PwuWRKY48 Confers Drought Tolerance in Populus wulianensis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Drought Treatment
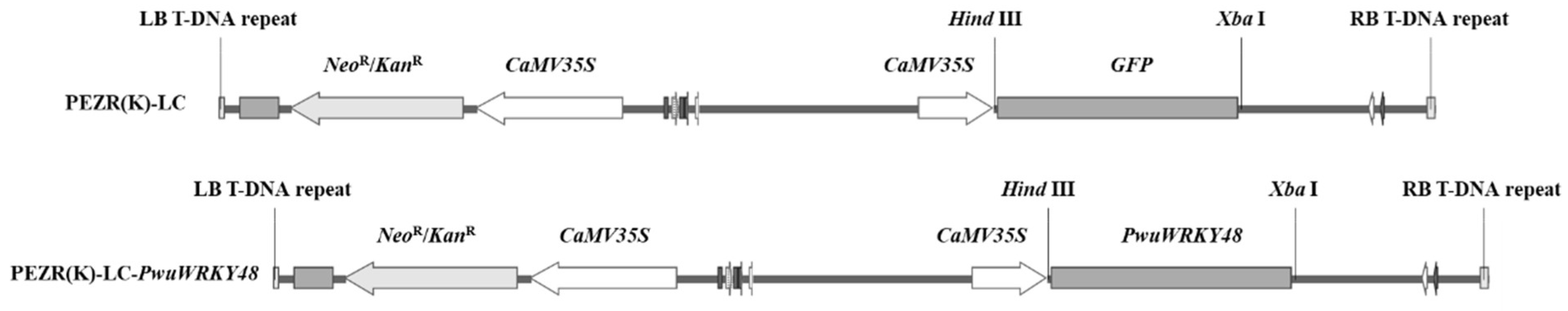
2.2. Gene Cloning and Vector Construction
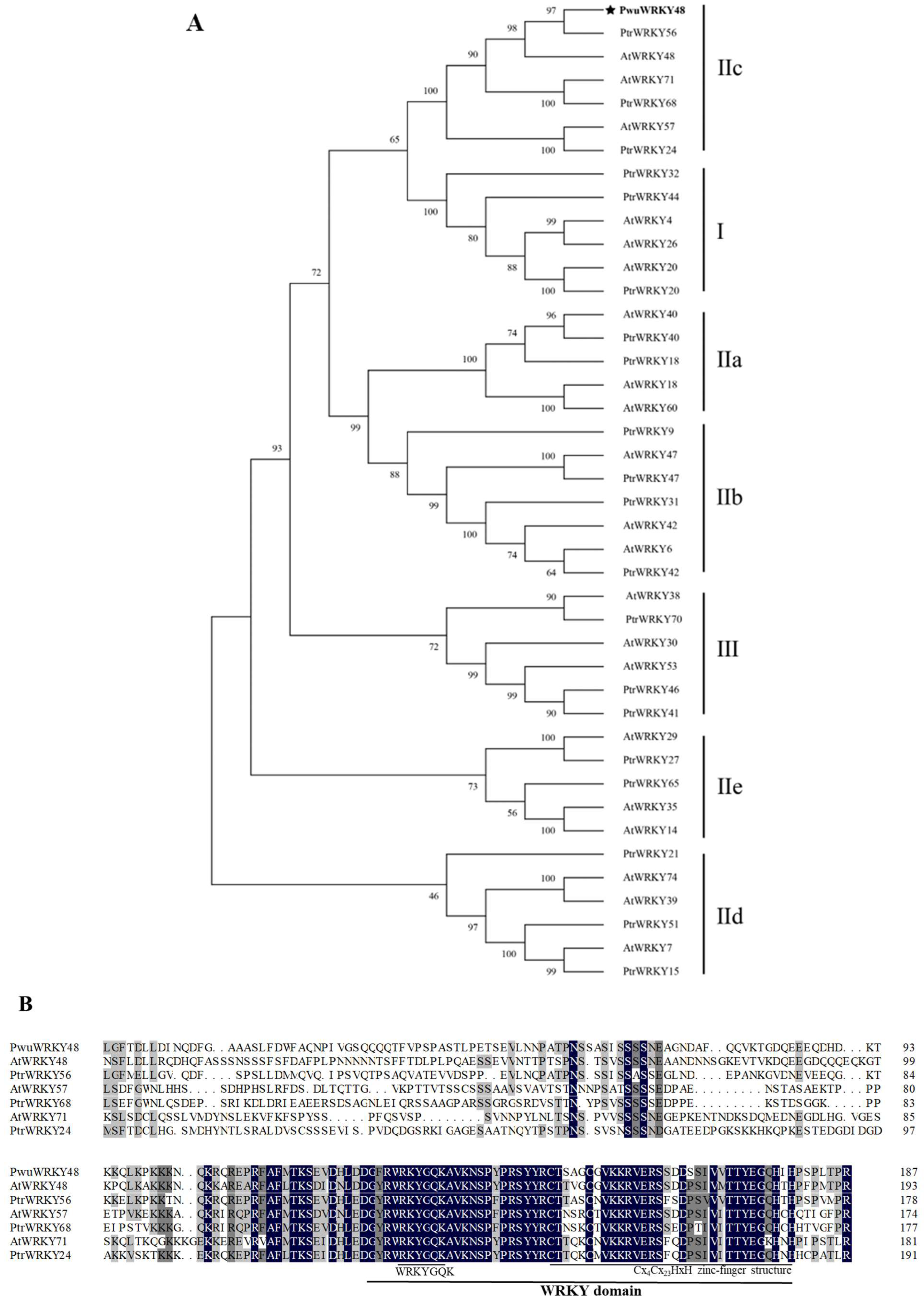
2.3. Gene Characterization and Phylogenetic Analysis
2.4. Expression Analysis of PwuWRKY48
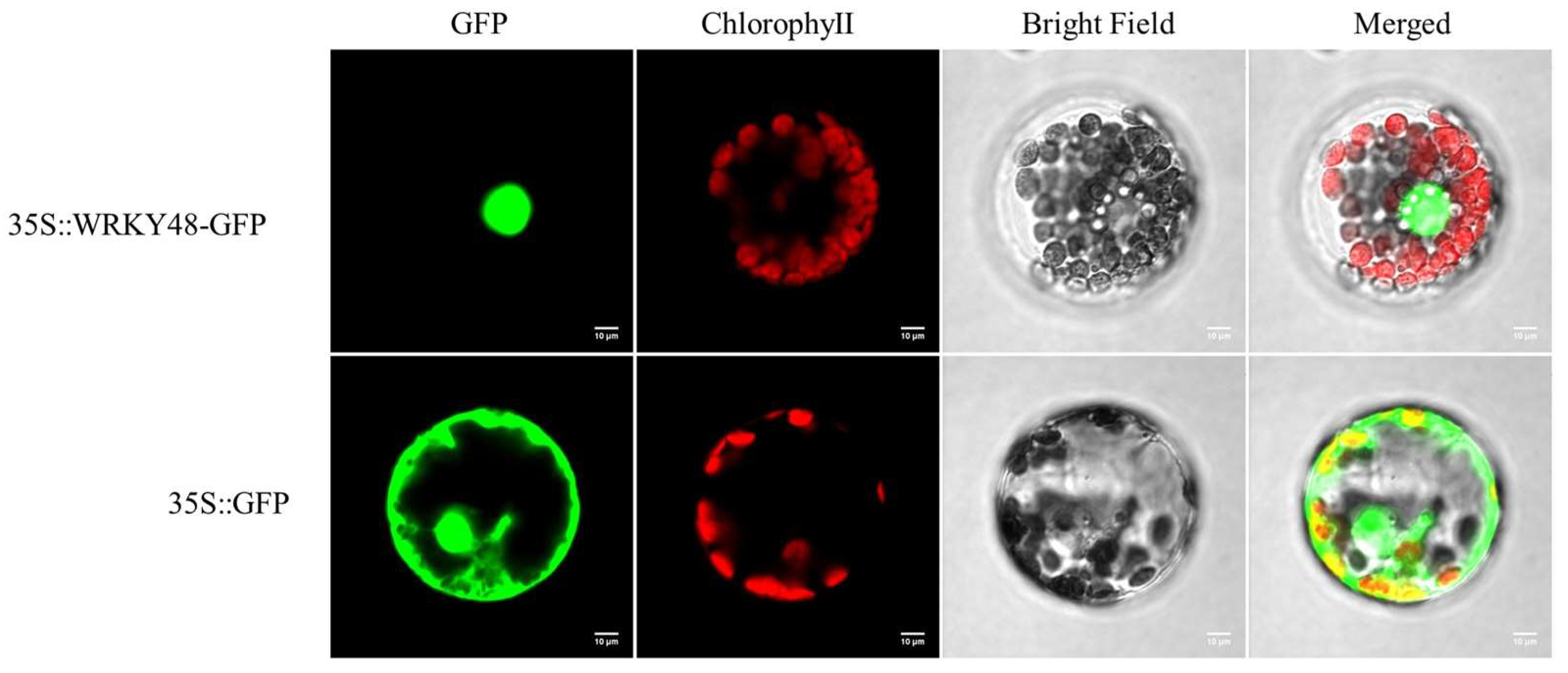
2.5. Subcellular Localization of PwuWRKY48
2.6. Promoter Cloning and Cis-Acting Element Identification
2.7. Gene Transformation
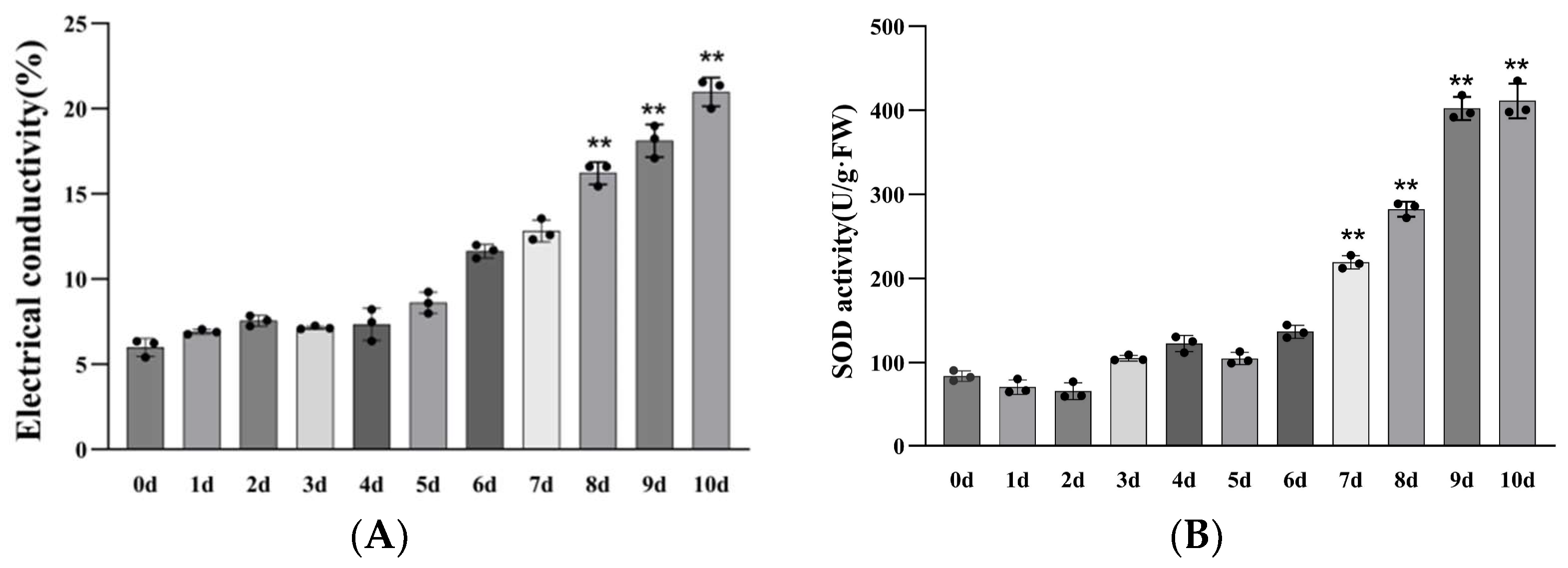
2.8. Drought Resistance Evaluation of the Transgenic Plants
3. Results
3.1. Expression Pattern of PwuWRKY48 Gene in Drought Treatment
3.2. Characterization of PwuWRKY48 Gene and Homologous Analysis
3.3. Identification of the Regulation Elements on PwuWRKY48 Promoter
3.4. Subcellular Localization of PwuWRKY48
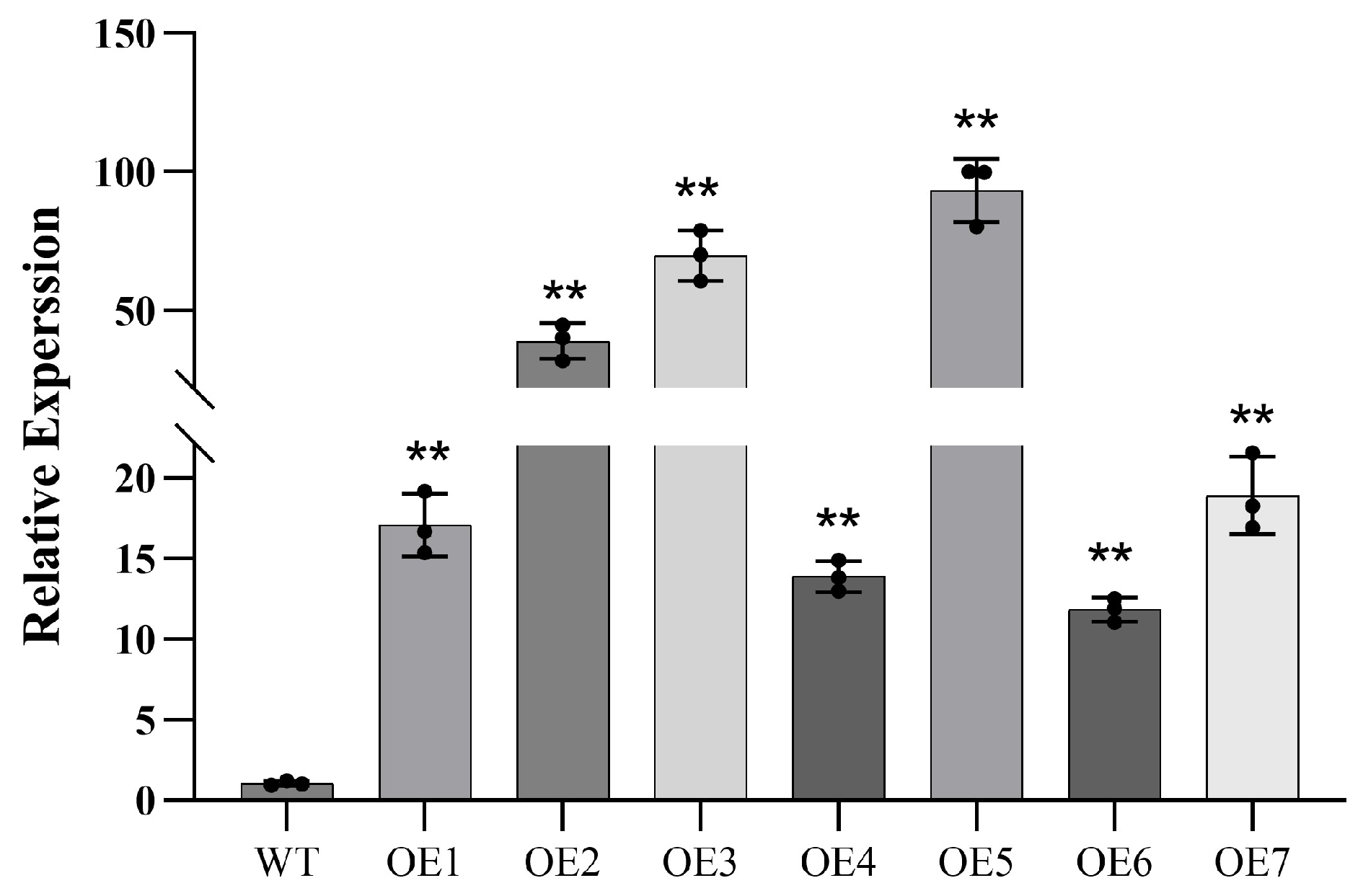
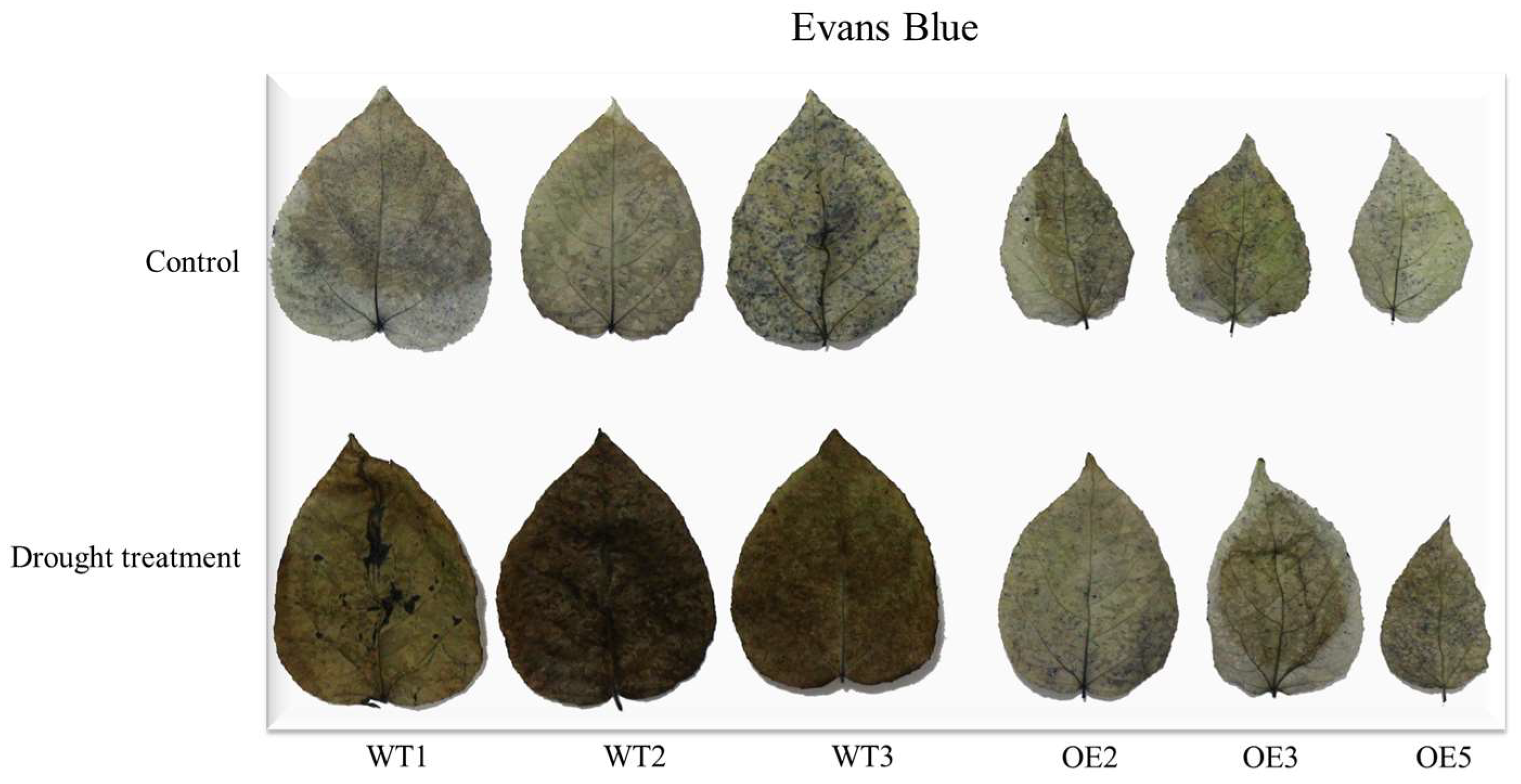
3.5. Drought Resistance Analysis of P. wulianensis Plants with PwuWRKY48 Overexpression
4. Discussion
4.1. Cis-Regulation of WRKY Expression
4.2. PwuWRKY48 Gene Advanced Drought Tolerance in Plants via Regulating Target Gene Expression
4.3. WRKY Genes Exhibited Various Resistance to Abiotic Stress
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wani, S.H.; Anand, S.; Singh, B.; Bohra, A.; Joshi, R. WRKY transcription factors and plant defense responses: Latest discoveries and future prospects. Plant Cell Rep. 2021, 40, 1071–1085. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, T.; Lin, Z.; Gu, B.; Xing, C.; Zhao, L.; Dong, H.; Gao, J.; Xie, Z.; Zhang, S.; et al. A WRKY transcription factor PbrWRKY53 from Pyrus betulaefolia is involved in drought tolerance and AsA accumulation. Plant Biotechnol. J. 2019, 17, 1770–1787. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, K.; Xu, Y.; Wang, L.; Liu, H.; Qin, Z.; Xiang, Y. The moso bamboo WRKY transcription factor, PheWRKY86, regulates drought tolerance in transgenic plants. Plant Physiol. Biochem. 2022, 170, 180–191. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Zhang, D.; Tang, X.; Li, Z.; Shen, C.; Han, X.; Deng, W.; Yin, W.; Xia, X. PtrWRKY75 overexpression reduces stomatal aperture and improves drought tolerance by salicylic acid-induced reactive oxygen species accumulation in poplar. Environ. Exp. Bot. 2020, 176, 104117. [Google Scholar] [CrossRef]
- Lu, K.; Liang, S.; Wu, Z.; Bi, C.; Yu, Y.-T.; Wang, X.-F.; Zhang, D.-P. Overexpression of an Arabidopsis cysteine-rich receptor-like protein kinase, CRK5, enhances abscisic acid sensitivity and confers drought tolerance. J. Exp. Bot. 2016, 67, 5009–5027. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, X.; Yang, F.; Fan, D.; Jiang, Y.; Luo, K. PtrWRKY19, a novel WRKY transcription factor, contributes to the regulation of pith secondary wall formation in Populus trichocarpa. Sci. Rep. 2016, 6, 18643. [Google Scholar] [CrossRef]
- Yu, X.; Lu, B.; Dong, Y.; Li, Y.; Yang, M. Cloning and functional identification of PeWRKY41 from Populus × euramericana. Ind. Crops Prod. 2022, 175, 114279. [Google Scholar] [CrossRef]
- Raven, P.H.; Hong, D.Y. (Eds.) Flora of China; Science Press & St, Beijing: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 1999; Volume 4. [Google Scholar]
- Zang, D.K. Rare and Endangered Plants in Shandong; China Forestry Press: Beijing, China, 2016; pp. 245–246. [Google Scholar]
- Wu, Q.; Zang, F.; Ma, Y.; Zheng, Y.; Zang, D. Analysis of genetic diversity and population structure in endangered Populus wulianensis based on 18 newly developed EST-SSR markers. Glob. Ecol. Conserv. 2020, 24, e01329. [Google Scholar] [CrossRef]
- Wu, Q.; Zang, F.; Xie, X.; Ma, Y.; Zheng, Y.; Zang, D. Full-length transcriptome sequencing analysis and development of EST-SSR markers for the endangered species Populus wulianensis. Sci. Rep. 2020, 10, 16249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, D.; Liu, L.J.; Han, Y.; Sun, T.; Wang, N.; Xie, X.M.; Dong, X.; Lu, Y.Z. Establishment of Generation System for Populus wulianensis and Control of Vitrification of Its Test-tube Seedlings. Mol. Plant Breed. 2019, 17, 6434–6446. [Google Scholar]
- Logemann, J.; Schell, J.; Willmitzer, L. Improved method for the isolation of RNA from plant tissues. Anal. Biochem. 1987, 163, 16–20. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yan, P.; Zeng, Y.; Shen, W.; Tuo, D.; Li, X.; Zhou, P. Nimble Cloning: A Simple, Versatile, and Efficient System for Standardized Molecular Cloning. Front. Bioeng. Biotechnol. 2020, 7, 460. [Google Scholar] [CrossRef] [PubMed]
- Drechsel, G.; Bergler, J.; Wippel, K.; Sauer, N.; Vogelmann, K.; Hoth, S. C-terminal armadillo repeats are essential and sufficient for association of the plant U-box armadillo E3 ubiquitin ligase SAUL1 with the plasma membrane. J. Exp. Bot. 2010, 62, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Abel, S.; Theologis, A. Transient transformation of Arabidopsis leaf protoplasts: A versatile experimental system to study gene expression. Plant J. 1994, 5, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, Y.Z.; Chen, L.S.; Peng, S.F.; Wang, X.N.; Yang, X.H.; Ma, L. Correlation analysis of SPAD value and chlorophyll content in leaves of Camelliaoleifera. J. Cent. South. Univ. For. Technol. 2013, 33, 77–80. [Google Scholar]
- Jia, C.; Wang, J.; Guo, B.; Li, X.; Yang, T.; Yang, H.; Li, N.; Wang, B.; Yu, Q. A group III WRKY transcription factor, SlWRKY52, positively regulates drought tolerance in tomato. Environ. Exp. Bot. 2023, 215, 105513. [Google Scholar] [CrossRef]
- Govind, G.; Vokkaliga ThammeGowda, H.; Jayaker Kalaiarasi, P.; Iyer, D.R.; Muthappa, S.K.; Nese, S.; Makarla, U.K. Identification and functional validation of a unique set of drought induced genes preferentially expressed in response to gradual water stress in peanut. Mol. Genet. Genom. 2009, 281, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhou, Y.; Zhai, H.; He, S.; Zhao, N.; Liu, Q. A Novel Sweetpotato WRKY Transcription Factor, IbWRKY2, Positively Regulates Drought and Salt Tolerance in Transgenic Arabidopsis. Biomolecules 2020, 10, 506. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-L.; Qu, L.; Guo, D.; Wang, Y.; Zhu, J.-H.; Peng, S.-Q. Histone deacetylase interacts with a WRKY transcription factor to regulate the expression of the small rubber particle protein gene from Hevea brasiliensis. Ind. Crop. Prod. 2020, 145, 111989. [Google Scholar] [CrossRef]
- Jing, Z.; Liu, Z. Genome-wide identification of WRKY transcription factors in kiwifruit (Actinidia spp.) and analysis of WRKY expression in responses to biotic and abiotic stresses. Genes. Genom. 2018, 40, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Duan, Y.; Yin, J.; Ye, S.; Zhu, J.; Zhang, F.; Lu, W.; Fan, D.; Luo, K. Genome-wide identification and characterization of the Populus WRKY transcription factor family and analysis of their expression in response to biotic and abiotic stresses. J. Exp. Bot. 2014, 65, 6629–6644. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, P.; Yu, J.; Wang, L.; Dossa, K.; Zhang, Y.; Zhou, R.; Wei, X.; Zhang, X. Genome-wide analysis of WRKY gene family in the sesame genome and identification of the WRKY genes involved in responses to abiotic stresses. BMC Plant Biol. 2017, 17, 152. [Google Scholar] [CrossRef]
- Yuan, Y.; Ren, S.; Liu, X.; Su, L.; Wu, Y.; Zhang, W.; Li, Y.; Jiang, Y.; Wang, H.; Fu, R.; et al. SlWRKY35 positively regulates carotenoid biosynthesis by activating the MEP pathway in tomato fruit. New Phytol. 2022, 234, 164–178. [Google Scholar] [CrossRef]
- Doll, J.; Muth, M.; Riester, L.; Nebel, S.; Bresson, J.; Lee, H.-C.; Zentgraf, U. Arabidopsis thaliana WRKY25 Transcription Factor Mediates Oxidative Stress Tolerance and Regulates Senescence in a Redox-Dependent Manner. Front. Plant Sci. 2020, 10, 1734. [Google Scholar] [CrossRef]
- Wu, X.; Chen, L.; Lin, X.; Chen, X.; Han, C.; Tian, F.; Wan, X.; Liu, Q.; He, F.; Chen, L.; et al. Integrating physiological and transcriptome analyses clarified the molecular regulation mechanism of PyWRKY48 in poplar under cadmium stress. Int. J. Biol. Macromol. 2023, 238, 124072. [Google Scholar] [CrossRef]
- Yan, J.Y.; Li, C.X.; Sun, L.; Ren, J.Y.; Li, G.X.; Ding, Z.J.; Zheng, S.J. A WRKY Transcription Factor Regulates Fe Translocation under Fe Deficiency. Plant Physiol. 2016, 171, 2017–2027. [Google Scholar] [CrossRef]
- Arraño-Salinas, P.; Domínguez-Figueroa, J.; Herrera-Vásquez, A.; Zavala, D.; Medina, J.; Vicente-Carbajosa, J.; Meneses, C.; Canessa, P.; Moreno, A.A.; Blanco-Herrera, F. WRKY7, -11 and -17 transcription factors are modulators of the bZIP28 branch of the unfolded protein response during PAMP-triggered immunity in Arabidopsis thaliana. Plant Sci. 2018, 277, 242–250. [Google Scholar] [CrossRef]
- Shang, Y.; Yan, L.; Liu, Z.-Q.; Cao, Z.; Mei, C.; Xin, Q.; Wu, F.-Q.; Wang, X.-F.; Du, S.-Y.; Jiang, T.; et al. The Mg-Chelatase H Subunit of Arabidopsis Antagonizes a Group of WRKY Transcription Repressors to Relieve ABA-Responsive Genes of Inhibition. Plant Cell 2010, 22, 1909–1935. [Google Scholar] [CrossRef]
- Gu, J.; Lv, F.; Gao, L.; Jiang, S.; Wang, Q.; Li, S.; Yang, R.; Li, Y.; Li, S.; Wang, P. A WRKY Transcription Factor CbWRKY27 Negatively Regulates Salt Tolerance in Catalpa bungei. Forests 2023, 14, 486. [Google Scholar] [CrossRef]
- Kiranmai, K.; Lokanadha Rao, G.; Pandurangaiah, M.; Nareshkumar, A.; Amaranatha Reddy, V.; Lokesh, U.; Venkatesh, B.; Anthony Johnson, A.M.; Sudhakar, C. A Novel WRKY Transcription Factor, MuWRKY3 (Macrotyloma uniflorum Lam. Verdc.) Enhances Drought Stress Tolerance in Transgenic Groundnut (Arachis hypogaea L.). Plants 2018, 9, 346. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, M.; Mu, Y.; Guan, L.; Wu, F.; Liu, K.; Li, M.; Wang, N.; Zhuang, Z.; Zhao, Y.; et al. PwuWRKY48 Confers Drought Tolerance in Populus wulianensis. Forests 2024, 15, 302. https://doi.org/10.3390/f15020302
Wang Y, Li M, Mu Y, Guan L, Wu F, Liu K, Li M, Wang N, Zhuang Z, Zhao Y, et al. PwuWRKY48 Confers Drought Tolerance in Populus wulianensis. Forests. 2024; 15(2):302. https://doi.org/10.3390/f15020302
Chicago/Turabian StyleWang, Yan, Mengtian Li, Yanjuan Mu, Lingshan Guan, Fusheng Wu, Kun Liu, Meng Li, Ning Wang, Zhenjie Zhuang, Yunchao Zhao, and et al. 2024. "PwuWRKY48 Confers Drought Tolerance in Populus wulianensis" Forests 15, no. 2: 302. https://doi.org/10.3390/f15020302





