X-ray Diffraction) Analysis
3.7. Gas Chromatography–Mass Spectrometry Analysis
3.8. Thermogravimetry Analysis
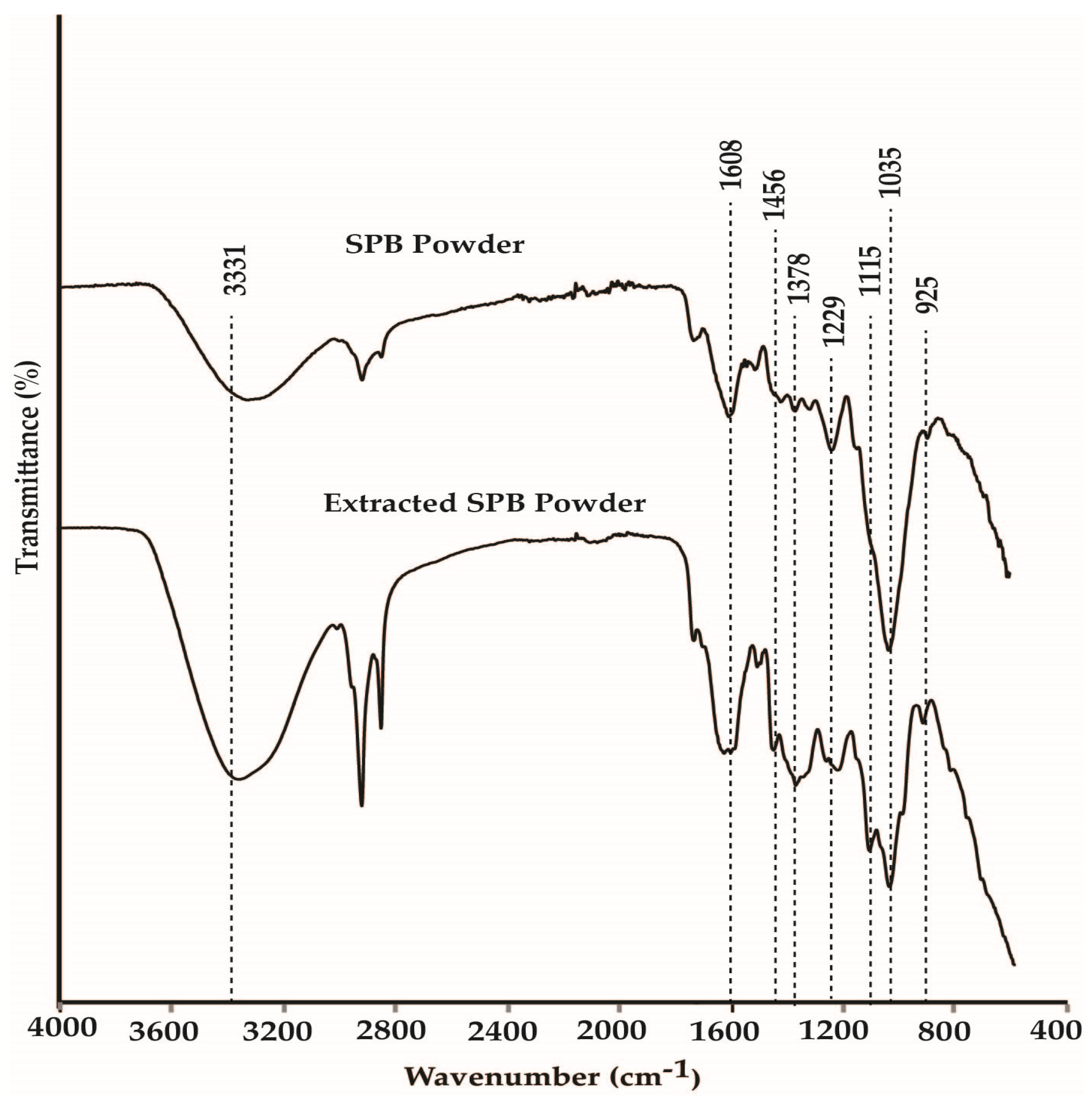
3.9. Fourier-Transform Infrared Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mogea, J.P. Four new species of Arenga (Palmae) from Indonesia. Reinwardtia J. Taxon. Bot. Plant Sociol. Ecol. 2004, 12, 181–189. [Google Scholar]
- Fadhilla, S.; Hakim, L.; Agustian, A.; Lubis, Y.S.; Siregar, A.W. The diversity of utilizations, tapping flow discharge, and conservation of sugar palm (Arenga longipes Mogea) cultivated in Langkat, North Sumatra, Indonesia. Biodiversitas 2023, 24, 122–132. [Google Scholar] [CrossRef]
- Haryoso, A.; Zuhud, E.A.M.; Hikmat, A.; Sunkar, A.; Darusman, D. Ethnobotany of sugar palm (Arenga pinnata) in the Sasak Community, Kekait Village, West Nusa Tenggara, Indonesia. Biodiversitas 2020, 21, 117–128. [Google Scholar] [CrossRef]
- Hakim, L.; Batubara, R.; Manurung, H.; Silitonga, V.W. Longitudinal and radial variability of anatomical properties, fiber morphology, and mechanical properties of fibrovascular bundle from Indonesian Arenga Longipes Mogea. Sp. Nov Frond. J. Nat. Fibers 2023, 20, 1–16. [Google Scholar] [CrossRef]
- Imraan, R.A.; Ilyas, A.S.; Norfarhana; Bangar, S.P.; Knight, V.F.; Norrrahim, M.N.F. Sugar palm (Arenga pinnata) fibers: New emerging natural fibre and its relevant properties, treatments and potential applications. J. Mater. Res. Technol. 2023, 24, 4551–4572. [Google Scholar] [CrossRef]
- Azhar, I.; Nasution, Z.; Delvian; Agussabti; Aulin, F.R.; Sembiring, M.R. Utilization of sugar palm (Arenga pinnata Merr) by the communities around the PT Toba Pulp Lestari. Proceeding of the International Conference on Agriculture, Environment and Food Security (ICAEFS). IOP Conf. Ser. Earth Environ. Sci. 2021, 782, 032017. [Google Scholar] [CrossRef]
- Sibarani, R. The local wisdom on Aren (Arenga pinnata) palm tree in Toba Batak tradition of North Sumatera at Lake Toba Area. J. Phys. Conf. Ser. 2018, 1116, 052060. [Google Scholar] [CrossRef]
- Zuhud, E.A.M.; Al-Manar, P.; Zuraida; Hidayati, S. Potency and Conservation of Aren (Arenga pinnata (Wurmb) Merr.) in Meru Betiri National Park, East Java-Indonesia. J. Manaj. Hutan Trop. 2018, 26, 212–221. [Google Scholar] [CrossRef]
- Farma, R.; Apriyani, I.; Awitdrus, A.; Taer, E.; Apriwandi, A. Hemicellulosa-derived Arenga pinnata bunches as free-standing carbon nanofber membranes for electrode material supercapacitors. Sci. Rep. 2022, 16, 2572. [Google Scholar] [CrossRef] [PubMed]
- Sumaiyah; Wirjosentono, B.; Karsono; Nasution, M.P.; Gea, S. Preparation and characterization of nanocrystalline cellulose from sugar palm bunch (Arenga pinnata (Wurmb) Merr.). Int. J. PharmTech Res. 2014, 6, 814–820. [Google Scholar]
- Huzaifah, M.R.M.; Sapuan, S.M.; Leman, Z.; Ishak, M.R. Comparative study on chemical composition, physical, tensile, and thermal properties of sugar palm fiber (Arenga pinnata) obtained from different geographical locations. BioResources 2017, 12, 9366–9382. [Google Scholar] [CrossRef]
- Hakim, L.; Widyorini, R.; Nugroho, W.D.; Prayitno, T.A. Anatomical, chemical, and mechanical properties of fibrovascular bundles of Salacca (snake fruit) frond. Bioresources 2019, 14, 7943–7957. [Google Scholar] [CrossRef]
- ASTM D 3379-75; Standard Test Method for Tensile Strength and Young’s Modulus for High-Modulus Single-Filamen Materials. ASTM International: West Conshohocken, PA, USA, 1989.
- ASTM D 882-18; Standard Test Method for Tensile Strength Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM D 1110-21; Standard Test Method for Water Solubilty of Wood. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D 1105-21; Standard Test Method for Preparation of Extractive-Free Wood. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D 1103-13; Standard Test Method for Alfa-Celluloce in Wood. ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM D 1104-13; Standard Test Method for Holocellulose in Wood. ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM D 1106-21; Standard Test Method for Acid Insoluble Lignin in Wood. ASTM International: West Conshohocken, PA, USA, 2021.
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the x-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Zhu, J.; Li, J.; Wang, C.; Wang, H. Anatomy of the Windmill Palm (Trachycarpus fortunei) and Its Application Potential. Forests 2019, 10, 1130. [Google Scholar] [CrossRef]
- Zhai, S.; Sugiyama, J.; Itoh, T.; Pan, B.; Li, D. Cell wall characterization of windmill palm (Trachycarpus fortunei) fibers and its functional implications. IAWA J. 2013, 34, 20–33. [Google Scholar] [CrossRef]
- Indriati, L.; Elyani, N.; Dina, S.F. Empty fruit bunches, potential fiber source for Indonesian pulp and paper industry. IOP Conf. Ser. Mater. Sci. Eng. 2020, 980, 012045. [Google Scholar] [CrossRef]
- Sunarti, S.; Praptoyo, H.; Nirsatmanto, A. Characteristics of fiber from the wood of Acacia auriculiformis × Acacia mangium hybrid with regard to pulp. J. Pemuliaan Tanam. Hutan 2016, 10, 135–143. [Google Scholar] [CrossRef]
- Hartono, R.; Farizky, F.; Sutiawan, J.; Sumardi, I.; Suhesti, E. Fiber Quality of Betung Bamboo (Dendrocalamus asper) from Forest Area with Special Purpose (FASP) Pondok Buluh, Simalungun, North Sumatera. IOP Conf. Ser. Earth Environ. Sci. 2022, 1115, 012085. [Google Scholar] [CrossRef]
- Sahari, J.; Sapuan, S.M.; Zainudin, E.S.; Maleque, M.A. Sugar palm tree: A versatile plant and novel source for biofibres, biomatrices, and biocomposites. Polym. Renew. Resour. 2012, 3, 61–78. [Google Scholar] [CrossRef]
- Khantayanuwong, S.; Yimlamai, P.; Chitbanyong, K.; Wanitpinyo, K.; Pisutpiched, S.; Sungkaew, S.; Sukyai, P.; Puangsin, B. Fiber morphology, chemical composition, and properties of kraft pulping handsheet made from four Thailand bamboo species. J. Nat. Fibers 2023, 20, 2150924. [Google Scholar] [CrossRef]
- Koronis, G.; Silva, A.; Fontul, M. Green composites: A review of adequate materials for automotive applications. Compos. Part B Eng. 2013, 44, 120–127. [Google Scholar] [CrossRef]
- Jones, D.; Ormondroyd, G.O.; Curling, S.F.; Popescu, C.M.; Popescu, M.C. Chemical compositions of natural fibres. In Advanced High Strength Natural Fibre Composites in Construction; Woodhead Publishing: Thorston, UK, 2017; pp. 23–58. [Google Scholar]
- Conte, A.A.; Sun, K.; Hu, X.; Beachley, V.Z. Effects of fiber density and strain rate on the mechanical properties of electrospun polycaprolactone nanofiber mats. Front. Chem. 2020, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Ahvenainen, P.; Kontro, I.; Svedström, K. Comparison of sample crystallinity determination methods by X-ray diffraction for challenging cellulose I materials. Cellulose 2016, 23, 1073–1086. [Google Scholar] [CrossRef]
- Siruru, H.; Syafii, W.; Wistara, I.N.J.; Pari, G. Characteristics of sago pith and sago bark waste from Seram Island, Maluku, Indonesia. Biodiversitas J. Biol. Divers. 2019, 20, 12. [Google Scholar] [CrossRef]
- Qanytah; Syamsu, K.; Fahma, F.; Pari, G. Structure analysis of three non-wood materials for liner paper. Nord. Pulp Pap. Res. J. 2019, 34, 453–466. [Google Scholar] [CrossRef]
- Bensidhom, G.; Arabiourrutia, M.; Trabelsi, A.B.H.; Cortazar, M.; Ceylan, S.; Olazar, M. Fast pyrolysis of date palm biomass using Py-GCMS. J. Energy Inst. 2021, 99, 229–239. [Google Scholar] [CrossRef]
- Babinszki, B.; Jakab, E.; Terjék, V.; Sebestyén, Z.; Várhegyi, G.; May, Z.; Czégény, Z. Thermal decomposition of biomass wastes derived from palm oil production. J. Anal. Appl. Pyrolysis 2021, 155, 105069. [Google Scholar] [CrossRef]
- Ishak, M.R.; Sapuan, S.M.; Leman, Z.; Rahman, M.Z.A.; Anwar, U.M.K. Characterization of sugar palm (Arenga pinnata) fibres: Tensile and thermal properties. J. Therm. Anal. Calorim. 2012, 109, 981–989. [Google Scholar] [CrossRef]
- El-Sayed, S.A.; Khass, T.M.; Mostafa, M.E. Thermal degradation behaviour and chemical kinetic characteristics of biomass pyrolysis using TG/DTG/DTA techniques. Biomass Convers. Biorefinery 2023, 4, 1–25. [Google Scholar] [CrossRef]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Balasundram, V.; Alias, N.; Ibrahim, N.; Kasmani, M.R.; Isha, R.; Hamid, A.M.K.; Hasbullah, H. Thermal Characterization of Malaysian Biomass via Thermogravimetric Analysis. J. Energy Saf. Technol. 2018, 1, 31–38. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Huang, X.; Kocaefe, D.; Kocaefe, Y.; Boluk, Y.; Pichette, A. Study of the degradation behavior of heat-treated jack pine (Pinus banksiana) under artificial sunlight irradiation. Polym. Degrad. Stab. 2012, 97, 1197–1214. [Google Scholar] [CrossRef]
- Gonultas, O.; Candan, Z. Chemical characterization and ftir spectroscopy of thermally compressed eucalyptus wood panels. Maderas Cienc. Tecnol. 2018, 20, 431–442. [Google Scholar] [CrossRef]
- Moosavinejad, S.M.; Madhoushi, M.; Vakili, M.; Rasouli, D. Evaluation of degradation in chemical compounds of wood in historical buildings using ft-ir and ft-raman vibrational spectroscopy. Maderas Cienc. Tecnol. 2019, 21, 381–392. [Google Scholar] [CrossRef]
- Xu, G.; Wang, L.; Liu, J.; Wu, J. FTIR and XPS analysis of the changes in bamboo chemical structure decayed by white-rot and brown-rot fungi. Appl. Surf. Sci. 2013, 280, 799–805. [Google Scholar] [CrossRef]
- Bodîrlău, R.; Teacă, C.A.; Resmeriţă, A.M.; Spiridon, I. Investigation of structural and thermal properties of different wood species treated with toluene-2,4-diisocyanate. Cellul. Chem. Technol. 2012, 46, 381–387. [Google Scholar]
- Poletto, M.; Pistor, V.; Zeni, M.; Zattera, A.J. Crystalline properties and decomposition kinetics of cellulose fibers in wood pulp obtained by two pulping processes. Polym. Degrad. Stab. 2011, 96, 679–685. [Google Scholar] [CrossRef]
- Popescu, M.C.; Popescu, C.M.; Lisa, G.; Sakata, Y. Evaluation of morphological and chemical aspects of different wood species by spectroscopy and thermal methods. J. Mol. Struct. 2011, 988, 65–72. [Google Scholar] [CrossRef]
- Mogea, J.P.; Seibert, B.; Smits, W. Multipurpose palm: The sugar palm (Arenga pinnata (Wurmb) Merr.). Agrofor. Syst. 1991, 13, 111–129. [Google Scholar] [CrossRef]
- Imlimthan, S.; Figueiredo, P.; Santos, H.A.; Sarparanta, M. Chapter 1—Introduction to lignocellulosic materials. In Lignin-Based Materials for Biomedical Applications; Hélder Santos, H.A., Figueiredo, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–34. ISBN 9780128203033. [Google Scholar] [CrossRef]
- Hakim, L.; Widyorini, R.; Nugroho, W.D.; Prayitno, T.A. Performance of citric acid-bonded oriented board from modified fibrovascular bundle of salacca (Salacca zalacca (Gaertn.) Voss) frond. Polymer 2021, 13, 4090. [Google Scholar] [CrossRef]
- Auriga, R.; Auriga, A.; Borysiuk, P.; Wilkowski, J.; Fornalczyk, O.; Ochmian, I. Lignocellulosic Biomass from Grapevines as Raw Material for Particleboard Production. Polymers 2022, 14, 2483. [Google Scholar] [CrossRef]
- Duangkham, S.; Thuadaij, P. Characterization of charcoal briquettes produced from blending rice straw and banana peel. Heliyon 2023, 9, e16305. [Google Scholar] [CrossRef]
- Przybysz, K.; Małachowska, E.; Martyniak, D.; Boruszewski, P.; Iłowska, J.; Kalinowska, H.; Przybysz, P. Yield of Pulp, Dimensional Properties of Fibers, and Properties of Paper Produced from Fast Growing Trees and Grasses. BioResources 2018, 13, 1372–1382. [Google Scholar] [CrossRef]
- Ferdous, T.; Ni, Y.; Quaiyyum, M.A.; Uddin, M.N.; Jahan, M.S. Non-Wood Fibers: Relationships of Fiber Properties with Pulp Properties. ACS Omega 2021, 6, 21613–21622. [Google Scholar] [CrossRef] [PubMed]
- Sugiharto, Y.E.C.; Lee, H.; Fitriana, A.D.; Lee, H.; Jeon, W.; Park, K.; Ahn, J.; Lee, H. Effect of decanoic acid and 10-hydroxydecanoic acid on the biotransformation of methyl decanoate to sebacic acid. AMB Expr. 2018, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Teixeira, S.; Teixeira, J. A Review of Biomass Thermal Analysis, Kinetics and Product Distribution for Combustion Modeling: From the Micro to Macro Perspective. Energies 2023, 16, 6705. [Google Scholar] [CrossRef]
- Sitek, T.; Pospíšil, T.; Poláčik, J.; Chýlek, R. Thermogravimetric analysis of solid biomass fuels and corresponding emission of fine particles. Energy 2021, 237, 121609. [Google Scholar] [CrossRef]

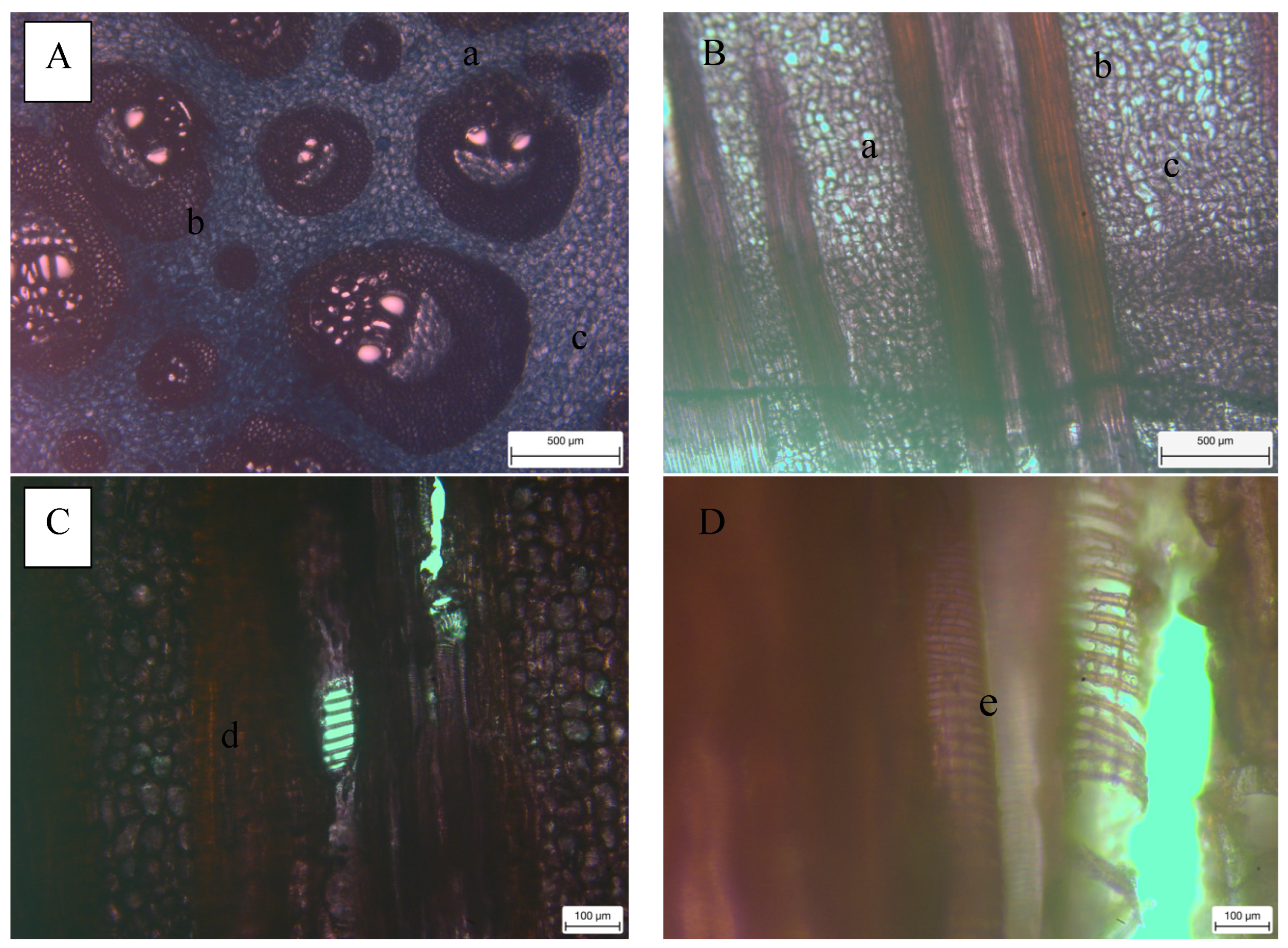
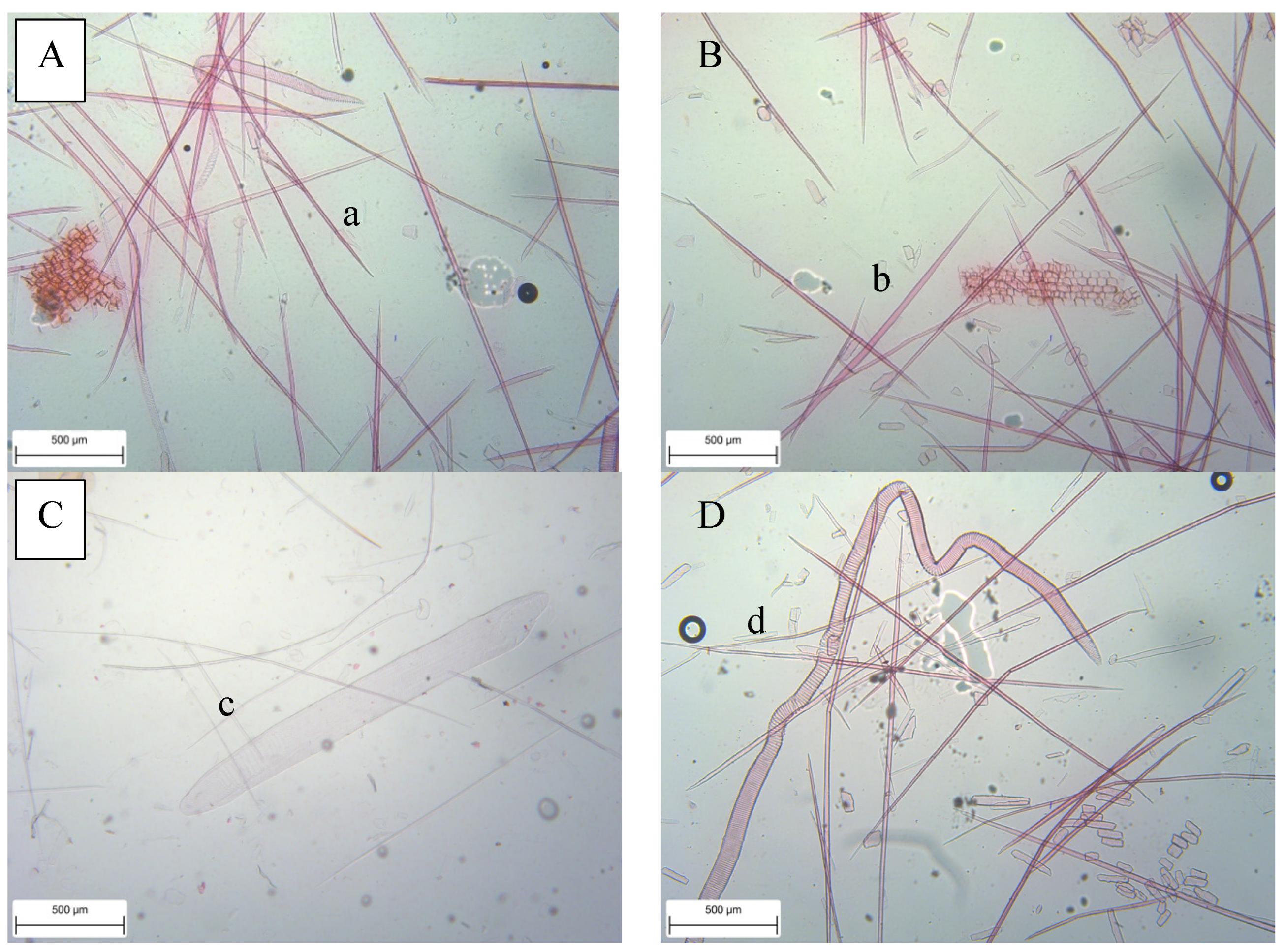
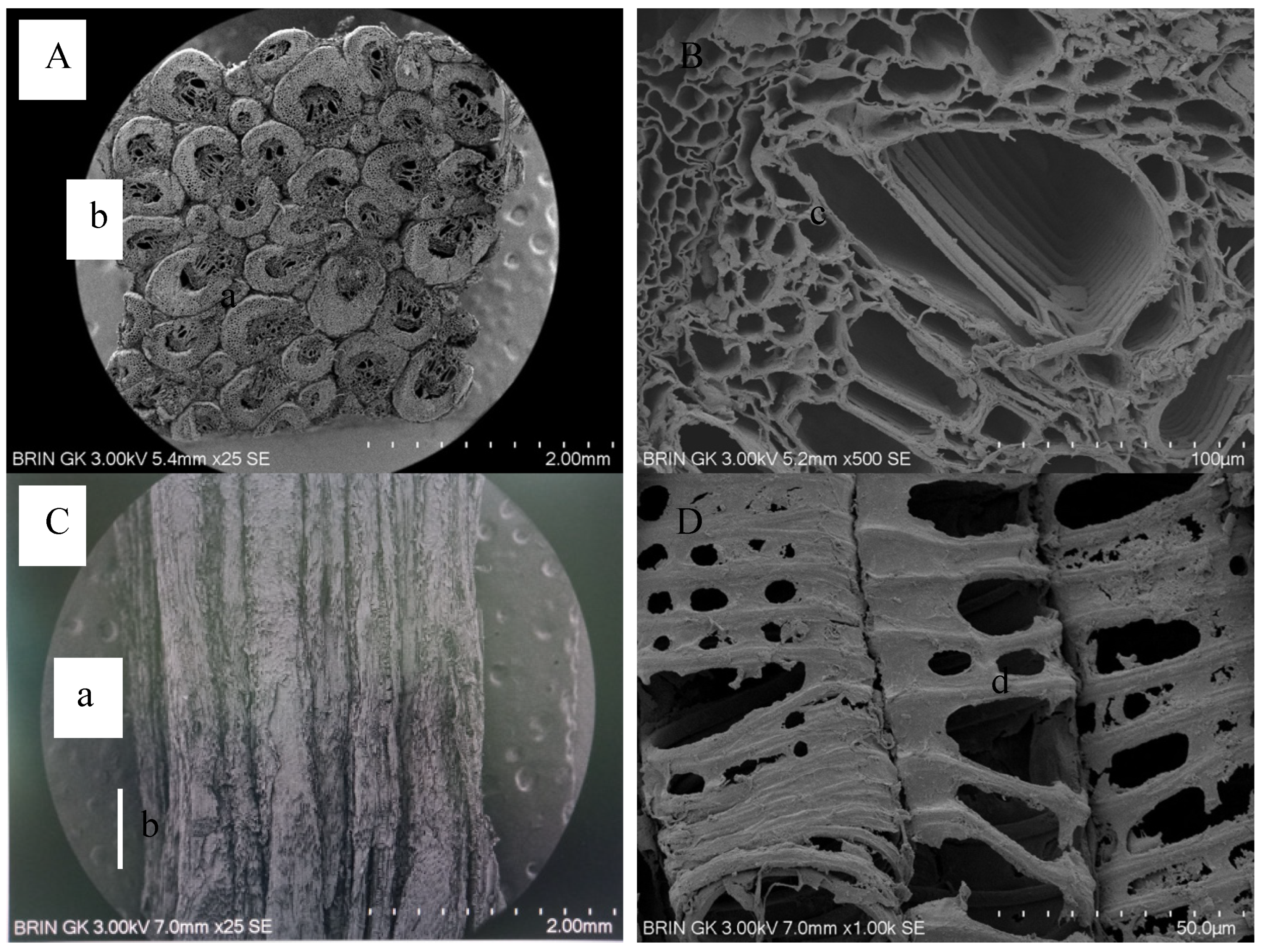


| Anatomical Characteristics | FVBs | |
|---|---|---|
| Small FVBs | Big FVBs | |
| Frequency of fibrovascular bundles per 4 mm2 | 4–6 | 4–6 |
| Total transverse area (mm2) | 0.08 ± 0.02 | 0.55 ± 0.14 |
| Vascular tissue area (mm2) | 0.03 ± 0.01 | 0.23 ± 0.05 |
| Non-vascular tissue area (mm2) | 0.04 ± 0.01 | 0.31 ± 0.10 |
| Ratio of vascular tissue area to total transverse area (%) | 46.30 | 42.74 |
| Ratio of non-vascular tissue area to total transverse area (%) | 53.69 | 57.25 |
| Ratio of vascular tissue area to non-vascular tissue area (%) | 86.00 | 74.00 |
| Fiber Morphology | Value |
|---|---|
| - Fiber length (mm) | 1.34 ± 0.41 |
| - Fiber diameter (μm) | 20.05 ± 3.81 |
| - Lumen diameter (μm) | 11.82 ± 2.95 |
| - Cell wall thickness (μm) | 4.12 ± 1.08 |
| Fiber Derivates | |
| - Runkel ratio | 0.75 ± 0.33 |
| - Felting Power | 68.96 ± 22.81 |
| - Muhlstep ratio | 64.74 ± 9.22 |
| - Coefficient of rigidity | 0.37 ± 0.20 |
| - Flexibility ratio | 0.54 ± 0.15 |
| Chemical Properties | Value |
|---|---|
| Hot water solubility (%) | 4.79 ± 0.84 |
| Cold water solubility (%) | 7.12 ± 0.68 |
| 1% NaOH solubility (%) | 9.81 ± 3.78 |
| Ethanol-benzene solubility (%) | 7.27 ± 2.38 |
| Holocellulose (%) | 68.51 ± 4.56 |
| Cellulose (%) | 45.31 ± 3.20 |
| Hemicellulose (%) | 23.21 ± 3.73 |
| Klason lignin (%) | 27.23 ± 4.23 |
| Ash content (%) | 1.39 ± 0.32 |
| Physical and Mechanical Properties | FVBs | |
|---|---|---|
| Small FVBs | Big FVBs | |
| Diameter (mm) | 0.047 ± 0.14 | 0.036 ± 0.01 |
| Density (g/cm3) | 0.35 ± 0.15 | 0.48 ± 0.11 |
| Tensile strength (MPa) | 221.85 ± 27.18 | 493.72 ± 24.92 |
| Young’s modulus (GPa) | 4.13 ± 0.74 | 6.52 ± 0.66 |
| Specific tensile strength (MPa) | 482.28 ± 181.20 | 1028.55 ± 226.54 |
| Specific Young’s modulus (GPa) | 11.80 ± 4.93 | 13.58 ± 6.01 |
| Peak | RT | Area (%) | Compound |
|---|---|---|---|
| 1 | 7.794 | 5.03 | 1-Dodecanamine, N,N-dimethyl- |
| 2 | 9.363 | 1.57 | 1-Tetradecanamine, N,N-dimethyl- |
| 3 | 9.79 | 2.45 | Tetradecanoic acid |
| 4 | 10.932 | 0.9 | Hexadecanoic acid, methyl ester |
| 5 | 11.186 | 14.18 | n-Hexadecanoic acid |
| 6 | 12.097 | 6.36 | 6-Octadecenoic acid, methyl ester, (Z)- |
| 7 | 12.247 | 1.61 | 1-Allyl-3-methylcyclohex-2-enol |
| 8 | 12.443 | 100 | cis-Vaccenic acid |
| 9 | 12.547 | 7.57 | Octadecanoic acid |
| 10 | 16.792 | 2.9 | Diisooctyl phthalate |
| 11 | 23.957 | - | 4-Butyl-N[(1E)-phenylmethylene]-aniline |
| 12 | 25.895 | - | Piperidine,1-[5-(1,3-benzodioxol-5-yl)-1-oxo-2,4-pentadienyl]- |
| 13 | 26.737 | - | 7-Dimethylocta-2,6-dien-1-yl palmitate |
| 14 | 28.94 | - | 3-hydoxyphthalide |
| 15 | 30.74 | - | Nonacosan-10-one |
| 16 | 31.651 | - | 2S-Chloro-2-(3′-methoxy-4′-hydroxybenzyl)-3S-(3″methoxy-4″-hydroxybenzyl)-.gamma.-butyrolactone |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakim, L.; Iswanto, A.H.; Herawati, E.; Batubara, R.; Lubis, Y.S.; Aini, E.N. Characterization of Indonesian Sugar Palm Bunch (Arenga longipes Mogea) Properties for Various Utilization Purposes. Forests 2024, 15, 239. https://doi.org/10.3390/f15020239
Hakim L, Iswanto AH, Herawati E, Batubara R, Lubis YS, Aini EN. Characterization of Indonesian Sugar Palm Bunch (Arenga longipes Mogea) Properties for Various Utilization Purposes. Forests. 2024; 15(2):239. https://doi.org/10.3390/f15020239
Chicago/Turabian StyleHakim, Luthfi, Apri Heri Iswanto, Evalina Herawati, Ridwanti Batubara, Yunida Syafriani Lubis, and Erlina Nurul Aini. 2024. "Characterization of Indonesian Sugar Palm Bunch (Arenga longipes Mogea) Properties for Various Utilization Purposes" Forests 15, no. 2: 239. https://doi.org/10.3390/f15020239
APA StyleHakim, L., Iswanto, A. H., Herawati, E., Batubara, R., Lubis, Y. S., & Aini, E. N. (2024). Characterization of Indonesian Sugar Palm Bunch (Arenga longipes Mogea) Properties for Various Utilization Purposes. Forests, 15(2), 239. https://doi.org/10.3390/f15020239








