Climatic Warming Caused a Transition in Tree Growth Sensitivity from Temperature to Moisture Conditions: Evidence from Multi-Species Tree-Ring Data in the Southeastern Tibetan Plateau
Abstract
:1. Introduction
2. Materials and Methods
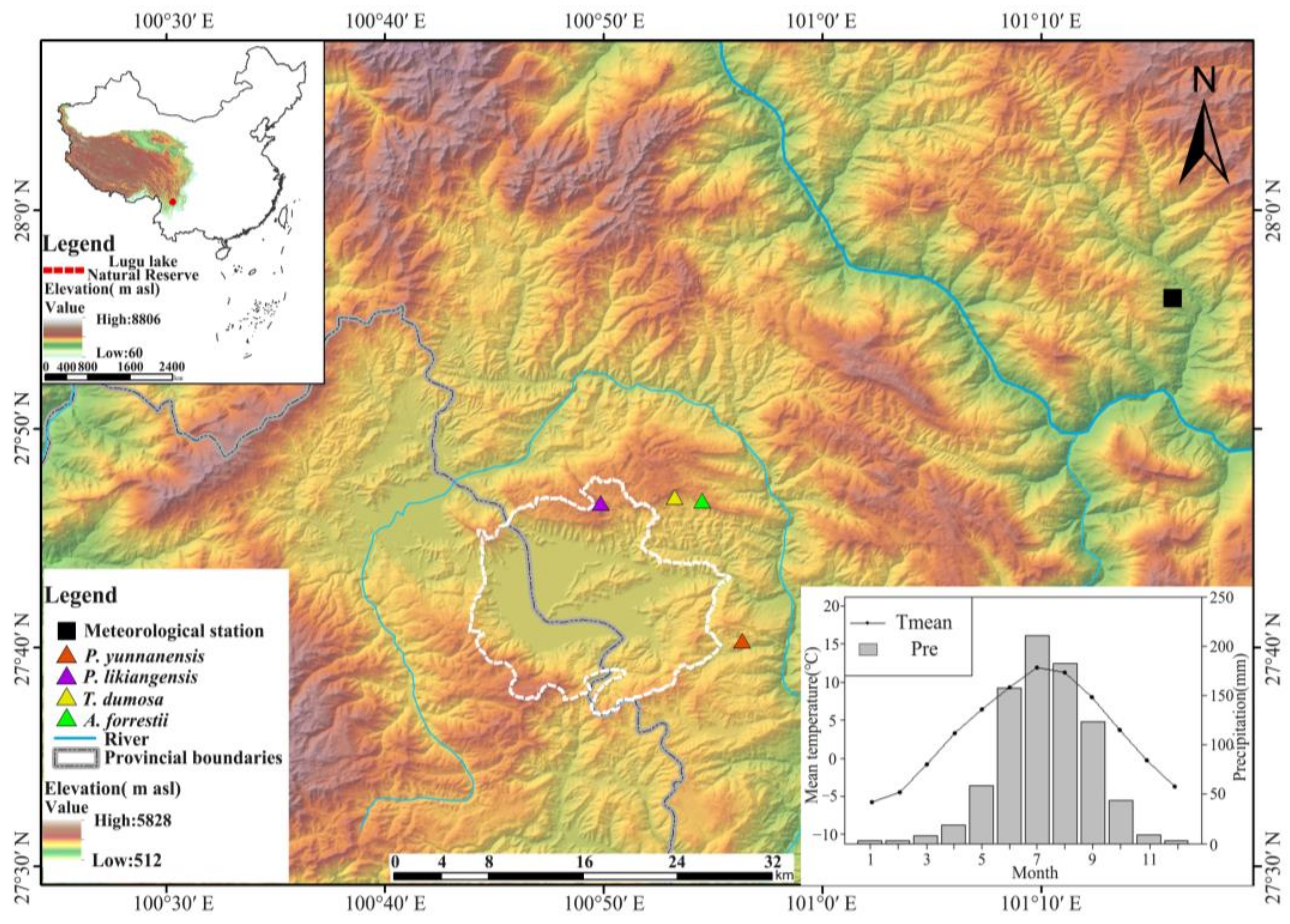
2.1. Study Area
2.2. Tree Ring Sampling and Measurements
2.3. Climate Data
2.4. Data Analysis
3. Results
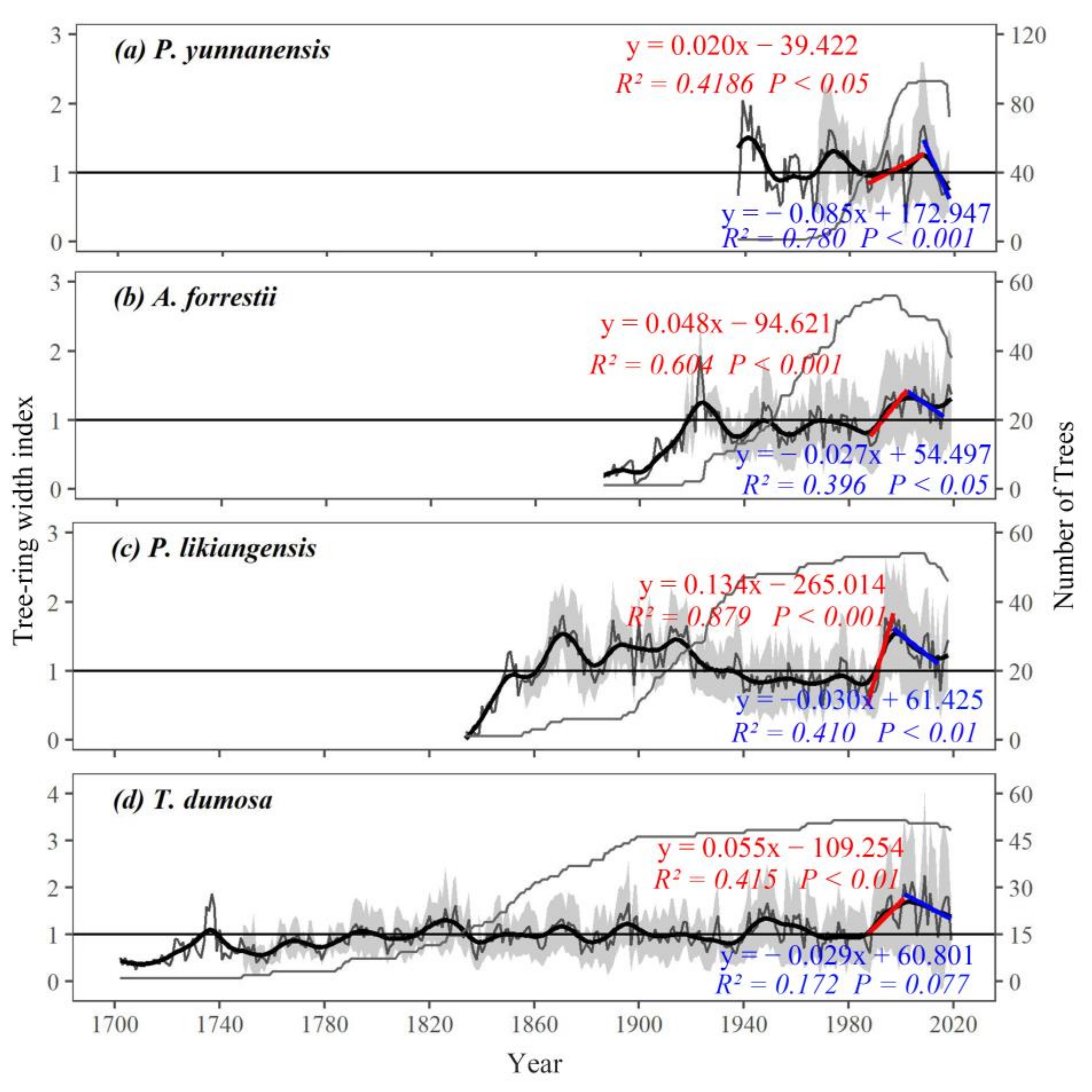
3.1. Chronological Characteristics and Growth Pattern
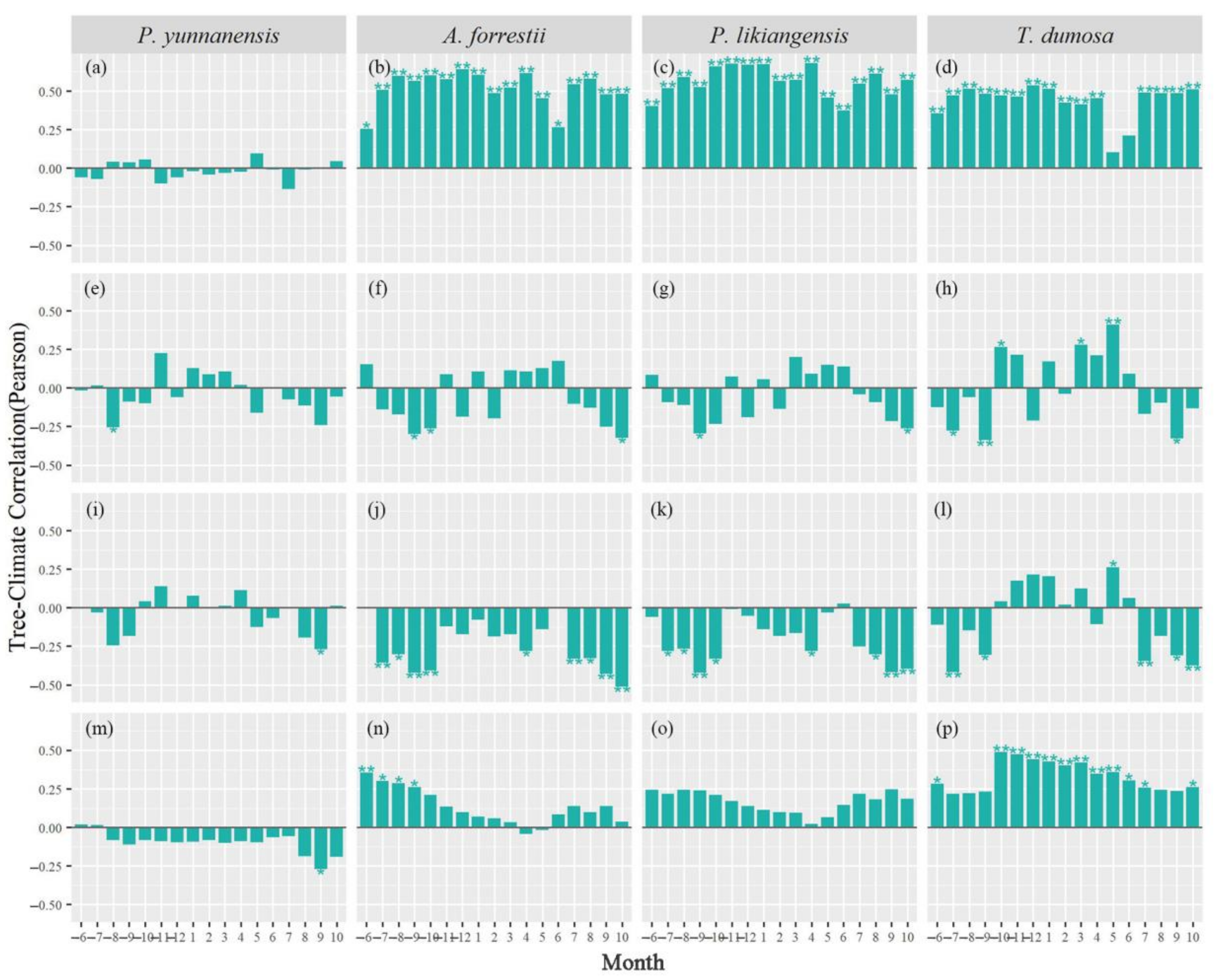
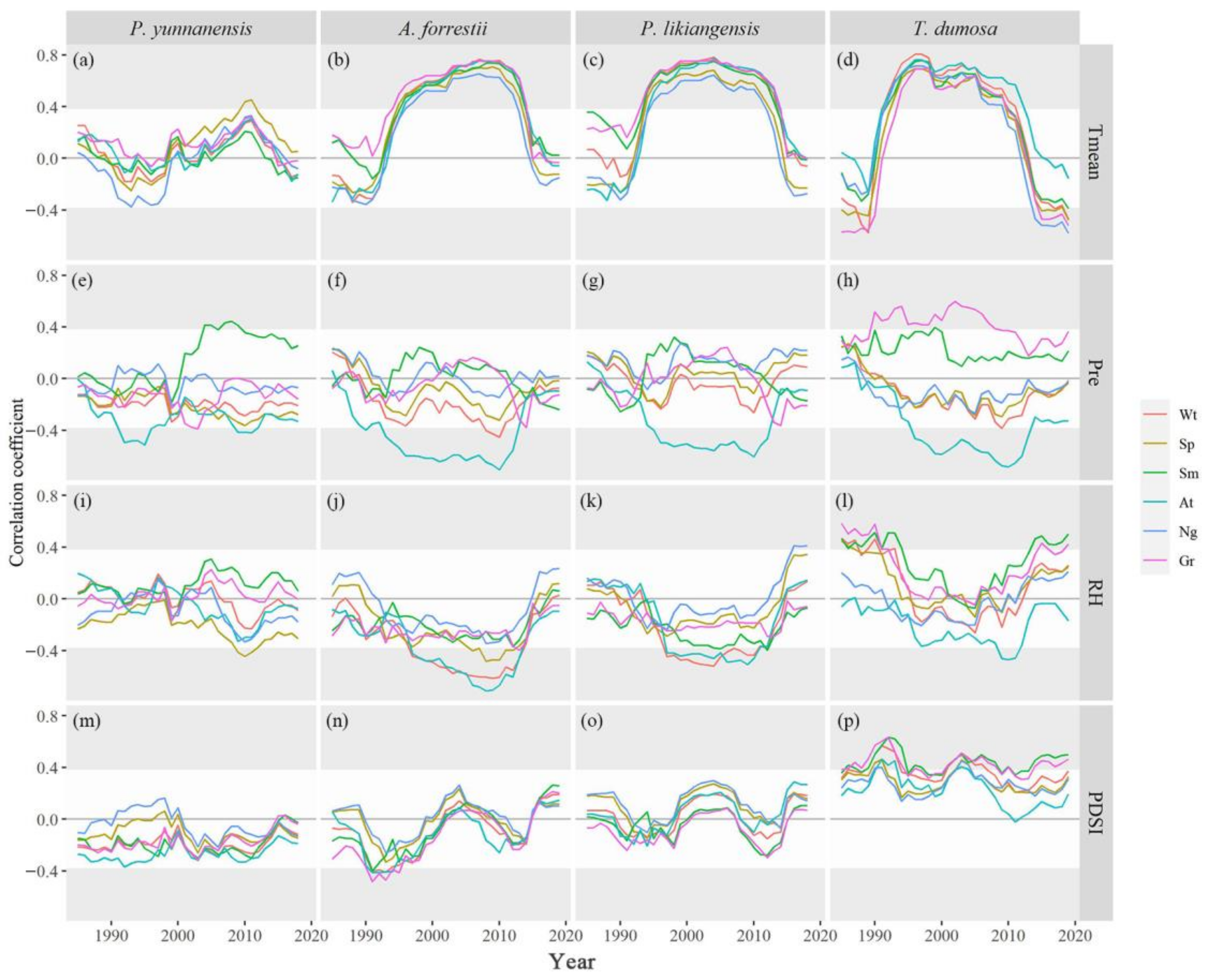
3.2. Tree Growth–Climate Single-Month Relationships
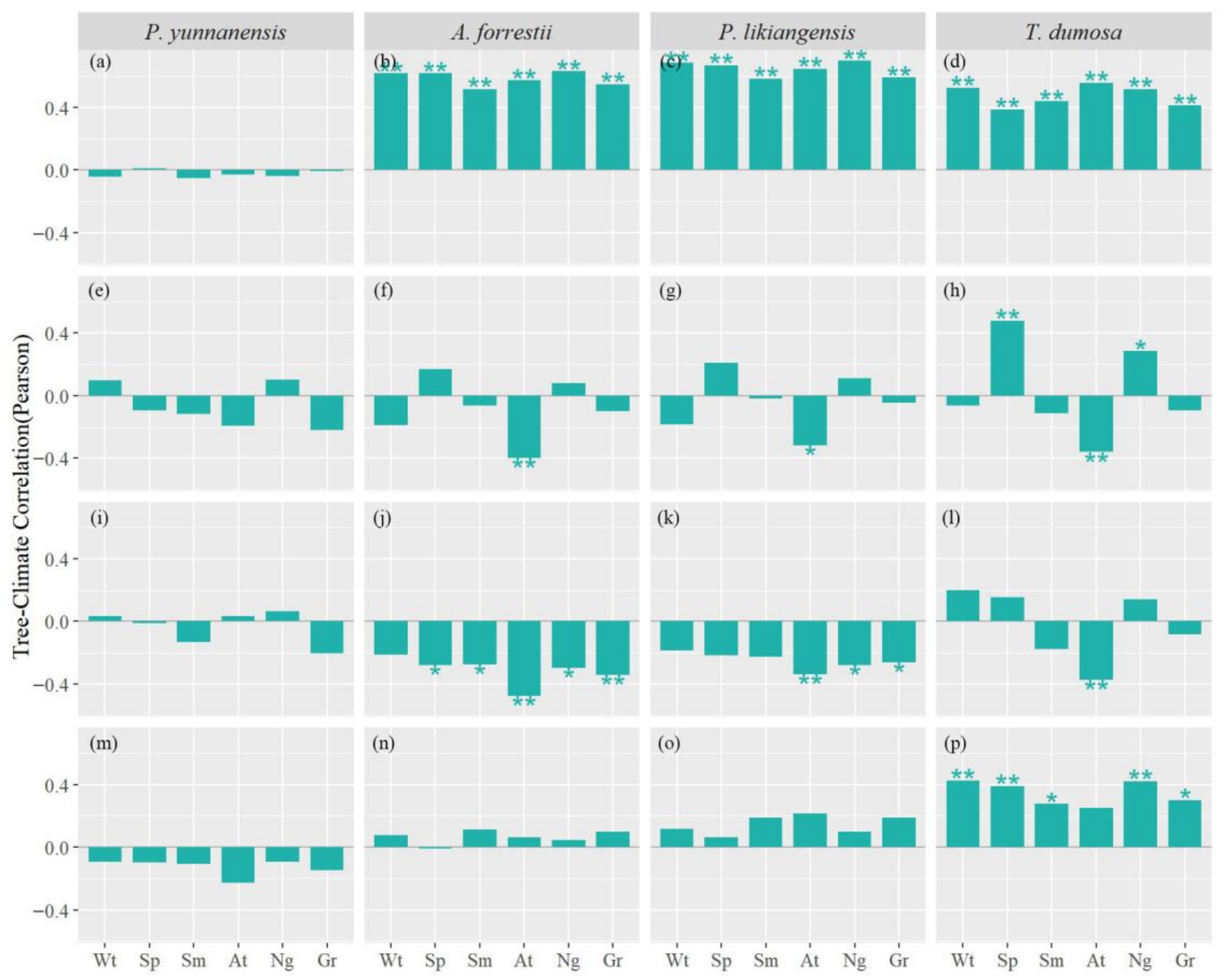
3.3. Tree Growth–Climate Seasonal and Interannual Relationships
3.4. Individual Tree Response to Climate
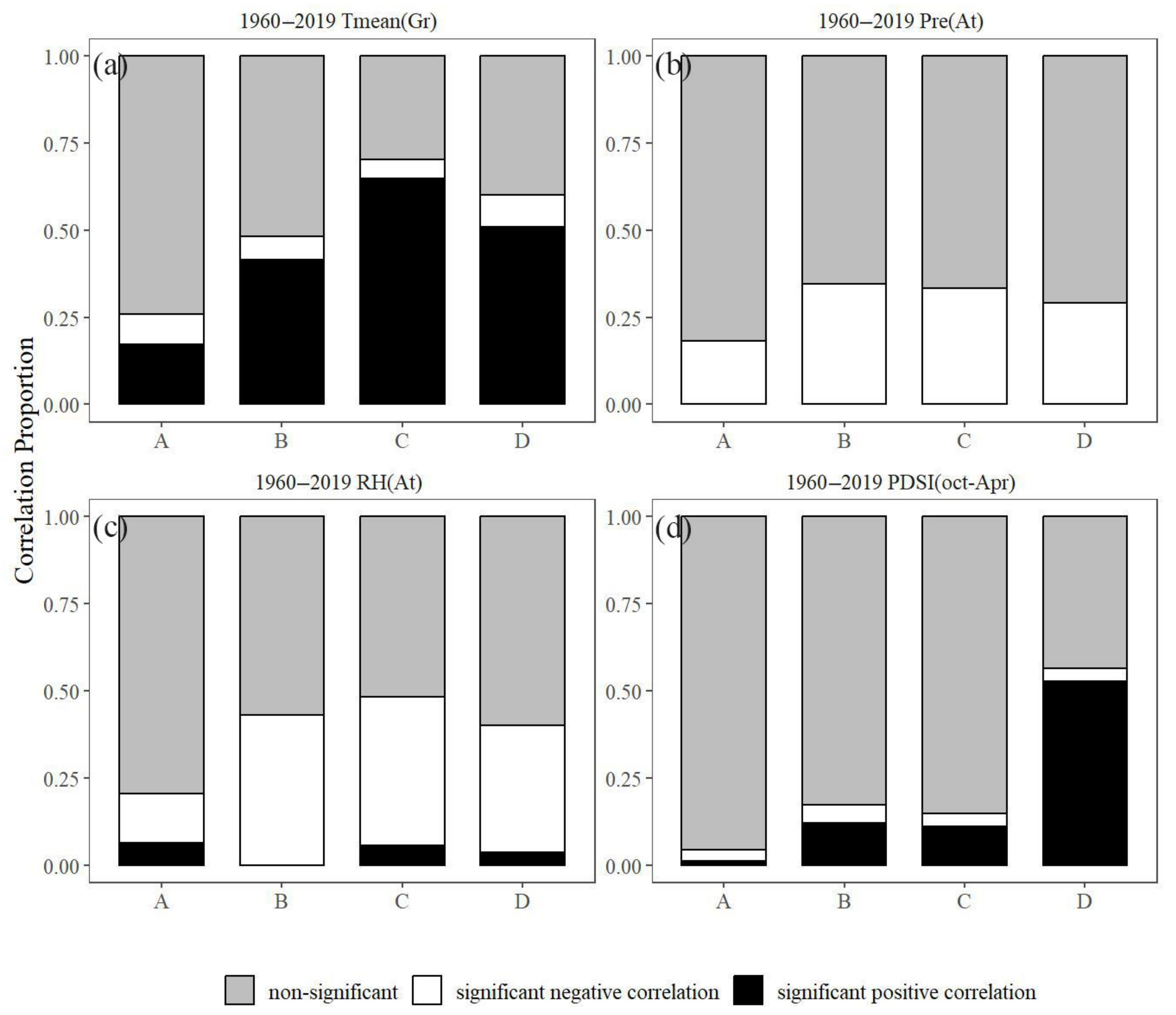
3.5. Stability of Climate–Growth Relationships
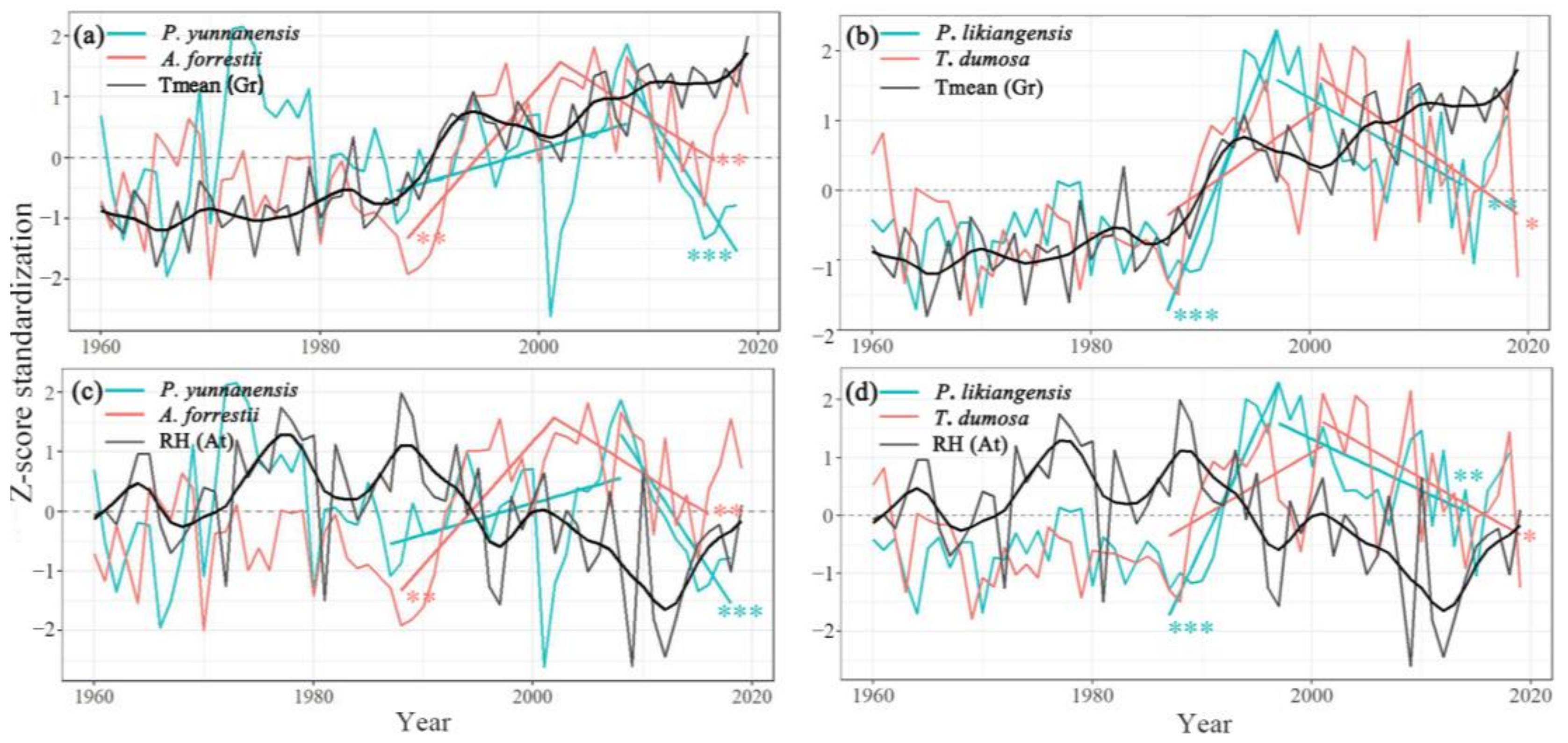
3.6. Standardization of Tree and Climate Data
4. Discussion
4.1. The Trend of Tree Growth and Its Influence on Climate
4.2. Response Stability of Tree Growth to Climate Change
4.3. Potential Variation in Forest Dynamics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.W.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K.; Blanco, G.; et al. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2023. [Google Scholar]
- Peng, Z.; Zhang, Y.; Zhu, L.; Lu, Q.; Mo, Q.; Cai, J.; Guo, M. Divergent Tree Growth and the Response to Climate Warming and Humidification in the Tianshan Mountains, China. Forests 2022, 13, 886. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A Global Overview of Drought and Heat-Induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Boisvenue, C.; Running, S.W. Impacts of Climate Change on Natural Forest Productivity—Evidence since the Middle of the 20th Century. Glob. Chang. Biol. 2006, 12, 862–882. [Google Scholar] [CrossRef]
- Adams, H.R.; Barnard, H.R.; Loomis, A.K. Topography Alters Tree Growth–Climate Relationships in a Semi-arid Forested Catchment. Ecosphere 2014, 5, 1–16. [Google Scholar] [CrossRef]
- Ackerman, F.; Goldblum, D. Lodgepole Pine and Interior Spruce Radial Growth Response to Climate and Topography in the Canadian Rocky Mountains, Alberta. Can. J. For. Res. 2021, 51, 986–1001. [Google Scholar] [CrossRef]
- Canham, C.D.; Murphy, L. The Demography of Tree Species Response to Climate: Sapling and Canopy Tree Growth. Ecosphere 2016, 7, e01474. [Google Scholar] [CrossRef]
- Fei, S.; Desprez, J.M.; Potter, K.M.; Jo, I.; Knott, J.A.; Oswalt, C.M. Divergence of Species Responses to Climate Change. Sci. Adv. 2017, 3, e1603055. [Google Scholar] [CrossRef]
- Community and Ecosystem Responses to Recent Climate Change|Philosophical Transactions of the Royal Society B: Biological Sciences. Available online: https://royalsocietypublishing.org/doi/10.1098/rstb.2010.0021 (accessed on 19 January 2024).
- Scherrer, D.; Massy, S.; Meier, S.; Vittoz, P.; Guisan, A. Assessing and Predicting Shifts in Mountain Forest Composition across 25 Years of Climate Change. Divers. Distrib. 2017, 23, 517–528. [Google Scholar] [CrossRef]
- Holtmeier, F.-K.; Broll, G. Sensitivity and Response of Northern Hemisphere Altitudinal and Polar Treelines to Environmental Change at Landscape and Local Scales: Treeline and Environmental Change. Glob. Ecol. Biogeogr. 2005, 14, 395–410. [Google Scholar] [CrossRef]
- Qi, Z.; Liu, H.; Wu, X.; Hao, Q. Climate-Driven Speedup of Alpine Treeline Forest Growth in the Tianshan Mountains, Northwestern China. Glob. Chang. Biol. 2015, 21, 816–826. [Google Scholar] [CrossRef]
- Silva, L.C.R.; Sun, G.; Zhu-Barker, X.; Liang, Q.; Wu, N.; Horwath, W.R. Tree Growth Acceleration and Expansion of Alpine Forests: The Synergistic Effect of Atmospheric and Edaphic Change. Sci. Adv. 2016, 2, e1501302. [Google Scholar] [CrossRef]
- Ning, Q.-R.; Gong, X.-W.; Li, M.-Y.; Hao, G.-Y. Differences in Growth Pattern and Response to Climate Warming between Larix olgensis and Pinus koraiensis in Northeast China Are Related to Their Distinctions in Xylem Hydraulics. Agric. For. Meteorol. 2022, 312, 108724. [Google Scholar] [CrossRef]
- Che, C.; Xiao, S.; Ding, A.; Peng, X.; Su, J. Growth Response of Plantations Hippophae rhamnoides Linn. on Different Slope Aspects and Natural Caragana opulens Kom. to Climate and Implications for Plantations Management. Ecol. Indic. 2022, 138, 108833. [Google Scholar] [CrossRef]
- Shi, P.; Sun, S.; Wang, M.; Li, N.; Wang, J.; Jin, Y.; Gu, X.; Yin, W. Climate Change Regionalization in China (1961–2010). Sci. China Earth Sci. 2014, 57, 2676–2689. [Google Scholar] [CrossRef]
- Kastridis, A.; Kamperidou, V.; Stathis, D. Dendroclimatological Analysis of Fir (A. borisii-regis) in Greece in the Frame of Climate Change Investigation. Forests 2022, 13, 879. [Google Scholar] [CrossRef]
- Holmes, R.L. Computer-Assisted Quality Control in Tree-Ring Dating and Measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Bunn, A.G. A Dendrochronology Program Library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Fan, Z.-X.; Bräuning, A.; Cao, K.-F.; Zhu, S.-D. Growth–Climate Responses of High-Elevation Conifers in the Central Hengduan Mountains, Southwestern China. For. Ecol. Manag. 2009, 258, 306–313. [Google Scholar] [CrossRef]
- Liang, E.; Wang, Y.; Piao, S.; Lu, X.; Camarero, J.J.; Zhu, H.; Zhu, L.; Ellison, A.M.; Ciais, P.; Peñuelas, J. Species Interactions Slow Warming-Induced Upward Shifts of Treelines on the Tibetan Plateau. Proc. Natl. Acad. Sci. USA 2016, 113, 4380–4385. [Google Scholar] [CrossRef]
- St George, S.; Ault, T.R. The Imprint of Climate within Northern Hemisphere Trees. Quat. Sci. Rev. 2014, 89, 1–4. [Google Scholar] [CrossRef]
- Zhu, H.; Zheng, Y.; Shao, X.; Liu, X.; Xu, Y.; Liang, E. Millennial Temperature Reconstruction Based on Tree-Ring Widths of Qilian juniper from Wulan, Qinghai Province, China. Sci. Bull. 2008, 53, 3914–3920. [Google Scholar] [CrossRef]
- Gou, X.; Chen, F.; Jacoby, G.; Cook, E.; Yang, M.; Peng, J.; Zhang, Y. Rapid Tree Growth with Respect to the Last 400 Years in Response to Climate Warming, Northeastern Tibetan Plateau. Int. J. Climatol. 2007, 27, 1497–1503. [Google Scholar] [CrossRef]
- Tierney, G.L.; Fahey, T.J.; Hardy, J.P.; Fitzhugh, R.D.; Driscoll, C.T. Soil Freezing Alters Fine Root Dynamics in a Northern Hardwood Forest. Biogeochemistry 2001, 56, 175–190. [Google Scholar] [CrossRef]
- Girardin, M.-P.; Tardif, J.C.; Flannigan, M.D.; Bergeron, Y. Synoptic-Scale Atmospheric Circulation and Boreal Canada Summer Drought Variability of the Past Three Centuries. J. Clim. 2006, 19, 1922–1947. [Google Scholar] [CrossRef]
- Keyimu, M.; Li, Z.; Jiao, L.; Chen, W.; Wu, X.; Fan, Z.; Zeng, F.; Fu, B. Radial Growth Response of Quercus Liaotungensis to Climate Change—A Case Study on the Central Loess Plateau, China. Trees 2022, 36, 1811–1822. [Google Scholar] [CrossRef]
- Jevšenak, J.; Tychkov, I.; Gričar, J.; Levanič, T.; Tumajer, J.; Prislan, P.; Arnič, D.; Popkova, M.; Shishov, V.V. Growth-Limiting Factors and Climate Response Variability in Norway Spruce (Picea abies L.) along an Elevation and Precipitation Gradients in Slovenia. Int. J. Biometeorol. 2021, 65, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Rebetez, M. Seasonal Relationship between Temperature, Precipitation and Snow Cover in a Mountainous Region. Theor. Appl. Clim. 1996, 54, 99–106. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, X.; Li, J.; Duan, X.; Jin, Y.; Chen, Z. Altitudinal Disparity in Growth of Dahurian Larch (Larix gmelinii Rupr.) in Response to Recent Climate Change in Northeast China. Sci. Total Environ. 2019, 670, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Shutova, E.; Wielgolaski, F.E.; Karlsen, S.R.; Makarova, O.; Berlina, N.; Filimonova, T.; Haraldsson, E.; Aspholm, P.E.; Flø, L.; Høgda, K.A. Growing Seasons of Nordic Mountain Birch in Northernmost Europe as Indicated by Long-Term Field Studies and Analyses of Satellite Images. Int. J. Biometeorol. 2006, 51, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.-L.; Zaw, Z.; Peng, X.-H.; Zhang, H.; Fu, P.-L.; Wang, W.-L.; Bräuning, A.; Fan, Z.-X. Tree Rings in Tsuga Dumosa Reveal Increasing Drought Variability in Subtropical Southwest China over the Past Two Centuries. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2023, 628, 111757. [Google Scholar] [CrossRef]
- Hong, Y.; Zhang, L.; Liu, X.; Aritsara, A.N.A.; Zeng, X.; Xing, X.; Lu, Q.; Wang, K.; Wang, Y.; Zhang, Y.; et al. Tree Ring Anatomy Indices of Pinus tabuliformis Revealed the Shifted Dominant Climate Factor Influencing Potential Hydraulic Function in Western Qinling Mountains. Dendrochronologia 2021, 70, 125881. [Google Scholar] [CrossRef]
- Castagneri, D.; Battipaglia, G.; Von Arx, G.; Pacheco, A.; Carrer, M. Tree-Ring Anatomy and Carbon Isotope Ratio Show Both Direct and Legacy Effects of Climate on Bimodal Xylem Formation in Pinus Pinea. Tree Physiol. 2018, 38, 1098–1109. [Google Scholar] [CrossRef]
- Sigdel, S.R.; Wang, Y.; Camarero, J.J.; Zhu, H.; Liang, E.; Peñuelas, J. Moisture-mediated Responsiveness of Treeline Shifts to Global Warming in the Himalayas. Glob. Chang. Biol. 2018, 24, 5549–5559. [Google Scholar] [CrossRef]
- Leal, S.; Eamus, D.; Grabner, M.; Wimmer, R.; Cherubini, P. Tree Rings of Pinus Nigra from the Vienna Basin Region (Austria) Show Evidence of Change in Climatic Sensitivity in the Late 20th Century. Can. J. For. Res. 2008, 38, 744–759. [Google Scholar] [CrossRef]
- Panthi, S.; Fan, Z.; Van Der Sleen, P.; Zuidema, P.A. Long-term Physiological and Growth Responses of Himalayan Fir to Environmental Change Are Mediated by Mean Climate. Glob. Chang. Biol. 2020, 26, 1778–1794. [Google Scholar] [CrossRef]
- Salzer, M.W.; Hughes, M.K.; Bunn, A.G.; Kipfmueller, K.F. Recent Unprecedented Tree-Ring Growth in Bristlecone Pine at the Highest Elevations and Possible Causes. Proc. Natl. Acad. Sci. USA 2009, 106, 20348–20353. [Google Scholar] [CrossRef]
- Drought Stress, Growth and Nonstructural Carbohydrate Dynamics of Pine Trees in a Semi-Arid Forest|Tree Physiology|Oxford Academic. Available online: https://academic.oup.com/treephys/article/34/9/981/2338180?login=false (accessed on 20 January 2024).
- Gazol, A.; Camarero, J.J.; Gutiérrez, E.; Popa, I.; Andreu-Hayles, L.; Motta, R.; Nola, P.; Ribas, M.; Sangüesa-Barreda, G.; Urbinati, C.; et al. Distinct Effects of Climate Warming on Populations of Silver Fir (Abies Alba) across Europe. J. Biogeogr. 2015, 42, 1150–1162. [Google Scholar] [CrossRef]
- Anderegg, L.D.L.; HilleRisLambers, J. Drought Stress Limits the Geographic Ranges of Two Tree Species via Different Physiological Mechanisms. Glob. Chang. Biol. 2016, 22, 1029–1045. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.K.; Bräuning, A.; Timonen, M.; Rautio, P. Growth Response of Scots Pines in Polar-Alpine Tree-Line to a Warming Climate. For. Ecol. Manag. 2017, 399, 94–107. [Google Scholar] [CrossRef]
- Wilmking, M.; Juday, G.P. Longitudinal Variation of Radial Growth at Alaska’s Northern Treeline—Recent Changes and Possible Scenarios for the 21st Century. Glob. Planet. Chang. 2005, 47, 282–300. [Google Scholar] [CrossRef]

| Item | P. yunnanensis | A. forrestii | P. likiangensis | T. dumosa |
|---|---|---|---|---|
| Longitude | 100.939° E | 100.908° E | 100.831° E | 100.888° E |
| Latitude | 27.672° N | 27.779° N | 27.777° N | 27.781° N |
| Elevation | 2849 m | 3520 m | 3643 m | 3380 m |
| Sample size (trees/cores) | 55/93 | 35/58 | 30/54 | 31/55 |
| Chronology span/A.D | 1937–2018 | 1886–2019 | 1834–1018 | 1702–2019 |
| Mean sensitivity (MS) | 0.272 | 0.280 | 0.241 | 0.281 |
| Signal-to-noise ratio (SNR) | 15.104 | 12.542 | 20.172 | 11.205 |
| Expressed population signal (EPS) | 0.938 | 0.926 | 0.953 | 0.918 |
| First order autocorrelation (AC1) | 0.837 | 0.568 | 0.811 | 0.722 |
| Gleichläufigkeit (GLK) | 0.983 | 0.624 | 0.662 | 0.642 |
| Mean inter-series correlation (Rbar) | 0.677 | 0.536 | 0.651 | 0.594 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, W.; Xie, C.; Shi, S.; Li, J.; Wang, G.; Bie, X. Climatic Warming Caused a Transition in Tree Growth Sensitivity from Temperature to Moisture Conditions: Evidence from Multi-Species Tree-Ring Data in the Southeastern Tibetan Plateau. Forests 2024, 15, 241. https://doi.org/10.3390/f15020241
Luo W, Xie C, Shi S, Li J, Wang G, Bie X. Climatic Warming Caused a Transition in Tree Growth Sensitivity from Temperature to Moisture Conditions: Evidence from Multi-Species Tree-Ring Data in the Southeastern Tibetan Plateau. Forests. 2024; 15(2):241. https://doi.org/10.3390/f15020241
Chicago/Turabian StyleLuo, Wenwen, Chengsheng Xie, Songlin Shi, Jingji Li, Guoyan Wang, and Xiaojuan Bie. 2024. "Climatic Warming Caused a Transition in Tree Growth Sensitivity from Temperature to Moisture Conditions: Evidence from Multi-Species Tree-Ring Data in the Southeastern Tibetan Plateau" Forests 15, no. 2: 241. https://doi.org/10.3390/f15020241
APA StyleLuo, W., Xie, C., Shi, S., Li, J., Wang, G., & Bie, X. (2024). Climatic Warming Caused a Transition in Tree Growth Sensitivity from Temperature to Moisture Conditions: Evidence from Multi-Species Tree-Ring Data in the Southeastern Tibetan Plateau. Forests, 15(2), 241. https://doi.org/10.3390/f15020241





