Effects of Stand Types on Ectomycorrhizal Fungal Community Composition and Structure of Pinus massoniana in Subtropical Mountain Forest Ecosystems
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Processing
2.3. Analysis of Soil Properties and Plant Diversity
2.4. Classification and Identification of EMF
2.5. Statistical Analysis
3. Results
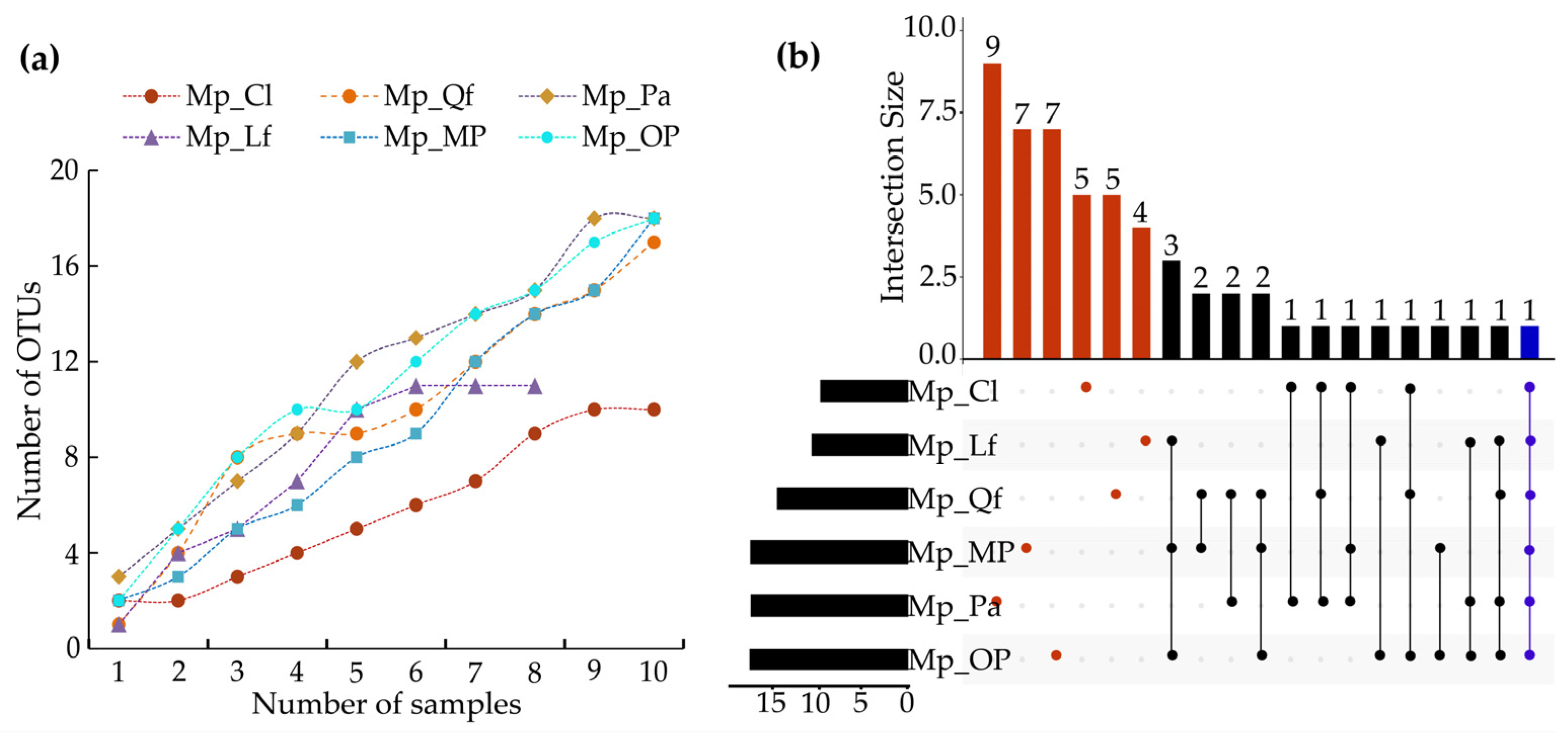
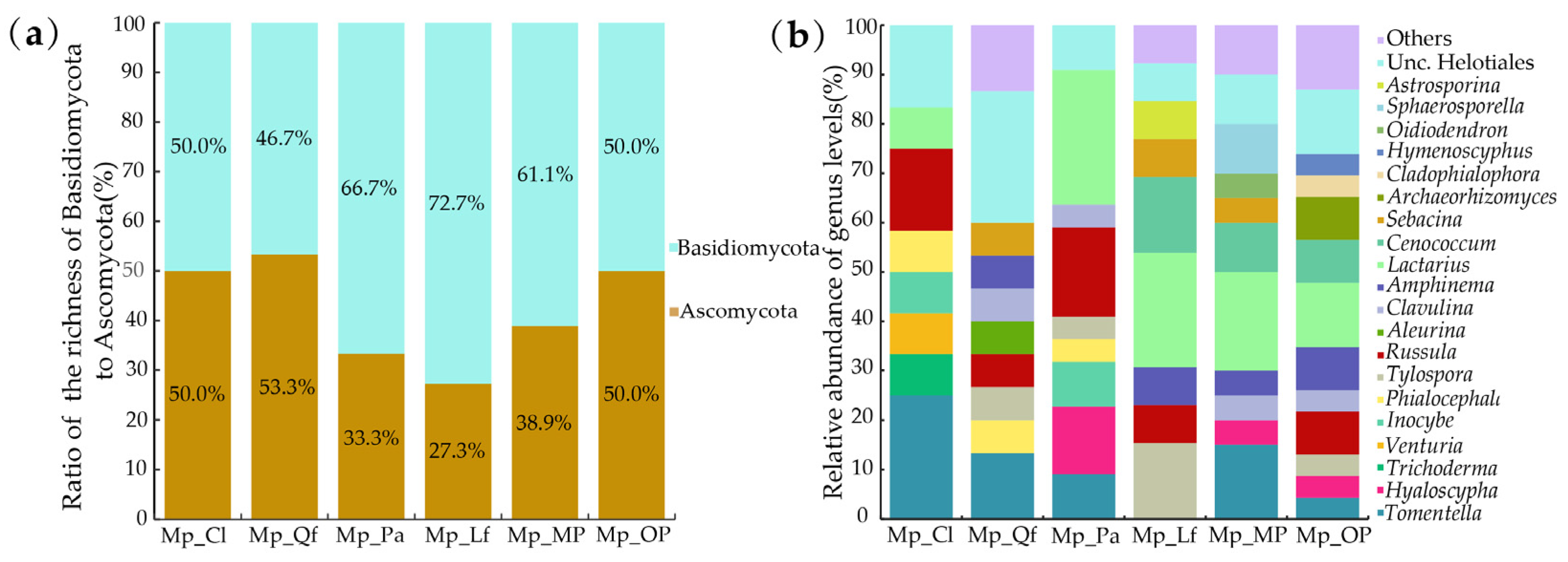
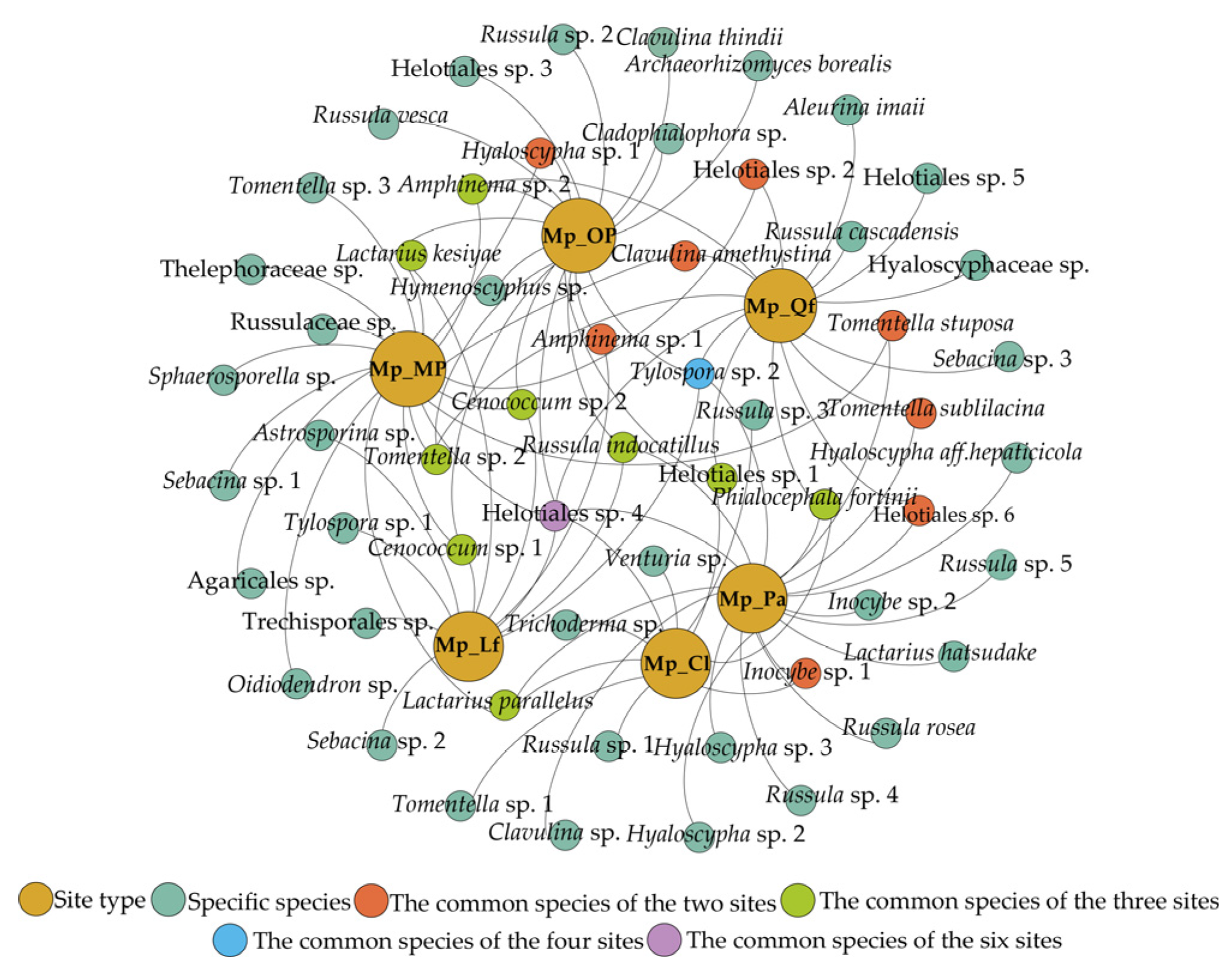
3.1. EMF Community Composition of P. massoniana
3.2. EMF Community Diversity of P. massoniana
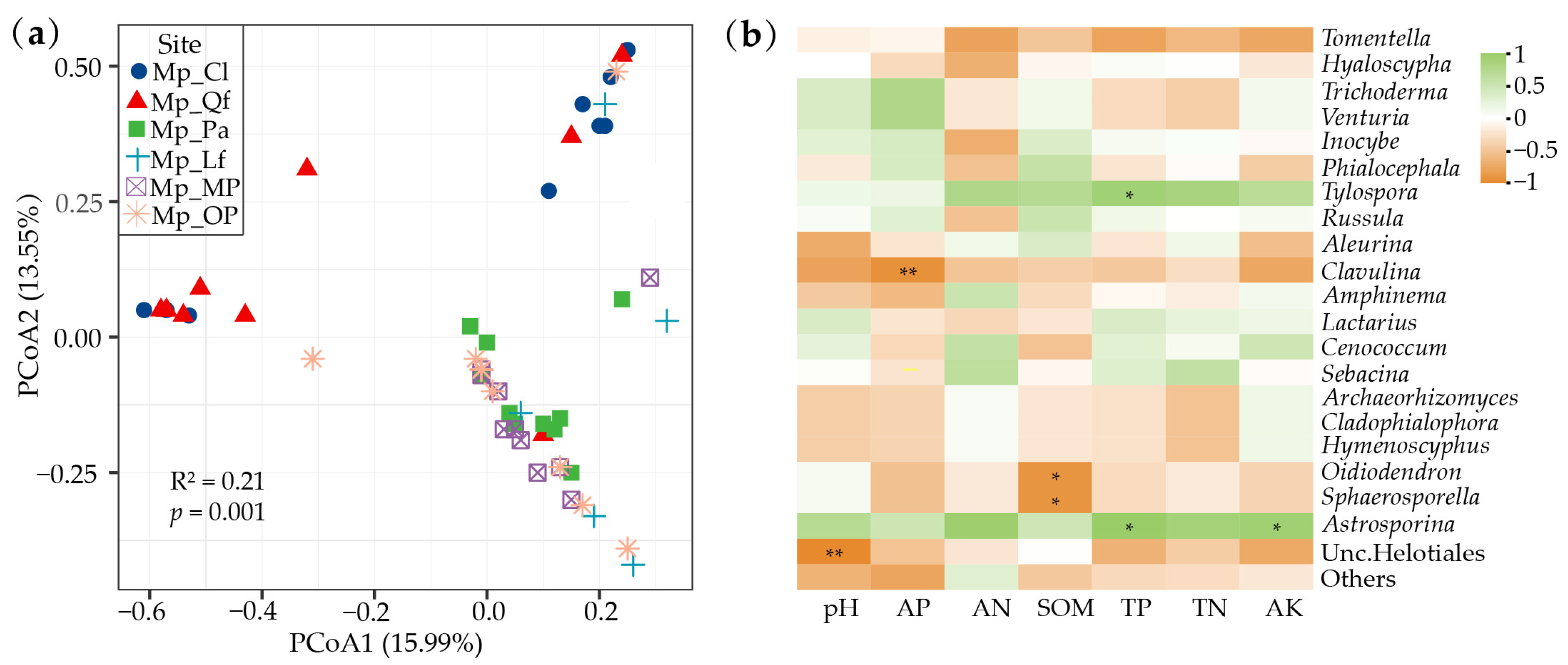
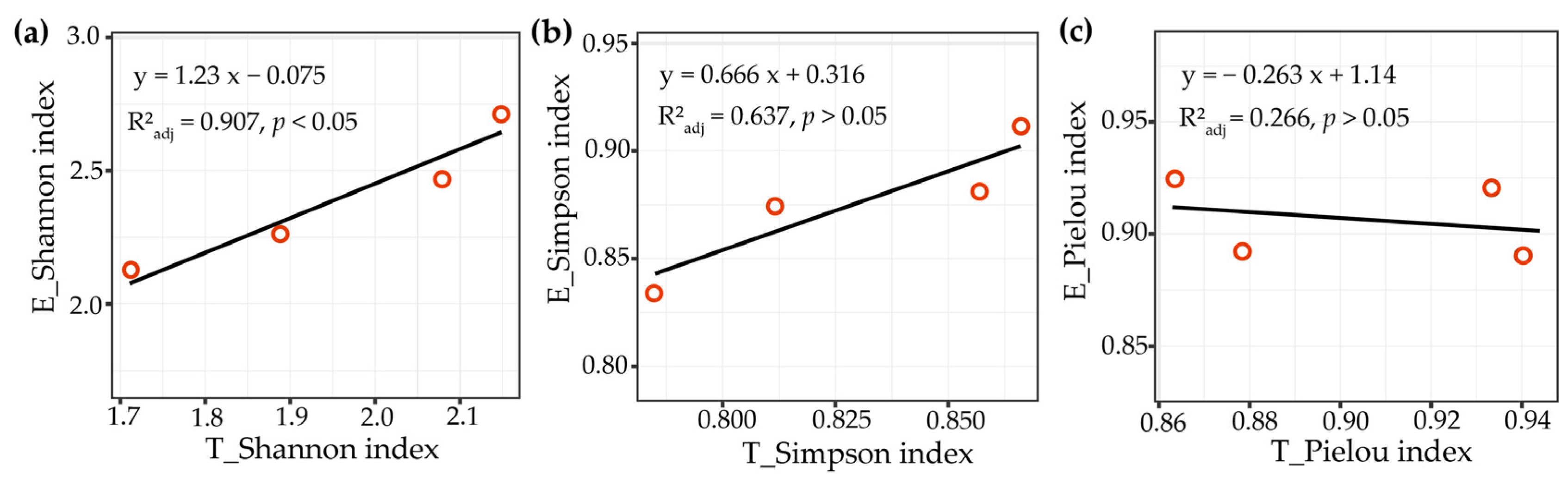
3.3. The Relationship between EMF Community of P. massoniana, Tree Species Diversity, and Soil Properties
4. Discussion
4.1. Composition and Structure of P. massoniana EMF Community
4.2. Diversity of P. massoniana EMF Community
4.3. Factors Influencing of P. massoniana EMF Community
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Zhang, X.; Xu, Y.; Babalola, B.; Xiang, S.; Zhao, Y.; Fan, Y. Fungal diversity and community assembly of ectomycorrhizal fungi associated with five pine species in Inner Mongolia, China. Front. Microbiol. 2021, 12, 646821. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, M.; Bardgett, R.D.; van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Ferlian, O.; Goldmann, K.; Eisenhauer, N.; Tarkka, M.T.; Buscot, F.; Heintz-Buschart, A. Distinct effects of host and neighbour tree identity on arbuscular and ectomycorrhizal fungi along a tree diversity gradient. ISME Commun. 2021, 1, 40. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.B.; Allen, M.F.; Helm, D.J.; Trappe, J.M.; Molina, R.; Rincon, E. Patterns and regulation of mycorrhizal plant and fungal diversity. Plant Soil 1995, 170, 47–62. [Google Scholar] [CrossRef]
- Tedersoo, L.; Mett, M.; Ishida, T.; Bahram, M. Phylogenetic relationships among host plants explain differences in fungal species richness and community composition in ectomycorrhizal symbiosis. New Phytol. 2013, 199, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Shi, N.; Liu, Y.; Peay, K.G.; Zheng, Y.; Ding, Q.; Mi, X.; Ma, K.; Wubet, T.; Buscot, F.; et al. Host plant genus-level diversity is the best predictor of ectomycorrhizal fungal diversity in a Chinese subtropical forest. Mol. Ecol. 2013, 22, 3403–3414. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Nara, K.; Hogetsu, T. Host effects on ectomycorrhizal fungal communities: Insight from eight host species in mixed conifer-broadleaf forests. New Phytol. 2007, 174, 430–440. [Google Scholar] [CrossRef]
- Deyn, G.B.; Putten, W. Linking aboveground and belowground diversity. Trends Ecol. Evol. 2005, 20, 625–633. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, Y.; Li, J.; Li, X.; Ruan, H.; Bhople, P.; Keiblinger, K.; Mao, L.; Liu, D. Interaction among soil nutrients, plant diversity and hypogeal fungal trophic guild modifies root-associated fungal diversity in coniferous forests of Chinese Southern Himalayas. Plant Soil 2022, 481, 395–408. [Google Scholar] [CrossRef]
- Kernaghan, G.; Widden, P.; Bergeron, Y.; Légaré, S.; Paré, D. Biotic and abiotic factors affecting diversity in boreal mixed-woods. Oikos 2003, 102, 497–504. [Google Scholar] [CrossRef]
- Otsing, E.; Anslan, S.; Ambrosio, E.; Koricheva, J.; Tedersoo, L. Tree species richness and neighborhood effects on ectomycorrhizal fungal richness and community structure in boreal Forest. Front. Microbiol. 2021, 12, 567961. [Google Scholar] [CrossRef] [PubMed]
- Arraiano-Castilho, R.; Bidartondo, M.I.; Niskanen, T.; Clarkson, J.J.; Brunner, I.; Zimmermann, S.; Senn-Irlet, B.; Frey, B.; Peintner, U.; Mrak, T.; et al. Habitat specialisation controls ectomycorrhizal fungi above the treeline in the European Alps. New Phytol. 2021, 229, 2901–2916. [Google Scholar] [CrossRef] [PubMed]
- Adamo, I.; Castaño, C.; Bonet, J.A.; Colinas, C.; Martínez De Aragón, J.; Alday, J.G. Soil physico-chemical properties have a greater effect on soil fungi than host species in Mediterranean pure and mixed pine forests. Soil Biol. Biochem. 2021, 160, 108320. [Google Scholar] [CrossRef]
- Chi, Y.; Wang, G.G.; Zhu, M.; Jin, P.; Hu, Y.; Shu, P.; Wang, Z.; Fan, A.; Qian, P.; Han, Y.; et al. Potentially suitable habitat prediction of Pinus massoniana Lamb. in China under climate change using Maxent model. Front. For. Glob. Chang. 2023, 6, 1144401. [Google Scholar] [CrossRef]
- Shao, C.; Duan, H.; Ding, G.; Luo, X.; Fu, Y.; Lou, Q. Physiological and biochemical dynamics of Pinus massoniana Lamb. seedlings under extreme drought stress and during recovery. Forests 2022, 13, 65. [Google Scholar] [CrossRef]
- Li, X.; Kang, W.; Liu, S.; Yin, H.; Lyu, Q.; Su, Y.; Liu, J.; Liu, J.; Fan, C.; Chen, G.; et al. Diversity of ectomycorrhizal fungal communities in four types of stands in Pinus massoniana plantation in the west of China. Forests 2021, 12, 719. [Google Scholar] [CrossRef]
- Huang, J.; Nara, K.; Lian, C.; Zong, K.; Peng, K.; Xue, S.; Shen, Z. Ectomycorrhizal fungal communities associated with Masson pine (Pinus massoniana Lamb.) in Pb–Zn mine sites of central south China. Mycorrhiza 2012, 22, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, M.; Zhang, J.; Wen, C.; Zhang, J. Effects of resin tapping on ectomycorrhizal fungal community composition and structure of Pinus massoniana in subtropical mountain forest ecosystems in southwestern China. For. Ecol. Manag. 2023, 540, 121030. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Zhao, X.; Feng, W.; Ding, G.; Quan, W. Effect of ectomycorrhizal fungi on the drought resistance of Pinus massoniana seedlings. J. Fungi 2023, 9, 471. [Google Scholar] [CrossRef]
- Sun, P.; Shen, Y.; Wang, L.; Chen, T.; Zhang, M.; Xiao, W.; Cheng, R. Photosynthetic product allocations of Pinus massoniana seedlings inoculated with ectomycorrhizal fungi along a nitrogen addition gradient. Front. Plant Sci. 2022, 13, 948676. [Google Scholar] [CrossRef]
- Guo, K.; Fang, J.; Wang, G.; Tang, Z.; Xie, Z.; Shen, Z.; Wang, R.; Qiang, S.; Liang, C.; Da, L.; et al. A revised scheme of vegetation classification system of China. Chin. J. Plant Ecol. 2020, 44, 111–127. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, S. Study on classification, distribition and succession of Pinus massoniana forest in Guangxi. Bull. Bot. Res. 2002, 22, 151–155. Available online: http://bbr.nefu.edu.cn/CN/Y2002/V22/I2/151 (accessed on 28 November 2023). (In Chinese).
- Yang, Y.; Xu, M.; Zhang, J.; Nie, K.; Wen, C.; Zhang, J. Characteristics of communities and soil physicochemical properties of different Pinus massoniana in central Guizhou. Res. Soil Water Conserv. 2022, 29, 119–126. (In Chinese) [Google Scholar] [CrossRef]
- Pulido-Chavez, F.; Alvarado, E.; Deluca, T.; Edmonds, R.; Glassman, S. High-severity wildfire reduces richness and alters composition of ectomycorrhizal fungi in low-severity adapted ponderosa pine forests. For. Ecol. Manag. 2021, 485, 118923. [Google Scholar] [CrossRef]
- Tedersoo, L.; Suvi, T.; Larsson, E.; Kõljalg, U. Diversity and community structure of ectomycorrhizal fungi in a wooded meadow. Mycol. Res. 2006, 110, 734–748. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, M.; Xiao, Z.; Jin, C. Structural and functional characteristics of soil microbial community in a Pinus massoniana forest at different elevations. PeerJ 2022, 10, e13504. [Google Scholar] [CrossRef]
- Lu, R. Soil Agrochemical Analysis Methods; China Agricultural Science and Technology Press: Beiing, China, 2000; pp. 1–638. (In Chinese) [Google Scholar]
- Fang, J.; Wang, X.; Shen, Z.; Tang, Z.; He, J.; Yu, D.; Jiang, Y.; Wang, Z.; Zheng, C.; Zhu, J.; et al. Methods and protocols for plant community inventory. Biodivers. Sci. 2009, 17, 533–548. (In Chinese) [Google Scholar]
- Agerer, R. Colour Atlas of Ectomycorrhizae; Einhorn-Verlag Eduard Dietenberger: Schwabisch Gmund, Germany, 1987. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- White, T.; Bruns, T.; Lee, S.; Taylor, J.; Innis, M.; Gelfand, D.; Sninsky, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: Cambridge, MA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar] [CrossRef]
- Yue, R.; Guo, M.; Ding, G.; Wang, Y. Ectomycorrhizal fungi associated with Pinus sylvestris var. mongolica were altered by soil environments with aging plantation in a semi-arid desert. Front. Environ. Sci. 2022, 10, 858452. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, J.; Zhu, X.; Zhao, B.; Liu, C.; Dong, J.; Hong, L.; Liu, Y.; Chen, Y.; Wen, Z. Diversity and community structure of ectomycorrhizal fungi in Pinus thunbergii coastal forests bordering the Yellow Sea of China. Braz. J. Microbiol. 2021, 52, 801–809. [Google Scholar] [CrossRef]
- Zhang, J.; Taniguchi, T.; Xu, M.; Du, S.; Liu, G.; Yamanaka, N. Ectomycorrhizal fungal communities of Quercus liaotungensis along different successional stands on the Loess Plateau, China. J. For. Res. 2014, 19, 395–403. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, M.; Zou, X.; Chen, J.; Ma, H.; Yang, L.; Zhang, J. Progress in the research of ectomycorrhizal fungi in the south of China. J. West China For. Sci. 2019, 48, 131–142. (In Chinese) [Google Scholar] [CrossRef]
- Looney, B.; Meidl, P.; Piatek, M.; Miettinen, O.; Martin, F.; Matheny, P.; Labbé, J. Russulaceae: A new genomic dataset to study ecosystem function and evolutionary diversification of ectomycorrhizal fungi with their tree associates. New Phytol. 2018, 218, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Corrales, A.; Koch, R.; Vasco, A.; Smith, M.; Ge, Z.; Henkel, T. Diversity and distribution of tropical ectomycorrhizal fungi. Mycologia 2022, 114, 919–933. [Google Scholar] [CrossRef] [PubMed]
- Tuo, Y.; Rong, N.; Hu, J.; Guiping, Z.; Wang, Y.; Zhang, Z.; Qi, Z.; Li, Y.; Zhang, B. Exploring the relationships between macrofungi diversity and major environmental factors in Wunvfeng National Forest Park in northeast China. J. Fungi 2022, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Gates, G.; Dunk, C.W.; Lebel, T.; May, T.W.; Kõljalg, U.; Jairus, T. Establishment of ectomycorrhizal fungal community on isolated Nothofagus cunninghamii seedlings regenerating on dead wood in Australian wet temperate forests: Does fruit-body type matter? Mycorrhiza 2009, 19, 403–416. [Google Scholar] [CrossRef]
- Gu, X.; Jia, H.; Wang, X.; Jiang, Y.; Li, J.; He, X. Differential aluminum tolerance and absorption characteristics in Pinus massoniana seedlings colonized with ectomycorrhizal fungi of Lactarius deliciosus and Pisolithus tinctorius. J. For. Res. 2023, 34, 1523–1533. [Google Scholar] [CrossRef]
- Jakucs, E.; Erős-Honti, Z. Morphological-anatomical characterization and identification of Tomentella ectomycorrhizas. Mycorrhiza 2008, 18, 277–285. [Google Scholar] [CrossRef]
- Zhao, P.; Guo, M.; Gao, G.; Zhang, Y.; Ding, G.; Yue, R.; Akhtar, M. Community structure and functional group of root-associated Fungi of Pinus sylvestris var. mongolica across stand ages in the Mu Us Desert. Ecol. Evol. 2020, 10, 3032–3042. [Google Scholar] [CrossRef]
- Ren, J.; Fang, S.; Lin, G.; Lin, F.; Yuan, Z.; Ye, J.; Wang, X.; Hao, Z.; Fortunel, C. Tree growth response to soil nutrients and neighborhood crowding varies between mycorrhizal types in an old-growth temperate forest. Oecologia 2021, 197, 523–535. [Google Scholar] [CrossRef]
- Bruns, T.D. Thoughts on the processes that maintain local species diversity of ectomycorrhizal fungi. Plant Soil 1995, 170, 63–73. [Google Scholar] [CrossRef]
- Ma, L.; Bongers, F.J.; Li, S.; Tang, T.; Yang, B.; Ma, K.; Liu, X. Species identity and composition effects on community productivity in a subtropical forest. Basic Appl. Ecol. 2021, 55, 87–97. [Google Scholar] [CrossRef]
- Liu, M.; Shen, Y.; Li, Q.; Xiao, W.; Song, X. Arbuscular mycorrhizal fungal colonization and soil pH induced by nitrogen and phosphorus additions affects leaf C:N:P stoichiometry in Chinese fir (Cunninghamia lanceolata) forests. Plant Soil 2021, 461, 421–440. [Google Scholar] [CrossRef]
- Adams, F.; Reddell, P.; Webb, M.; Shipton, W. Arbuscular mycorrhizas and ectomycorrhizas on Eucalyptus grandis (Myrtaceae) trees and seedlings in native forests of tropical north-eastern Australia. Aust. J. Bot. 2006, 54, 271–281. [Google Scholar] [CrossRef]
- Chen, Y.; Brundrett, M.C.; Dell, B. Effects of ectomycorrhizas and vesicular-arbuscular mycorrhizas, alone or in competition, on root colonization and growth of Eucalyptus globulus and E. urophylla. New Phytol. 2000, 146, 545–555. [Google Scholar] [CrossRef]
- Waldrop, M.P.; Zak, D.R.; Blackwood, C.B.; Curtis, C.D.; Tilman, D. Resource availability controls fungal diversity across a plant diversity gradient. Ecol. Lett. 2006, 9, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Miao, N.; Liu, S. Habitats shape root-associated fungal and bacterial communities of Minjiang fir saplings. J. For. Res. 2023, 34, 1491–1502. [Google Scholar] [CrossRef]
- Bennett, J.; Maherali, H.; Reinhart, K.; Lekberg, Y.; Hart, M.; Klironomos, J. Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science 2017, 355, 181–184. [Google Scholar] [CrossRef]
- Griffiths, R.P.; Baham, J.E.; Caldwell, B.A. Soil solution chemistry of ectomycorrhizal mats in forest soil. Soil Biol. Biochem. 1994, 26, 331–337. [Google Scholar] [CrossRef]
- Miyauchi, S.; Kiss, E.; Kuo, A.; Drula, E.; Kohler, A.; Sanchez-Garcia, M.; Morin, E.; Andreopoulos, B.; Barry, K.; Bonito, G.; et al. Large-scale genome sequencing of mycorrhizal fungi provides insights into the early evolution of symbiotic traits. Nat. Commun. 2020, 11, 5125. [Google Scholar] [CrossRef]
- Shah, F.; Nicolas, C.; Bentzer, J.; Ellstrom, M.; Smits, M.; Rineau, F.; Canback, B.; Floudas, D.; Carleer, R.; Lackner, G.; et al. Ectomycorrhizal fungi decompose soil organic matter using oxidative mechanisms adapted from saprotrophic ancestors. New Phytol. 2016, 209, 1705–1719. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Zhang, T.A.; Chen, H.Y.H.; Ruan, H. Global negative effects of nitrogen deposition on soil microbes. ISME J. 2018, 12, 1817–1825. [Google Scholar] [CrossRef]
- Teste, F.; Laliberté, E.; Lambers, H.; Auer, Y.; Kramer, S.; Kandeler, E. Mycorrhizal fungal biomass and scavenging declines in phosphorus-impoverished soils during ecosystem retrogression. Soil Biol. Biochem. 2016, 92, 119–132. [Google Scholar] [CrossRef]
- Ling, Y.; Dehua, F.; Zhihui, W.; Xingyuan, W.; Jianguo, H. Effects of potassium on the secretion of proton and oxalate by ectomycorrhizal fungi and the concentrations of nitrogen, phosphorus and potassium in their hyphae. Acta Ecol. Sin. 2001, 21, 254–258. (In Chinese) [Google Scholar] [CrossRef]
- Huang, F.; Zhang, W.; Gan, X.; Huang, Y.; Guo, Y.; Wen, X. Changes in vegetation and soil properties during recovery of a subtropical forest in South China. J. Mt. Sci. 2018, 15, 46–58. [Google Scholar] [CrossRef]
- Gamfeldt, L.; Snäll, T.; Bagchi, R.; Jonsson, M.; Gustafsson, L.; Kjellander, P.; Ruiz-Jaen, M.; Fröberg, M.; Stendahl, J.; Philipson, C.; et al. Higher levels of multiple ecosystem services are found in forests with more tree species. Nat. Commun. 2013, 4, 1340. [Google Scholar] [CrossRef] [PubMed]
- Urbanová, M.; Šnajdr, J.; Baldrian, P. Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol. Biochem. 2015, 84, 53–64. [Google Scholar] [CrossRef]
- Saitta, A.; Anslan, S.; Bahram, M.; Brocca, L.; Tedersoo, L. Tree species identity and diversity drive fungal richness and community composition along an elevational gradient in a Mediterranean ecosystem. Mycorrhiza 2018, 28, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Hertzog, L.; Boonyarittichaikij, R.; Dekeukeleire, D.; Groote, S.; van Schrojenstein Lantman, I.; Sercu, B.; Smith, H.K.; de la Peña, E.; Vandegehuchte, M.; Bonte, D.; et al. Forest fragmentation modulates effects of tree species richness and composition on ecosystem multifunctionality. Bull. Ecol. Soc. Am. 2019, 100, e02653. [Google Scholar] [CrossRef]
- Stadt, K.J.; Huston, C.; Coates, K.D.; Feng, Z.; Dale, M.R.T.; Lieffers, V.J. Evaluation of competition and light estimation indices for predicting diameter growth in mature boreal mixed forests. Ann. For. Sci. 2007, 64, 477–490. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, P.; Shen, H. Competition intensity affects growing season nutrient dynamics in Korean pine trees and their microhabitat soil in mixed forest. For. Ecol. Manag. 2023, 539, 121018. [Google Scholar] [CrossRef]
- Jian, C. Comparison of ecological habits between Pinus massoniana and Cunninghamia lanceolata. Hunan For. Sci. Technol. 1992, 2, 31–35. (In Chinese) [Google Scholar]
- Yan, J.; Li, K.; Peng, X.; Huang, Z.; Liu, S.; Zhang, Q. The mechanism for exclusion of Pinus massoniana during the succession in subtropical forest ecosystems: Light competition or stoichiometric homoeostasis? Sci. Rep. 2015, 5, 10994. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.; Feng, L.; Zhou, T.; Zhang, H.; Li, H.; Bai, G.; Meng, X.; Li, Z.; Zhao, G. Phylogeography and population dynamics of an endemic oak (Quercus fabri Hance) in subtropical China revealed by molecular data and ecological niche modeling. Tree Genet. Genomes 2019, 16, 2. [Google Scholar] [CrossRef]
- Cavender-Bares, J.M.; González Rodríguez, A.; Pahlich, A.; Koehler, K.; Deacon, N.J. Phylogeography and climatic niche evolution in live oaks (Quercus series Virentes) from the tropics to the temperate zone. J. Biogegr. 2011, 38, 962–981. [Google Scholar] [CrossRef]
| Sites | P. massoniana Associations | |||||
|---|---|---|---|---|---|---|
| Mp_Cl | Mp_Qf | Mp_Pa | Mp_Lf | Mp_MP | Mp_OP | |
| Latitude/Longitude | 106°59′27.39″ E, 27°5′30.25″ N | 106°59′14.80″ E, 27°6′9.22″ N | 106°59′7.78″ E, 27°6′9.22″ N | 106°59′7.78″ E, 27°6′34.00″ N | 106°59′15.28″ E, 27°16′34.98″ N | 106°59′24.00″ E, 27°16′45.21″ N |
| Age (a) | 30–40 | 30–40 | 30–40 | 20–30 | 20–30 | 150–200 |
| Altitude (m) | 1132 | 1162 | 1156 | 1080 | 977 | 943 |
| Aspect (°) | 175 | 23 | 48 | 229 | 128 | 136 |
| Slope (°) | 10 | 10 | 8 | 5 | 12 | 8 |
| Canopy Coverage (%) | 85 | 93 | 95 | 72 | 88 | 68 |
| LAI | 1.50 | 1.54 | 1.45 | 1.30 | 1.65 | 1.49 |
| pH | 4.76 ± 0.05 b | 4.34 ± 0.02 e | 4.66 ± 0.04 c | 4.88 ± 0.04 a | 4.65 ± 0.02 c | 4.45 ± 0.01 d |
| SOM (g/kg) | 49.34 ± 0.31 b | 54.10 ± 2.38 a | 53.04 ± 5.03 a | 56.57 ± 1.06 a | 27.26 ± 1.70 c | 41.74 ± 5.22 b |
| TN (g/kg) | 0.98 ± 0.04 a | 1.18 ± 0.01 a | 1.21 ± 0.01 a | 1.38 ± 0.02 a | 1.07 ± 0.02 a | 0.98 ± 0.04 a |
| TP (g/kg) | 0.40 ± 0.01 b | 0.43 ± 0.01 b | 0.54 ± 0.04 a b | 0.68 ± 0.04 a | 0.40 ± 0.01 b | 0.40 ± 0.01 b |
| AN (mg/kg) | 5.19 ± 0.63 b | 6.08 ± 0.30 ab | 3.92 ± 1.36 b | 7.69 ± 0.65 a | 5.25 ± 0.59 b | 5.19 ± 0.63 b |
| AP (mg/kg) | 17.81 ± 3.23 a | 11.32 ± 1.72 ab | 12.91 ± 3.44 ab | 15.98 ± 2.68 ab | 9.35 ± 1.14 b | 10.43 ± 1.44 b |
| AK (mg/kg) | 59.00 ± 9.54 b | 39.00 ± 0.00 d | 52.33 ± 1.15 b c | 82.67 ± 0.58 a | 44.33 ± 0.58 cd | 60.00 ± 1.00 b |
| T_Shanon index | 1.71 | 2.07 | 2.15 | 1.90 | 0.47 | 0.60 |
| T_Simpson index | 0.79 | 0.86 | 0.87 | 0.81 | 0.29 | 0.41 |
| T_Pielou index | 0.88 | 0.94 | 0.93 | 0.86 | 0.68 | 0.87 |
| Number | OTUs | Sequence Length (bp) | The Alignment Number | Identity (%) | Genbank ID |
|---|---|---|---|---|---|
| 1 | Agaricales sp. | 789 | FJ266729 | 97.36 | OR467492 |
| 2 | Aleurina imaii | 672 | MG871292 | 98.15 | OR469906 |
| 3 | Amphinema sp. 1 | 592 | LC013707 | 98.37 | OR482662 |
| 4 | Amphinema sp. 2 | 606 | JN943925 | 99.15 | OR482663 |
| 5 | Archaeorhizomyces borealis | 499 | NR_126144 | 98.8 | OR482664 |
| 6 | Astrosporina sp. | 533 | JQ991646 | 99.06 | OR483811 |
| 7 | Cenococcum sp. 1 | 592 | LC095124 | 94.90 | OR482665 |
| 8 | Cenococcum sp. 2 | 545 | AB769888 | 98.45 | OR482666 |
| 9 | Cladophialophora sp. | 636 | LC229676 | 98.66 | OR482667 |
| 10 | Clavulina amethystina | 695 | MK422194 | 99.23 | OR482668 |
| 11 | Clavulina sp. | 695 | ON794325 | 94.14 | OR482669 |
| 12 | Clavulina thindii | 463 | MG892054 | 98.15 | OR482670 |
| 13 | Helotiales sp. 1 | 587 | KP866121 | 98.96 | OR482671 |
| 14 | Helotiales sp. 2 | 641 | KP866122 | 96.54 | OR482672 |
| 15 | Helotiales sp. 3 | 512 | KP866123 | 99.45 | OR482673 |
| 16 | Helotiales sp. 4 | 566 | KX440153 | 98.92 | OR482674 |
| 17 | Helotiales sp. 5 | 461 | MG670433 | 98.81 | OR483812 |
| 18 | Helotiales sp. 6 | 460 | AB636433 | 99.22 | OR483813 |
| 19 | Hyaloscypha aff.hepaticicola | 562 | AB847066 | 99.42 | OR482675 |
| 20 | Hyaloscypha sp. 1 | 583 | OQ430740 | 99.79 | OR482676 |
| 21 | Hyaloscypha sp. 2 | 586 | MT522552 | 99.26 | OR482677 |
| 22 | Hyaloscypha sp. 3 | 904 | OQ207649 | 99.77 | OR482678 |
| 23 | Hyaloscyphaceae sp. | 571 | KU141214 | 99.30 | OR482679 |
| 24 | Hymenoscyphus sp. | 550 | KF679808 | 98.29 | OR482680 |
| 25 | Inocybe sp. 1 | 584 | MT237516 | 91.55 | OR482681 |
| 26 | Inocybe sp. 2 | 584 | LC175093 | 98.28 | OR482682 |
| 27 | Lactarius hatsudake | 709 | EF685076 | 99.57 | OR482683 |
| 28 | Lactarius kesiyae | 732 | KR025614 | 99.17 | OR482684 |
| 29 | Lactarius parallelus | 743 | MH984997 | 98.84 | OR482685 |
| 30 | Oidiodendron sp. | 568 | EU888629 | 99.46 | OR482686 |
| 31 | Phialocephala fortinii | 901 | KX440179 | 99.20 | OR482687 |
| 32 | Russula cascadensis | 653 | MT522568 | 99.67 | OR482688 |
| 33 | Russula indocatillus | 684 | MN581483 | 99.51 | OR482689 |
| 34 | Russula rosea | 693 | MZ221554 | 99.23 | OR482690 |
| 35 | Russula sp. 1 | 690 | MK770275 | 98.77 | OR482691 |
| 36 | Russula sp. 2 | 663 | OQ421796 | 99.75 | OR482692 |
| 37 | Russula sp. 3 | 671 | OQ430675 | 98.65 | OR482693 |
| 38 | Russula sp. 4 | 690 | LC367779 | 98.12 | OR482694 |
| 39 | Russula sp. 5 | 702 | KU205301 | 93.64 | OR482695 |
| 40 | Russula vesca | 676 | HM189953 | 97.64 | OR482696 |
| 41 | Russulaceae sp. | 683 | FJ454965 | 97.39 | OR482697 |
| 42 | Sebacina sp. 1 | 623 | OM236634 | 97.81 | OR482698 |
| 43 | Sebacina sp. 2 | 642 | KP013014 | 91.72 | OR482699 |
| 44 | Sebacina sp. 3 | 646 | KF000417 | 94.51 | OR482700 |
| 45 | Sphaerosporella sp. | 602 | MW476527 | 98.00 | OR482701 |
| 46 | Thelephoraceae sp. | 660 | AB634273 | 99.07 | OR482702 |
| 47 | Tomentella sp. 1 | 687 | MN970734 | 98.88 | OR482703 |
| 48 | Tomentella sp. 2 | 666 | KP866136 | 99.39 | OR482704 |
| 49 | Tomentella sp. 3 | 662 | JX630406 | 96.91 | OR482705 |
| 50 | Tomentella stuposa | 669 | MK602778 | 97.30 | OR482706 |
| 51 | Tomentella sublilacina | 660 | OQ430790 | 99.70 | OR482707 |
| 52 | Trechisporales sp. | 645 | LC436083 | 99.81 | OR482708 |
| 53 | Trichoderma sp. | 609 | MK870953 | 99.67 | OR482709 |
| 54 | Tylospora sp. 1 | 605 | AB456677 | 98.66 | OR482710 |
| 55 | Tylospora sp. 2 | 598 | KF007260 | 99.50 | OR482711 |
| 56 | Venturia sp. | 562 | MT522585 | 99.61 | OR482712 |
| Diversity Index | Mp_Cl | Mp_Qf | Mp_Pa | Mp_Lf | Mp_MP | Mp_OP |
|---|---|---|---|---|---|---|
| Shannon | 2.062 | 2.385 | 2.651 | 2.216 | 2.728 | 2.815 |
| Simpson | 0.833 | 0.864 | 0.908 | 0.870 | 0.923 | 0.934 |
| Pielou | 0.896 | 0.881 | 0.917 | 0.924 | 0.944 | 0.974 |
| Chao1 | 13.750 | 33.333 | 63.500 | 39.000 | 34.500 | 53.000 |
| Sites | Similarity Index | |||||
|---|---|---|---|---|---|---|
| Mp_Cl | Mp_Qf | Mp_Pa | Mp_Lf | Mp_MP | Mp_OP | |
| Mp_Cl | 0.107 | 0.125 | 0.045 | 0.069 | 0.065 | |
| Mp_Qf | 0.222 | 0.132 | 0.071 | 0.135 | 0.128 | |
| Mp_Pa | 0.286 | 0.303 | 0.094 | 0.125 | 0.098 | |
| Mp_Lf | 0.095 | 0.154 | 0.207 | 0.125 | 0.189 | |
| Mp_MP | 0.148 | 0.313 | 0.286 | 0.286 | 0.163 | |
| Mp_OP | 0.211 | 0.294 | 0.162 | 0.467 | 0.211 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, D.; Xu, M.; Yang, Y.; Zhang, J. Effects of Stand Types on Ectomycorrhizal Fungal Community Composition and Structure of Pinus massoniana in Subtropical Mountain Forest Ecosystems. Forests 2024, 15, 258. https://doi.org/10.3390/f15020258
Jiang D, Xu M, Yang Y, Zhang J. Effects of Stand Types on Ectomycorrhizal Fungal Community Composition and Structure of Pinus massoniana in Subtropical Mountain Forest Ecosystems. Forests. 2024; 15(2):258. https://doi.org/10.3390/f15020258
Chicago/Turabian StyleJiang, Dandan, Ming Xu, Yunli Yang, and Jian Zhang. 2024. "Effects of Stand Types on Ectomycorrhizal Fungal Community Composition and Structure of Pinus massoniana in Subtropical Mountain Forest Ecosystems" Forests 15, no. 2: 258. https://doi.org/10.3390/f15020258
APA StyleJiang, D., Xu, M., Yang, Y., & Zhang, J. (2024). Effects of Stand Types on Ectomycorrhizal Fungal Community Composition and Structure of Pinus massoniana in Subtropical Mountain Forest Ecosystems. Forests, 15(2), 258. https://doi.org/10.3390/f15020258






