Ecological Restoration in Eastern Canada Using Four Early-Successional Species on Severely Degraded Sites Using a Factorial of Site-Preparation Treatments: Growth and Biomass over Two Years
Abstract
:1. Introduction
2. Material and Methods
2.1. Site-Preparation Treatments
2.2. Plant Materials
2.3. Soil Properties
2.4. Plant Measurements
2.5. Statistical Analysis
3. Results
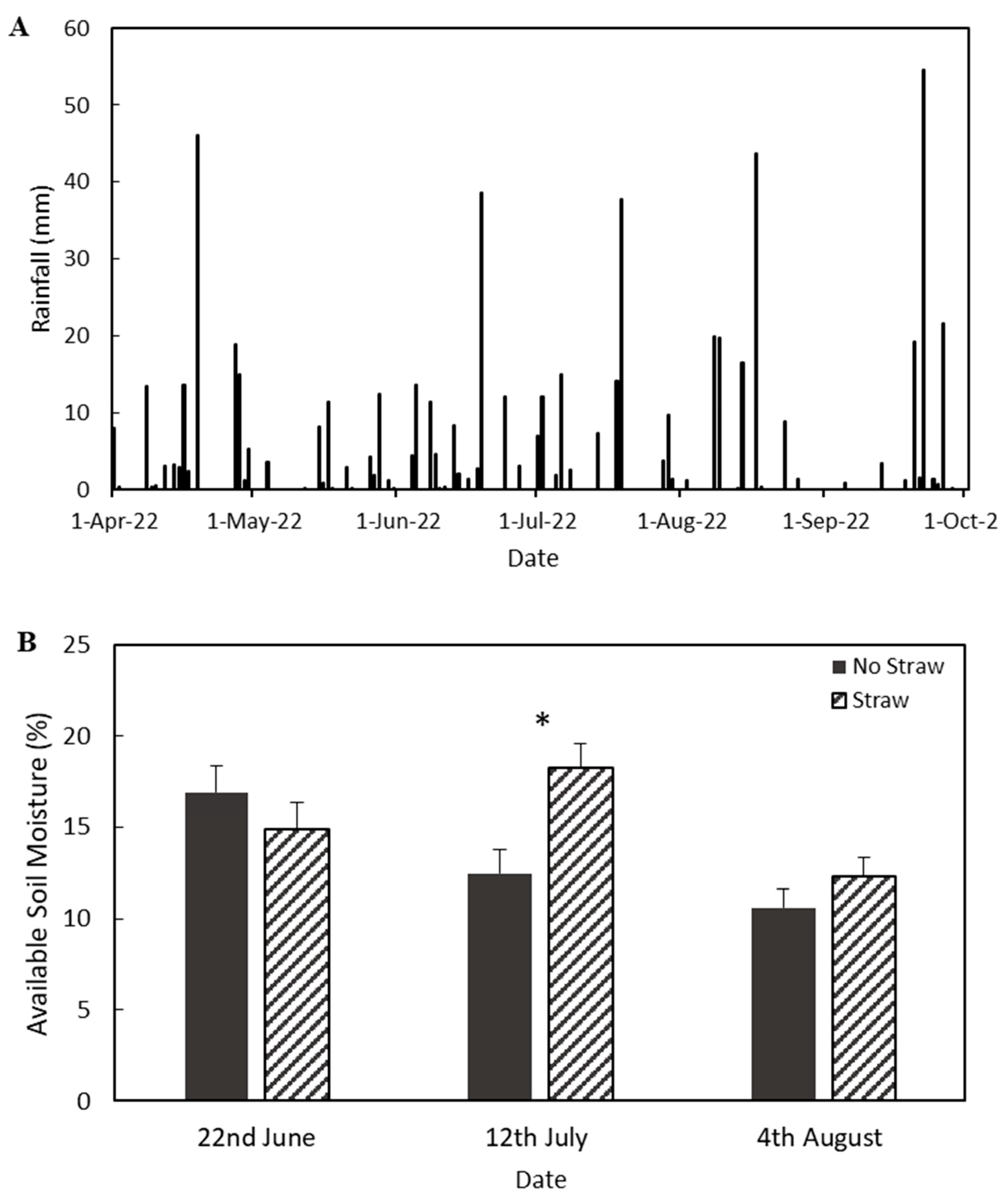
3.1. Soil Moisture
3.2. First-Year Height Growth
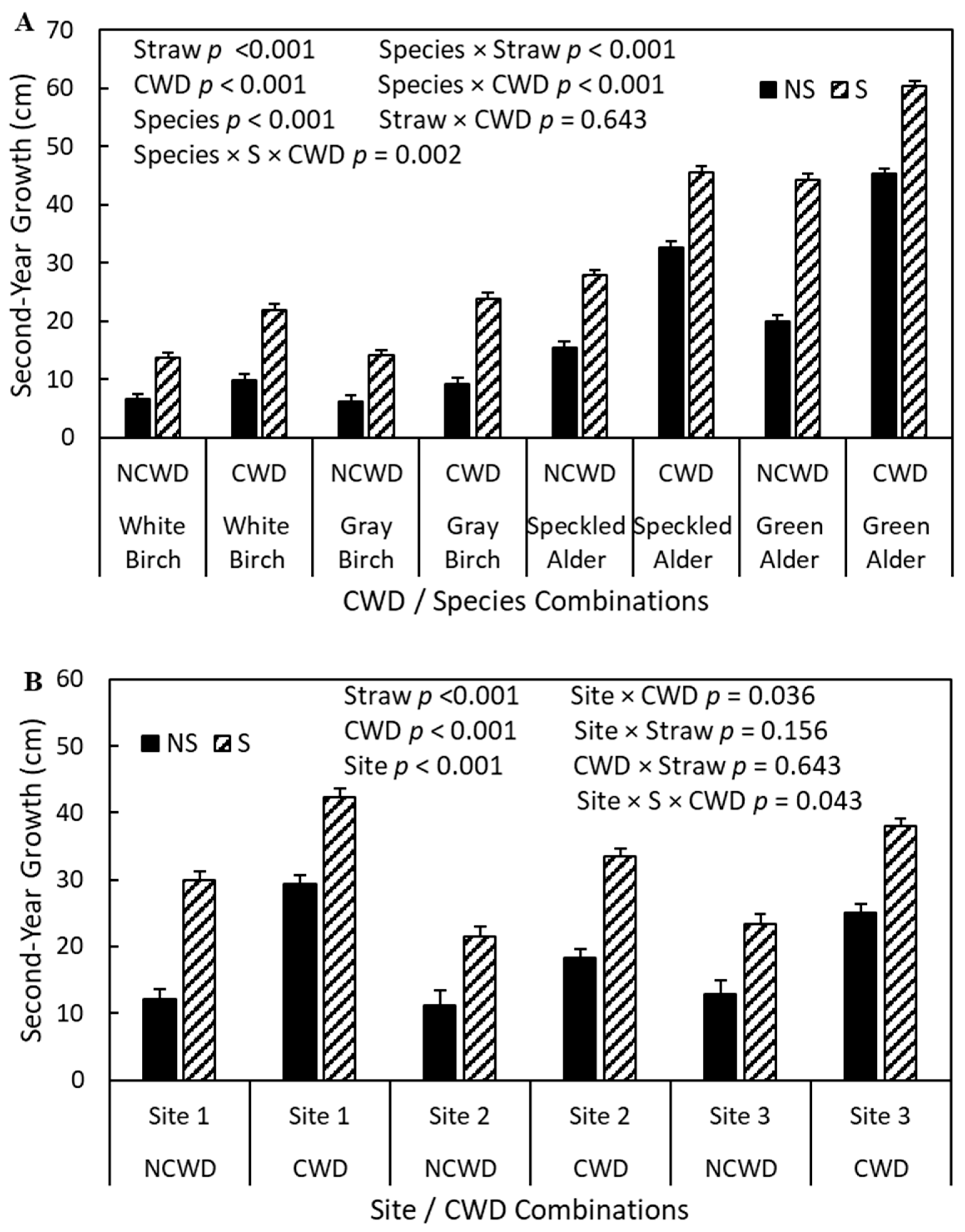
3.3. Second-Year Height Growth
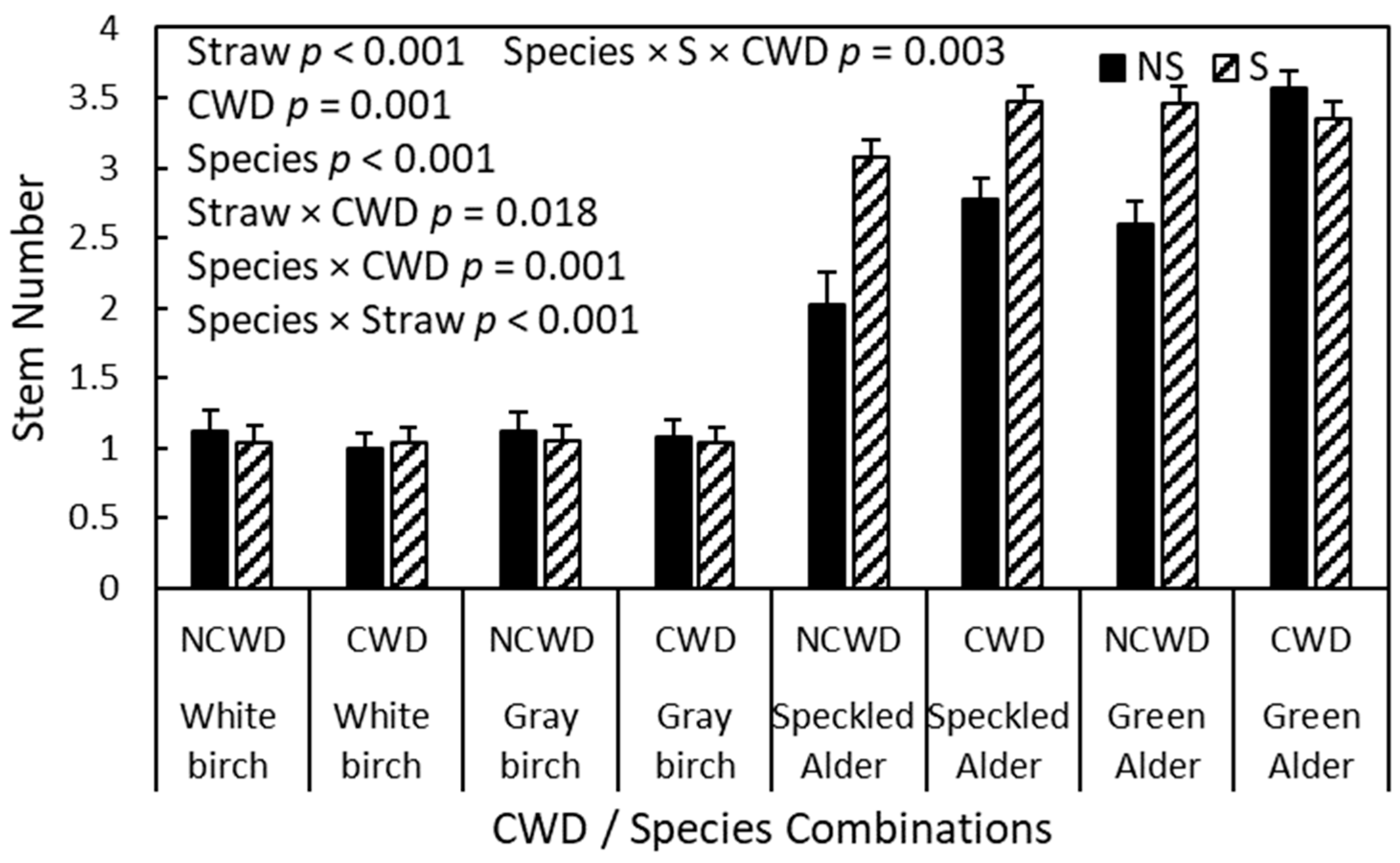
3.4. Second-Year Stem Number
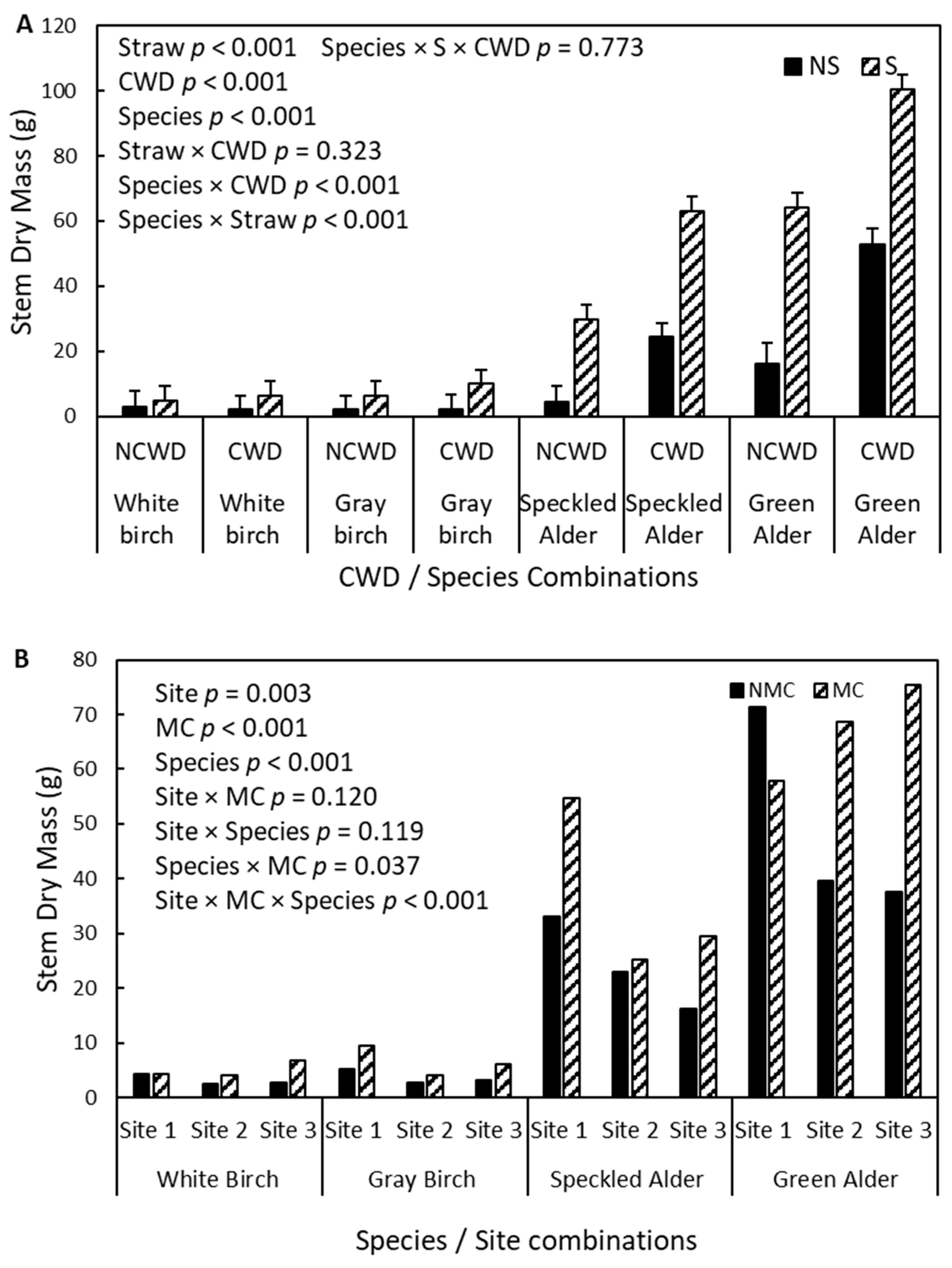
3.5. Second-Year Stem Dry Mass
4. Discussion
4.1. First-Year Height Growth
4.2. Second-Year Height Growth
4.3. Second-Year Stem Number
4.4. Second-Year Stem Dry Mass
5. Application
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patric, J.H. Soil Erosion in the Eastern Forest. J. For. 1976, 74, 671–677. [Google Scholar]
- Ryan, S.E.; Grant, G.E. Downstream Effects of Timber Harvesting on Channel Morphology in Elk River Basin, Oregon. J. Environ. Qual. 1991, 20, 60. [Google Scholar] [CrossRef]
- Abrams, M.D. Adaptations and responses to drought in Quercus species of North America. Tree Physiol. 1990, 7, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Trenberth, K.; Dai, A.; van der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Fathi, A.; Tari, D.B. Effect of Drought Stress and its Mechanism in Plants. Int. J. Life Sci. 2016, 10, 1–6. [Google Scholar] [CrossRef]
- Amissah, L.; Mohren, G.M.J.; Kyereh, B.; Poorter, L. The Effects of Drought and Shade on the Performance, Morphology and Physiology of Ghanaian Tree Species. PLoS ONE 2015, 10, e0121004. [Google Scholar] [CrossRef]
- Asghar, M.A.; Du, J.; Jiang, H.; Li, Y.; Sun, X.; Shang, J.; Liu, J.; Liu, W.; Imran, S.; Iqbal, N.; et al. Shade pretreatment enhanced drought resistance of soybean. Environ. Exp. Bot. 2020, 171, 103952. [Google Scholar] [CrossRef]
- Galea, D.; Major, J.E. First-Year Mortality of Four Early-Successional Species on Severely Degraded Sites in Eastern Canada as Influenced by a Factorial of Site Preparation Treatments. Forests 2024, 15, 143. [Google Scholar] [CrossRef]
- Polster, D.F. Successional reclamation in Western Canada: New light on an old subject. In Proceedings of the Canadian Land Reclamation Association and American Society for Surface Mining and Reclamation Conference, Calgary, AB, Canada, 27–31 August 1989. [Google Scholar]
- Jochimsen, M.E.A. Reclamation of colliery mine spoil founded on natural succession. Water Air Soil Pollut. 1996, 91, 99–108. [Google Scholar] [CrossRef]
- Bradshaw, A. Restoration of mined lands—Using natural processes. Ecol. Eng. 1997, 8, 255–269. [Google Scholar] [CrossRef]
- Šourková, M.; Frouz, J.; Fettweis, U.; Bens, O.; Hüttl, R.F.; Šantrůčková, H. Soil development and properties of microbial biomass succession in reclaimed post mining sites near Sokolov (Czech Republic) and near Cottbus (Germany). Geoderma 2005, 129, 73–80. [Google Scholar] [CrossRef]
- Frazier, C.S.; Graham, R.C. Pedogenic Transformation of Fractured Granitic Bedrock, Southern California. Soil Sci. Soc. Am. J. 2000, 64, 2057. [Google Scholar] [CrossRef]
- Landeweert, R.; Hoffland, E.; Finlay, R.D.; Kuyper, T.W.; van Breemen, N. Linking plants to rocks: Ectomycorrhizal fungi mobilize nutrients from minerals. Trends Ecol. Evol. 2001, 16, 248–254. [Google Scholar] [CrossRef]
- Bornyasz, M.A.; Graham, R.C.; Allen, M.F. Ectomycorrhizae in a soil-weathered granitic bedrock regolith: Linking matrix resources to plants. Geoderma 2005, 126, 141–160. [Google Scholar] [CrossRef]
- Rajakaruna, N.; Boyd, R.S. Edaphic Factor. In Encyclopedia of Ecology; Jorgensen, S.E., Fath, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 2, pp. 1201–1207. [Google Scholar]
- Skrindo, A.B. Natural Revegetation from Indigenous Soil. Ph.D. Thesis, Norwegian University of Life Sciences, Ås, Norway, 2005. [Google Scholar]
- Fox, J.E.D. Rehabilitation of Mined Lands, Review Artick. Forest Abstract. Commonw. For. Bur. 1984, 9, 565–600. [Google Scholar]
- Swanson, M.E.; Franklin, J.F.; Beschta, R.L.; Crisafulli, C.M.; Dellasala, D.A.; Hutto, R.L.; Lindenmayer, D.B.; Swanson, F.J. The forgotten stage of forest succession: Early-successional ecosystems on forest sites. Front. Ecol. Environ. 2011, 9, 117–125. [Google Scholar] [CrossRef]
- Lanner, R.M. Trees of the Great Basin: A Natural History; University of Nevada Press: Reno, NV, USA, 1983; 215p. [Google Scholar]
- Monsen, S.B.; Stevens, R.; Shaw, N.L. Restoring Western Ranges and Wildlands; Gen. Tech. Rep. RMRS-GTR-136-vol-2; Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2004; pp. 597–699.
- Son, Y.; Lee, Y.Y.; Lee, C.Y.; Yi, M.J. Nitrogen fixation, soil nitrogen availability, and biomass in pure and mixed plantations of alder and pine in central Korea. J. Plant Nutr. 2007, 30, 1841–1853. [Google Scholar] [CrossRef]
- Ste-Marie, C.; Pare, D.; Gagnon, D. The contrasting effects of aspen and jack pine on soil nutritional properties depend on parent material. Ecosystems 2007, 10, 1299–1310. [Google Scholar] [CrossRef]
- Hosie, R.C. Native Trees of Canada, 7th ed.; Canadian Forestry Service, Department of Fisheries and Forestry: Ottawa, ON, Canada, 1969. [Google Scholar]
- Vogel, W.G. A Guide for Revegetating Coal Mine Soils in the Eastern United States; Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Broomall, PA, USA, 1981; Volume 68, 190p.
- Blundon, D.J.; Dale, M.R.T. Dinitrogen fixation (Acetylene reduction) in primary succession near Mount Robson, British Columbia, Canada. Arct. Alp. Res. 1990, 22, 255–263. [Google Scholar] [CrossRef]
- Haeussler, S.; Coates, D. Autecological characteristics of selected species that compete with conifers in British Columbia: A literature review. Land Manag. Rep. 1986, 33, 180. [Google Scholar]
- DeHond, P.E.; Campbell, C.S. Multivariate analyses of hybridization between Betula cordifolia and B. populifolia (Betulaceae). Can. J. Bot. 1989, 67, 2252–2260. [Google Scholar] [CrossRef]
- Chapman, W.K.; Bessette, A.E. Trees and Shrubs of the Adirondacks; North Country Books: Utica, NY, USA, 1990; 131p. [Google Scholar]
- Furlow, J.J. The systematics of the American species of Alnus (Betulaceae) Part 1. Rhodora 1979, 81, 1–121. [Google Scholar]
- Armstrong, R.C.; Heston, K. Control of woody invasion of a kettle bog. Restor. Manag. Notes 1982, 1, 18. [Google Scholar]
- Brisson, J.; Cogliastro, A.; Robert, M. Controlling speckled alder (Alnus incana ssp. rugosa) invasion in a wetland reserve of southern Quebec. Nat. Areas J. 2006, 26, 78–83. [Google Scholar] [CrossRef]
- Bakuzis, E.V.; Hansen, H.L. Ecographs of shrubs and other undergrowth species of Minnesota forest communities. Minn. For. Notes 1962, 117, 1–2. [Google Scholar]
- Ashby, W.C. Soil Ripping and Herbicides Enhance Tree and Shrub Restoration on Stripmines. Restor. Ecol. 1997, 5, 169–177. [Google Scholar] [CrossRef]
- Polster, D.F. Natural Processes: The Application of Natural Systems for the Reclamation of Drastically Disturbed Sites. In Proceedings of the B.C. Technical and Research Committee on Reclamation, BC Mine Reclamation Symposium, Cranbrook, BC, Canada, 14–17 September 2009. [Google Scholar]
- Lambermont, J.; Lebon, G. Erosion of Cohesive Soils. J. Hydraul. Res. 1978, 16, 27–44. [Google Scholar] [CrossRef]
- Richardson, B.Z.; Pratt, M.M. Environmental Effects of Surface Mining of Minerals Other Than Coal: Annotated Bibliography and Summary Report; Intermountain Forest and Range Experiment Station, U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1980; Volume 95. [Google Scholar]
- Novak, M.D.; Chen, W.J.; Orchansky, A.L.; Ketler, R. Turbulent exchange processes within and above a straw mulch. Part II: Thermal and moisture regimes. Agric. For. Meteorol. 2000, 102, 155–171. [Google Scholar] [CrossRef]
- Adekalu, K.; Olorunfemi, I.; Osunbitan, J. Grass mulching effect on infiltration, surfacerunoff and soil loss of three agricultural soils in Nigeria. Bioresour. Technol. 2007, 98, 912–917. [Google Scholar] [CrossRef]
- Edwards, L.; Burney, J.R.; Richter, G.; MacRae, A.H. Evaluation of compost and straw mulching on soil-loss characteristics in erosion plots of potatoes in Prince Edward Island, Canada. Agric. Ecosyst. Environ. 2000, 81, 217–222. [Google Scholar] [CrossRef]
- Balwinder-Singh; Humphreys, E.; Eberbach, P.L.; Katupitiya, A.; Yadvinder-Singh; Kukal, S.S. Growth, yield, and water productivity of zero till wheat as affected by rice straw mulch and irrigation schedule. Field Crops Res. 2011, 121, 209–225. [Google Scholar] [CrossRef]
- Rahma, A.E.; Warrington, D.N.; Lei, T. Efficacy of wheat straw mulching in reducing soil and water losses from three typical soils of the Loess Plateau, China. Int. Soil Water Conserv. Res. 2019, 7, 335–345. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Liu, D.; Li, Z.; Zhang, G.; Tao, Y.; Pan, J.; Chen, F. Straw Mulching Reduces the Harmful Effects of Extreme Hydrological and Temperature Conditions in Citrus Orchards. PLoS ONE 2014, 9, e87094. [Google Scholar] [CrossRef]
- Pyper, M.; Vinge, T. A Visual Guide to Handling Woody Materials for Forested Land Reclamation; Oil Sands Research and Information Network; University of Alberta, School of Energy and the Environment: Edmonton, AB, Canada, 2012; 10p. [Google Scholar] [CrossRef]
- Jumpponen, A.; Väre, H.; Mattson, K.G.; Ohtonen, R.; Trappe, J.M. Characterization of ‘safe sites’ for pioneers in primary succession on recently deglaciated terrain. J. Ecol. 1999, 87, 98–105. [Google Scholar] [CrossRef]
- Wells, J.M.; Boddy, L. Wood decay, and phosphorus and fungal biomass allocation, in mycelial cord systems. New Phytol. 1990, 116, 285–295. [Google Scholar] [CrossRef]
- McKeague, J.A. Manual of Soil Sampling and Methods of Analysis, 2nd ed.; Canadian Society of Soil Science: Pinawa, MB, Canada, 1978. [Google Scholar]
- Hicks, C.R. Fundamental Concepts of Design of Experiments, 2nd ed.; Holt, Reinhart and Winston: New York, NY, USA, 1982; pp. 55–57. [Google Scholar]
- Orman, O.; Adamus, M.; Szewczyk, J. Regeneration processes on coarse woody debris in mixed forests: Do tree germinants and seedlings have species-specific responses when grown on coarse woody debris? J. Ecol. 2016, 104, 1809–1818. [Google Scholar] [CrossRef]
- Ekblad, A.; Huss-Danell, K. Nitrogen Fixation by Alnus incana and Nitrogen Transfer from A. incana to Pinus sylvestris Influenced by Macronutrients and Ectomycorrhiza. New Phytol. 1995, 131, 453–459. [Google Scholar] [CrossRef]
- Fessenden, R.J. Use of Actinorrhizal Plants for Land Reclamation and Amenity Planting in the USA and Canada; Syncrude Canada: Fort McMurray, AB, Canada, 1979. [Google Scholar]
- Brisebois, A.; Major, J.E. Effects of CO2 Treatments on Functional Carbon Efficiencies and Growth of Forest Tree Seedlings: A Study of Four Early-Successional Deciduous Species. Forests 2024, 15, 193. [Google Scholar] [CrossRef]
- Tao, Z.; Li, C.; Li, J.; Ding, Z.; Xu, J.; Sun, X.; Zhou, P.; Zhao, M. Tillage and straw mulching impacts on grain yield and water use efficiency of spring maize in Northern Huang–Huai–Hai Valley. Crop J. 2015, 3, 445–450. [Google Scholar] [CrossRef]
- Goulet, F. Frost heaving in forest tree seedings: A review. New For. 1995, 9, 67–94. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Impact of Mulches on Landscape Plants and the Environment—A Review. J. Environ. Hortic. 2007, 25, 239–249. [Google Scholar] [CrossRef]
- Wu, J.-B.; Guan, D.-X.; Han, S.-J.; Zhang, M.; Jin, C.-J. Ecological functions of coarse woody debris in forest ecosystem. J. For. Res. 2005, 16, 247–252. [Google Scholar] [CrossRef]
- Hutnik, R.J.; Cunningham, F.E. Paper birch (Betula papyrifera Marsh.). In Silvics of forest trees of the United States; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Upper Darby, PA, USA, 1965; pp. 93–98. [Google Scholar]
- Farrar, J.L. Canadian Forest Service. In Trees in Canada; Fitzhenry & Whiteside Ltd.: Markham, ON, Canada, 1995. [Google Scholar]
- Brisebois, A.; Major, J.E. Morphological and Allometric Variation of Four Coppiced Early-Successional Species, Used in Land Restoration, under CO2 and Soil Moisture Treatments, Natural Resources Canada; Canadian Forest Service: Fredericton, NB, Canada, 2024; to be submitted. [Google Scholar]
- Rokich, D.P.; Dixon, K.W.; Sivasithamparam, K.; Meney, K.A. Smoke, mulch, and seed broadcasting effects on woodland restoration in Western Australia. Restor. Ecol. 2002, 10, 185–194. [Google Scholar] [CrossRef]
- Schwencke, J. Recent advances in Frankia physiology and biochemistry with notes on practical implications. Microb. Interact. Agric. For. 1988, 1, 121–148. [Google Scholar]
- Ingestad, T.; Lund, A.-B. Nitrogen Stress in Birch Seedlings. I. Growth Technique and Growth. Physiol. Plant. 1979, 45, 137–148. [Google Scholar] [CrossRef]
- Leak, W.B. Relationship of Species and Site Index to Habitat in the White Mountains of New Hampshire; USDA Forest Service, Northeastern Forest Experiment Station: Broomall, PA, USA, 1978; Volume 397, 9p.




| Species | Source Year | Provenance | Country | Latitude (N) | Longitude (W) | Elevation (m) | |
|---|---|---|---|---|---|---|---|
| Betula papyrifera | White birch | 1998 | Wayeton, NB | CAN | 47.21667 | −65.93333 | 300 |
| Betula papyrifera | White birch | 1998 | Jewetts Creek, NB | CAN | 45.83333 | −66.98333 | 50 |
| Betula populifolia | Gray birch | 2008 | Newmarket, NB | CAN | 45.80501 | −66.95634 | 149 |
| Betula populifolia | Gray birch | 2008 | Newmarket, NB | CAN | 45.83147 | −66.97115 | 130 |
| Betula populifolia | Gray birch | 2008 | Newmarket, NB | CAN | 45.8076 | −66.96825 | 141 |
| Betula populifolia | Gray birch | 2008 | Newmarket, NB | CAN | 45.83486 | −66.96272 | 125 |
| Betula populifolia | Gray birch | 1999 | Bai-du-vin, NB | CAN | 47.03333 | −65.16666 | 5 |
| Alnus viridis subsp. crispa | Green alder | 2002 | West Quaco, NB | CAN | 45.33 | −65.53 | 65 |
| Alnus viridis subsp. crispa | Green alder | 1999 | Lower Prince William, NB | CAN | 45.87 | −67 | 20 |
| Alnus incana subsp. rugosa | Speckled alder | 1983 | Enmore, PEI | CAN | 46.58 | −64.05 | 10 |
| Alnus incana subsp. rugosa | Speckled alder | 1983 | Vallyfield, PEI | CAN | 46.13 | −62.72 | 45 |
| Alnus incana subsp. rugosa | Speckled alder | 1983 | Shediac, NB | CAN | 46.23 | −64.6 | 15 |
| ID | Organic Matter (%) | Total Nitrogen (%) | Carbon (%) | C:N Ratio | pH | Phosphorus (meq/100 g) | Potassium (meq/100 g) |
| Site 1 | 1.27 ± 0.91 a | 0.16 ± 0.03 a | 4.33 ± 2.15 a | 26.18 ± 9.14 b | 4.83 ± 0.11 b | 3.71 ± 1.91 a | 0.14 ± 0.08 a |
| Site 2 | 0.61 ± 0.33 b | 0.11 ± 0.02 b | 3.21 ± 1.61 b | 29.26 ± 14.13 b | 4.85 ± 0.13 ab | 2.54 ± 1.24 b | 0.13 ± 0.07 a |
| Site 3 | 0.63 ± 0.27 b | 0.08 ± 0.01 c | 4.65 ± 1.58 a | 60.54 ± 19.55 a | 4.92 ± 0.16 a | 3.73 ± 1.47 a | 0.11 ± 0.06 a |
| ID | Calcium (meq/100 g) | Magnesium (ppm) | Clay (%) | Silt (%) | Sand (%) | Rocks (%) | Average Depth (cm) |
| Site 1 | 0.34 ± 0.31 b | 0.18 ± 0.11 b | 16.19 ± 2.74 a | 42.38 ± 6.27 a | 41.66 ± 8.42 b | 31.75 ± 15.59 b | 31.93 ± 12.18 a |
| Site 2 | 0.94 ± 0.86 a | 0.35 ± 0.23 b | 12.97 ± 4.57 b | 34.22 ± 8.28 b | 52.75 ± 12.32 a | 39.88 ± 15.99 a | 11.64 ± 7.27 b |
| Site 3 | 1.73 ± 2.24 a | 0.66 ± 0.85 a | 12.09 ± 4.3 b | 28.88 ± 7.94 c | 59.16 ± 11.95 a | 50.06 ± 19.01 a | 14.94 ± 9.78 b |
| First-Year Growth | ||||
|---|---|---|---|---|
| Source of Variation | df | MS | VC (%) | p-Value |
| MC | 1 | 2229.036 | 1.8 | <0.001 |
| S | 1 | 11,329.324 | 9.1 | <0.001 |
| CWD | 1 | 3766.011 | 3.0 | <0.001 |
| SITE | 2 | 2312.886 | 3.7 | <0.001 |
| SPECIES | 3 | 2616.067 | 6.3 | <0.001 |
| BLOCK(SITE) | 9 | 461.287 | 3.3 | <0.001 |
| S × MC | 1 | 124.613 | 0.1 | 0.151 |
| CWD × MC | 1 | 27.561 | 0.0 | 0.499 |
| SITE × MC | 2 | 840.471 | 1.3 | <0.001 |
| SPECIES × MC | 3 | 37.453 | 0.1 | 0.602 |
| CWD × S | 1 | 0.012 | 0.0 | 0.989 |
| SPECIES × S | 3 | 504.543 | 1.2 | <0.001 |
| SITE × S | 2 | 29.887 | 0.0 | 0.610 |
| SPECIES × CWD | 3 | 217.799 | 0.5 | 0.013 |
| SITE × CWD | 2 | 214.289 | 0.3 | 0.029 |
| SITE × SPECIES | 6 | 76.581 | 0.4 | 0.269 |
| CWD × S × MC | 1 | 11.183 | 0.0 | 0.667 |
| SPECIES × S × MC | 3 | 47.021 | 0.1 | 0.506 |
| SITE × S × MC | 2 | 151.289 | 0.2 | 0.082 |
| SPECIES × CWD × MC | 3 | 78.941 | 0.2 | 0.271 |
| SITE × CWD × MC | 2 | 195.077 | 0.3 | 0.040 |
| SITE × SPECIES × MC | 6 | 42.277 | 0.2 | 0.650 |
| SPECIES × CWD × S | 3 | 100.517 | 0.2 | 0.173 |
| SITE × CWD × S | 2 | 740.767 | 1.2 | <0.001 |
| SITE × SPECIES × S | 6 | 143.817 | 0.7 | 0.037 |
| SITE × SPECIES × CWD | 6 | 63.679 | 0.3 | 0.388 |
| Error | 1347 | 60.386 | 65.2 | |
| R2 | 0.59 | |||
| Second-Year Growth | ||||
|---|---|---|---|---|
| Source of Variation | df | MS | VC (%) | p-Value |
| MC | 1 | 7523.429 | 1.4 | <0.001 |
| S | 1 | 42,715.569 | 8.2 | <0.001 |
| CWD | 1 | 38,554.355 | 7.4 | <0.001 |
| SITE | 2 | 4746.407 | 1.8 | <0.001 |
| SPECIES | 3 | 58,212.778 | 33.5 | <0.001 |
| BLOCK(SITE) | 9 | 439.142 | 0.8 | 0.012 |
| S × MC | 1 | 33.568 | 0.0 | 0.671 |
| CWD × MC | 1 | 477.750 | 0.1 | 0.109 |
| SITE × MC | 2 | 495.616 | 0.2 | 0.069 |
| SPECIES × MC | 3 | 582.258 | 0.3 | 0.025 |
| CWD × S | 1 | 39.746 | 0.0 | 0.643 |
| SPECIES × S | 3 | 1420.096 | 0.8 | <0.001 |
| SITE × S | 2 | 345.167 | 0.1 | 0.156 |
| SPECIES × CWD | 3 | 3966.841 | 2.3 | <0.001 |
| SITE × CWD | 2 | 620.287 | 0.2 | 0.036 |
| SITE × SPECIES | 6 | 453.808 | 0.5 | 0.023 |
| CWD × S × MC | 1 | 55.890 | 0.0 | 0.583 |
| SPECIES × S × MC | 3 | 504.243 | 0.3 | 0.043 |
| SITE × S × MC | 2 | 28.388 | 0.0 | 0.858 |
| SPECIES × CWD × MC | 3 | 36.562 | 0.0 | 0.898 |
| SITE × CWD × MC | 2 | 279.602 | 0.1 | 0.222 |
| SITE × SPECIES × MC | 6 | 292.118 | 0.3 | 0.151 |
| SPECIES × CWD × S | 3 | 939.252 | 0.5 | 0.002 |
| SITE × CWD × S | 2 | 583.284 | 0.2 | 0.043 |
| SITE × SPECIES × S | 6 | 183.842 | 0.2 | 0.490 |
| SITE × SPECIES × CWD | 6 | 31.091 | 0.0 | 0.985 |
| Error | 1140 | 185.361 | 40.5 | |
| R2 | 0.80 | |||
| Stem Number * | ||||
|---|---|---|---|---|
| Source of Variation | df | MS | VC (%) | p-Value |
| MC | 1 | 1.078 | 0.0 | 0.333 |
| S | 1 | 19.094 | 0.7 | <0.001 |
| CWD | 1 | 12.409 | 0.5 | 0.001 |
| SITE | 2 | 0.571 | 0.0 | 0.608 |
| SPECIES | 3 | 365.217 | 42.0 | <0.001 |
| BLOCK(SITE) | 9 | 1.184 | 0.4 | 0.413 |
| S × MC | 1 | <0.001 | 0.0 | 0.990 |
| CWD × MC | 1 | 11.625 | 0.4 | 0.002 |
| SITE × MC | 2 | 1.416 | 0.1 | 0.292 |
| SPECIES × MC | 3 | 0.210 | 0.0 | 0.908 |
| CWD × S | 1 | 6.506 | 0.2 | 0.018 |
| SPECIES × S | 3 | 10.181 | 1.2 | <0.001 |
| SITE × S | 2 | 0.596 | 0.0 | 0.596 |
| SPECIES × CWD | 3 | 6.382 | 0.7 | 0.001 |
| SITE × CWD | 2 | 0.666 | 0.1 | 0.561 |
| SITE × SPECIES | 6 | 0.334 | 0.1 | 0.942 |
| CWD × S × MC | 1 | 1.285 | 0.0 | 0.291 |
| SPECIES × S × MC | 3 | 0.615 | 0.1 | 0.658 |
| SITE × S × MC | 2 | 5.712 | 0.4 | 0.007 |
| SPECIES × CWD × MC | 3 | 8.182 | 0.9 | <0.001 |
| SITE × CWD × MC | 2 | 3.824 | 0.3 | 0.036 |
| SITE × SPECIES × MC | 6 | 1.118 | 0.3 | 0.442 |
| SPECIES × CWD × S | 3 | 5.314 | 0.6 | 0.003 |
| SITE × CWD × S | 2 | 2.841 | 0.2 | 0.085 |
| SITE × SPECIES × CWD | 6 | 0.504 | 0.1 | 0.854 |
| SITE × SPECIES × S | 6 | 0.904 | 0.2 | 0.620 |
| Error | 1140 | 1.149 | 50.2 | |
| R2 | 0.80 | |||
| Stem Dry Mass | ||||
|---|---|---|---|---|
| Source of Variation | df | MS | VC (%) | p-Value |
| MC | 1 | 6402.516 | 1.3 | <0.001 |
| S | 1 | 41,532.943 | 8.6 | <0.001 |
| CWD | 1 | 22,465.595 | 4.7 | <0.001 |
| SITE | 2 | 2758.520 | 1.1 | 0.003 |
| SPECIES | 3 | 58,811.420 | 36.6 | <0.001 |
| BLOCK(SITE) | 9 | 1185.972 | 2.2 | 0.007 |
| S × MC | 1 | 1054.536 | 0.2 | 0.130 |
| CWD × MC | 1 | 76.506 | 0.0 | 0.683 |
| SITE × MC | 2 | 975.820 | 0.4 | 0.120 |
| SPECIES × MC | 3 | 1309.781 | 0.8 | 0.037 |
| CWD × S | 1 | 447.437 | 0.1 | 0.323 |
| SPECIES × S | 3 | 10,179.717 | 6.3 | <0.001 |
| SITE × S | 2 | 1278.509 | 0.5 | 0.063 |
| SPECIES × CWD | 3 | 7142.417 | 4.4 | <0.001 |
| SITE × CWD | 2 | 50.165 | 0.0 | 0.896 |
| SITE × SPECIES | 6 | 780.715 | 1.0 | 0.119 |
| CWD × S × MC | 1 | 23.304 | 0.0 | 0.822 |
| SPECIES × S × MC | 3 | 600.204 | 0.4 | 0.270 |
| SITE × S × MC | 2 | 152.089 | 0.1 | 0.717 |
| SPECIES × CWD × MC | 3 | 84.651 | 0.1 | 0.906 |
| SITE × CWD × MC | 2 | 28.424 | 0.0 | 0.940 |
| SITE × SPECIES × MC | 6 | 1757.219 | 2.2 | 0.001 |
| SPECIES × CWD × S | 3 | 170.518 | 0.1 | 0.773 |
| SITE × CWD × S | 2 | 435.551 | 0.2 | 0.387 |
| SITE × SPECIES × CWD | 6 | 141.620 | 0.2 | 0.932 |
| SITE × SPECIES × S | 6 | 937.691 | 1.2 | 0.180 |
| Error | 288 | 457.215 | 27.3 | |
| R2 | 0.74 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galea, D.; Major, J.E. Ecological Restoration in Eastern Canada Using Four Early-Successional Species on Severely Degraded Sites Using a Factorial of Site-Preparation Treatments: Growth and Biomass over Two Years. Forests 2024, 15, 245. https://doi.org/10.3390/f15020245
Galea D, Major JE. Ecological Restoration in Eastern Canada Using Four Early-Successional Species on Severely Degraded Sites Using a Factorial of Site-Preparation Treatments: Growth and Biomass over Two Years. Forests. 2024; 15(2):245. https://doi.org/10.3390/f15020245
Chicago/Turabian StyleGalea, Dominic, and John E. Major. 2024. "Ecological Restoration in Eastern Canada Using Four Early-Successional Species on Severely Degraded Sites Using a Factorial of Site-Preparation Treatments: Growth and Biomass over Two Years" Forests 15, no. 2: 245. https://doi.org/10.3390/f15020245
APA StyleGalea, D., & Major, J. E. (2024). Ecological Restoration in Eastern Canada Using Four Early-Successional Species on Severely Degraded Sites Using a Factorial of Site-Preparation Treatments: Growth and Biomass over Two Years. Forests, 15(2), 245. https://doi.org/10.3390/f15020245





