Abstract
Investigating the mechanisms by which plants adapt to low phosphorus content in ecosystems is crucial for nutrient dynamics division. Our study investigated the growth adaptation strategies of Pinus massoniana seedlings to low phosphorus conditions, including nutrient and non-structural carbohydrate (NSC) allocation, nutrient stoichiometry, and changes in nutrient resorption efficiency along a fact-based gradient. Our results showed that the total biomass and aboveground biomass proportion increased with substrate phosphorus content, reaching maximum biomass in the one-time phosphorus treatment. The nutrient concentration of components remained relatively stable, with the allocating preference to roots and needles under low phosphorus conditions. NSC was allocated as starch in fine roots and as soluble sugar in needles. Seedlings did not show signs of phosphorus limitation, even in the non-phosphorus group. The nitrogen resorption efficiency to phosphorus resorption efficiency ratio (NRE: PRE) of needles significantly varied between the high and low phosphorus treatments. In response to phosphorus deficiency, seedlings demonstrated homeostatic adjustments to maintain the relative stability of nutrient concentration. Fine roots and needles were prioritized to ensure nutrient uptake and photosynthetic product production. Additionally, it was necessary to differentiate the indicative function of nitrogen/phosphorus for various species and components, and NRE: PRE potentially provides a sensitive indicator of nutrient limitation status.
1. Introduction
Phosphorus (P) is an essential element that plays a crucial role in plant growth, metabolism, and photosynthesis [1,2]. However, soil P is susceptible to leaching and loss through precipitation [3], and it can be easily immobilized by organic matter, metal ions, or clay minerals under acidic conditions [4]. Moreover, human activities have exacerbated these effects [5], leading to reduced availability of low P in the soil, which often shows a decreasing trend [6]. Increasingly, ecosystems are shifting from nitrogen (N) limitation or N-P co-limitation to P limitation [6,7], especially in tropical and subtropical regions [8,9]. Consequently, plants are experiencing increasing P stress.
To cope with low P environments, plants have evolved a variety of adaptive mechanisms to thrive in low P environments, including the allocation of photosynthetic products and nutrient resorption, to ensure growth and development. Non-structural carbohydrates (NSCs) serve as a direct energy source for various physiological processes [10,11]. Changes in NSC concentration and its components, including starch and soluble sugar, can provide insights into plant utilization strategies [12,13]. An increase in starch concentration indicates enhanced carbohydrate storage, while elevated soluble sugar concentration implies improved energy supply. Previous studies demonstrated that the addition of P accelerates NSC accumulation in coniferous trees [14]. And higher starch concentration has been observed in Pinus massoniana seedlings under stressful conditions [15]. However, there is a scarcity of research on the impact of P on the allocation of NSC in plants. Studying the response of NSC to P is essential for a better understanding of the intricate growth and physiological process mechanisms in plants.
Plant nutrient concentration, stoichiometric ratios, and allocation patterns are direct reflections of plant nutrient availability and acquisition strategies, reflecting the trade-offs faced by plants in obtaining aboveground and belowground resources, and are often used as indicators of plant nutrient limitations [16,17]. The 14/16 ratio was initially used in studies at the community level to assess whether the community is limited by N, P, or both [16]. It has been widely applied in research at various scales. However, due to differences in research scales, vegetation types, study areas, tissue types, and many other factors, the nutrient concentration and ratios may vary intensely [16,18,19], leading to controversies in defining thresholds. Increasingly, studies have redefined nutrient limitation thresholds for different regions [20,21], scales [18,21], and plant organs [22,23]. In contrast to the nutrient ratio indicator, the nutrient manipulation experiment explores plant performance based on the actual nutrient conditions, allowing for the examination of nutrient ratio changes in truly nutrient-limited environments. In particular, the establishment of low P experiments is of great significance for verifying the sensitivity and true reflection of N:P thresholds in plant nutrient conditions, thereby contributing to practical applications in P-limited environments. It also helps to address the limitations of nutrient resource acquisition and allocation at the individual level in the study of nutrient limitation indicators.
The transfer of nutrients between different organs, especially nutrient resorption and reallocation from senescent organs to new organs with physiological activity, is the key process for plants to improve nutrient utilization efficiency, maintain stable nutrient levels, and reduce reliance on soil nutrients [24,25]. It is widely believed that plants exhibit higher nutrient resorption efficiency (NuRE) under nutrient-limited conditions [25,26], but some studies have suggested a positive correlation between NuRE and nutrient supply [24]. The impact of leaf nutrient concentration and stoichiometry on NuRE has been widely reported, and different researchers have categorized the main control factors of NuRE into three strategies: nutrient concentration control, nutrient limitation control, and stoichiometry control [26]. However, it is currently unclear which strategy plays a dominant role under special low P conditions. Thus, further clarification is needed regarding the relationship between NuRE and nutrient limitation status.
Pinus massoniana is a common afforestation tree species in the subtropical region of China, and its soil P content is relatively low in its main distribution area, where the soil available P content varied from 0.35 to 7.55 mg kg−1 [27]. The soil P was recognized as a key indicator to control the growth and productivity of P. massoniana plantations, which were sensitive to available P content in soil [28]. However, under such low P soil conditions, P. massoniana would still maintain relative high productivity [28,29]. Therefore, whether soil P limits the growth of P. massoniana and which level of P limits their growth remains unclear. In this study, we aim to investigate the variations in nutrient and NSC concentration in different components and explore nutrient allocation and resorption strategies of P. massoniana seedlings under a low P gradient that conforms to the actual soil nutrient status. The following questions needed to be answered: (1) the nutrient adaptation status of P. massoniana seedlings in different components under low P conditions; (2) the allocation of NSC and its components in different components under low P conditions; and (3) the adaptation mechanisms of nutrients and photosynthetic products above and below the ground and their interactions in P. massoniana seedlings.
2. Materials and Methods
2.1. Materials and Experimental Design
Two-year-old seedlings of P. massoniana from the seed orchard in Ma’anshan Forest Farm, Duyun City, Guizhou Province, were obtained as experimental material. In December 2020, the P. massoniana seedlings were transplanted to the Forest Ecosystem State Positioning Observation Station, which is located in the Three Gorges Reservoir area, Zigui County, Hubei Province (110°54′ E, 30°53′ N, altitude 296 m), for nursery cultivation. In early May 2021, seedlings with consistent size and uniform growth were individually transplanted into pots (height 17 cm, diameter 16.5 cm) for nutrient control cultivation. The substrate was a mixture of quartz sand, vermiculite, and pumice in a ratio of 7:2:1, with a bulk density of approximately 1.15 g·cm−3. Before transplantation, the substrate was acid-washed with a 3% dilute hydrochloric acid solution, soaked for one week to remove impurities, and then balanced with a nutrient solution. All selected seedlings were placed on open-air nursery beds at the positioning station for experimental cultivation and subsequent observations.
To investigate the impact of different P content level, six P content treatments (200 seedlings were prepared for each treatment) were established according to surface soil (0–20 cm) available P content in the whole distribution of P. massoniana forests (2.235 mg·kg−1) [30]: no P (0 mg·kg−1, 0 AP), 1/4 times of available P (0.581 mg·kg−1, 1/4 AP), 1/2 times of available P (1.162 mg·kg−1, 1/2 AP), 1 time of available P (2.325 mg·kg−1, AP), 2 times of available P (4.650 mg·kg−1, 2 AP), and 4 times of available P (9.299 mg·kg−1, 4 AP). The nutrient requirements for each plant in each pot were calculated based on the substrate weight with approximately 3 kg per pot. To avoid the toxic effects of high ion concentrations on plants, nutrient solution was evenly applied to the pots five times within the first month after planting. Each application was spaced apart by 7 days to minimize fluctuations in nutrient concentrations among the substrate and plant tissues. Additionally, to maintain the effectiveness of ions, macro- and micro-elements were added separately. The contents of macroelements were shown in Table 1. The microelement nutrient solution was provided by a modified Hoagland nutrient solution [31], in which the concentrations of Fe3+, Zn2+, Mn2+, Cu2+, Mo6+, and B4+ were 7.149 mg·kg−1, 0.131 mg·kg−1, 0.110 mg·kg−1, 0.032 mg·kg−1, 0.048 mg·kg−1, and 0.270 mg·kg−1. The solution pH was maintained between 5.3 and 6.0. To address the poor water retention of the quartz sand substrate, a misting irrigation device was employed to irrigate and cool the seedlings after sunset, ensuring sufficient water supply. Additionally, shading nets were also used to minimize evaporation of moisture from the substrate. This nutrient control experiment started in early May 2021 and finished on 31 August 2021.

Table 1.
Contents of macroelements in different phosphorus treatments.
2.2. Plant Sampling
In the end of August 2021, four seedlings were randomly selected from each treatment, harvested by components, including new needles (current-year leaves), old needles (two-year-old leaves), new twigs (current-year twigs), old twigs (two-year-old twigs), stem, fine roots (≤2 mm), and coarse roots (>2 mm). All samples were then placed in a drying oven at 105 °C for 15 min, followed by drying at 65 °C until a constant weight was reached. The constant weight was recorded as biomass (M). The dried samples were ground using a ball mill (WL-48, Tianjin, China), passed through a 60 mesh size sieve and sealed for further analysis.
2.2.1. Nutrients and NSC Measurement and Reserves Calculation
For different components, N (g·kg−1) concentration was determined using an elemental analyzer (Elementar vario Macro cube, Shanghai, China), while total P (g·kg−1) concentration was measured using the phosphomolybdate colorimetry assay [32]. Soluble sugar and starch concentrations were determined using the anthrone colorimetric method [33] and calculated based on dry weight (DW) (% DW). NSC concentration was the sum of soluble sugar and starch concentrations.
The reserves of N, P, and NSC can be used to estimate nutrient allocation and predict growth changes [34,35]. The total stocks of N, P, and NSC, hereafter referred to as reserves, were calculated using the following formula:
where C represents the concentration of nutrients and substance of different components; M represents the biomass of different components; i represents N, P, soluble sugar, starch, and NSC; and j represents needles and roots.
Reserve = Cij × Mj,
2.2.2. Nutrient Resorption Efficiency
The P resorption efficiency (PRE) and N resorption efficiency (NRE) were calculated by determining the concentration of P or N in mature green needles (PG, NG) and senesced needles (PS, NS) [36,37,38]. These calculations were based on a mass loss correction factor (MLCF) of 0.745 for coniferous species, as suggested by Vergutz [39]. The formulas were as follows:
PRE (%) = (1 − PS × MLCF/PG) × 100%
NRE (%) = (1 − NS × MLCF/NG) × 100%
2.3. Data Analysis
All data were statistically analyzed and plotted using R 4.2.3 software (http://www.r-project.org/ (accessed on 15 March 2023)). Statistical analysis initially used exploratory data analysis (EDA) to provide tests on distribution and variance homogeneity, and generalized linear mixed models (GLMM) to confirm differences between nutrient composition of plant components. In the EDA process, the normality of the data was tested using the Shapiro–Wilk test, and the homogeneity of variances was assessed using the Bartlett test to ensure the data met the assumptions for further analysis. Linear mixed-effects model (LMM) out of GLMM was adopted to analyze the effects of P treatment on the biomass, N and P concentrations, N:P ratio, and NSC and its components concentrations, as well as the effects on nutrient reserves, NSC reserves, PRE, NRE, and NRE:PRE, with the repetition block regarded as a random factor. One-way analysis of variation (ANOVA) was conducted to investigate the differences in biomass, nutrient concentration, nutrient reserve, NSC concentration, and NuRE of seedlings under different P treatments. Post hoc comparisons were performed using the least significant difference (LSD) test.
3. Results
3.1. Variations in Biomass of Different Components with Substrate Phosphorus Treatment
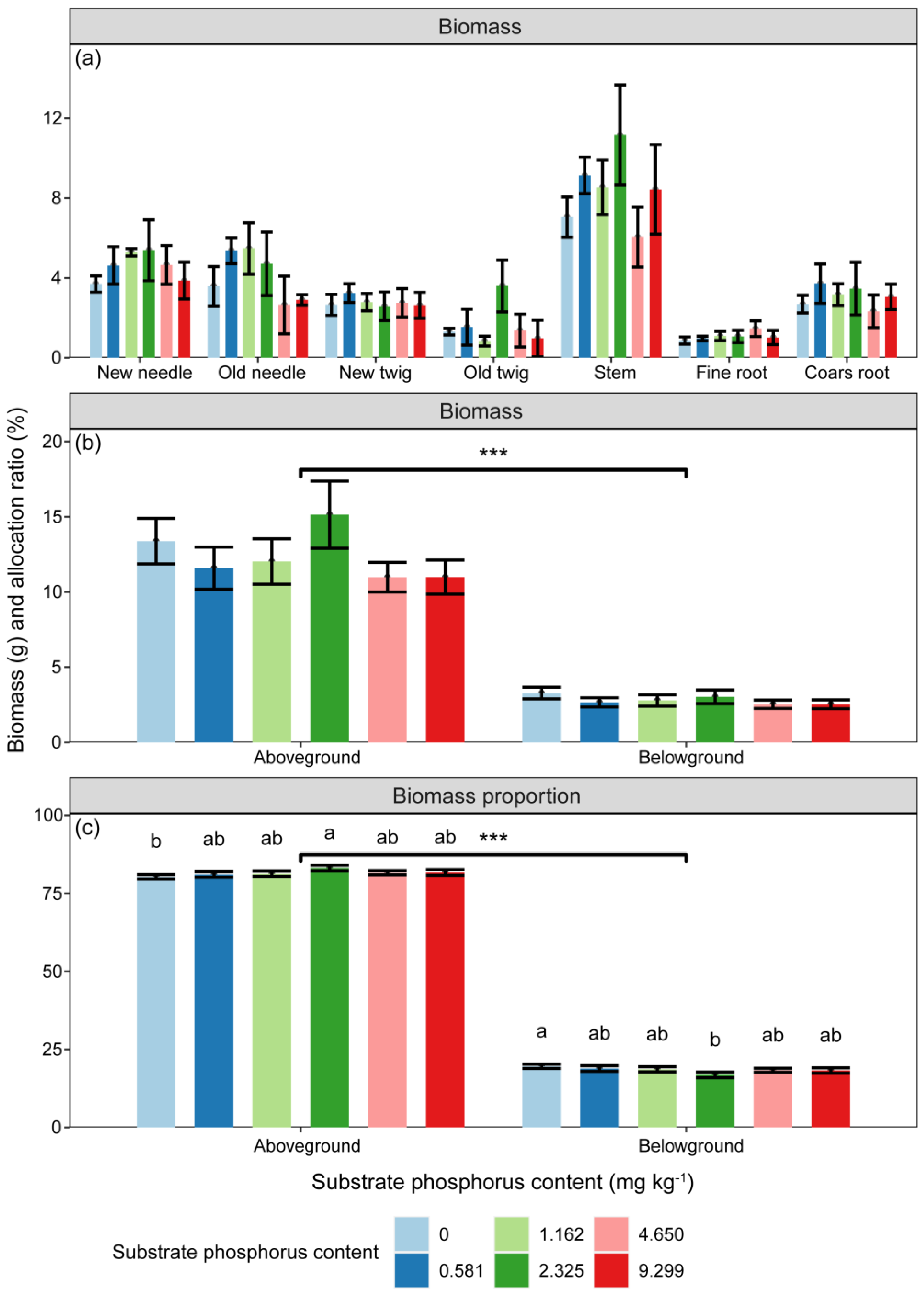
The biomass of P. massoniana seedlings was significantly influenced by substrate P treatment and component types (Table 2, p < 0.05). However, the differences in biomass among P treatments in any component did not reach a significant level (Figure 1, p > 0.05). The biomass of different components varied with increasing substrate P content. The biomass of new needles, old needles, and stems increased and then decreased with increasing substrate P content, while that of new twigs and coarse roots decreased. The total biomass reached its highest value in the 1/2 AP and AP treatments (Figure 1). Aboveground biomass was higher than belowground biomass (p < 0.001), but no difference among treatments was observed (Figure 1). However, there were significant differences in biomass allocation ratios among P treatments (Figure 1, p < 0.05). The allocation proportion of biomass to aboveground showed an increasing trend with substrate P content, while the highest allocation to belowground biomass occurred under 0 AP (Figure 1).

Table 2.
Results (F-value) of one-way analysis of variation (ANOVA) of substrate phosphorus treatment, component types and their interactions on biomass, nutrient concentration, nutrient reserve, stoichiometric ratio, non-structural carbohydrates concentration, non-structural carbohydrates reserve, and nutrient resorption efficiency. Significance levels are indicated by * (p < 0.05), ** (p < 0.01), and *** (p < 0.001).
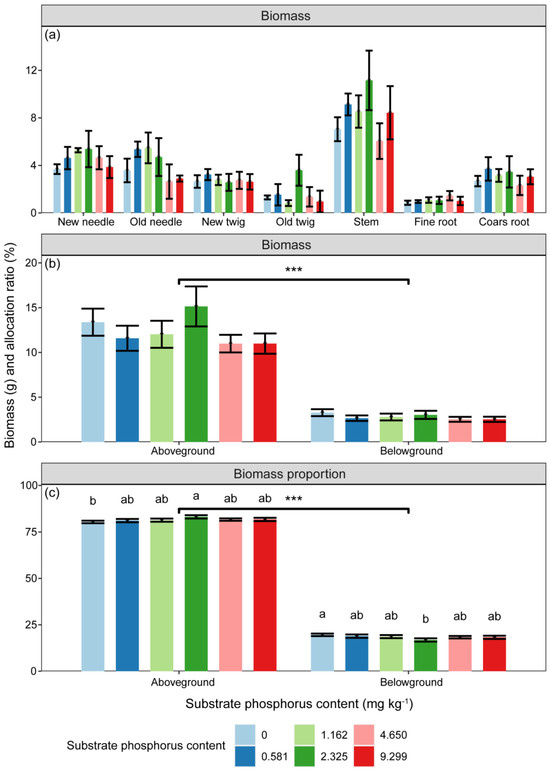
Figure 1.
Variations in (a,b) component biomass (g) and (c) aboveground and belowground biomass allocation ratios (%) with substrate phosphorus treatment. The differences in biomass and biomass allocation ratio between substrate phosphorus treatments are tested by LSD method, and the significant differences at the 0.05 level are marked with lowercase letters. Significant relationships between aboveground and belowground parts are denoted with an asterisk (p < 0.001, ***).
3.2. Changes in the Concentration of N, P, and NSC from Different Components with Substrate Phosphorus Treatment
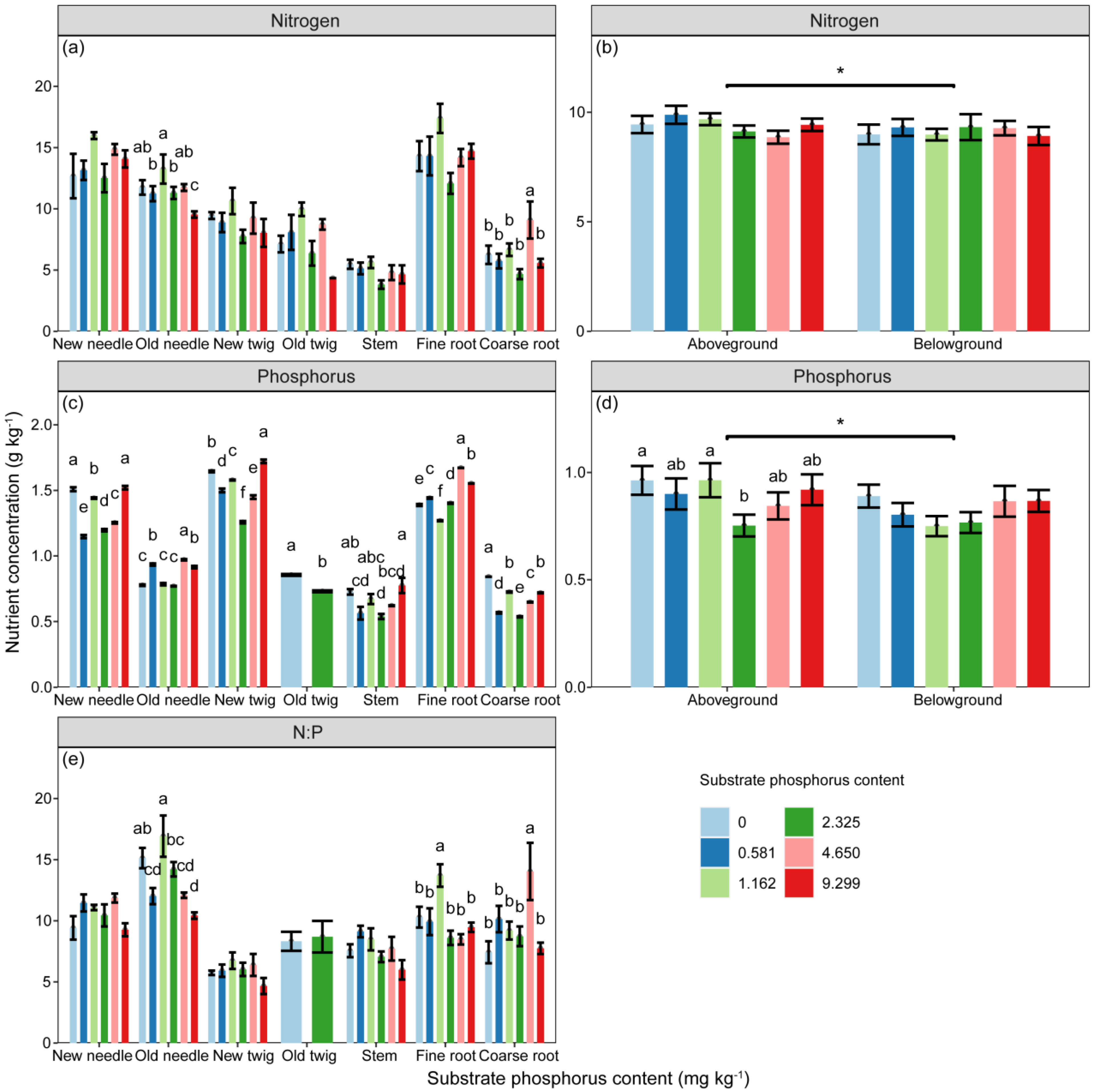
The concentrations of N and P, as well as the ratio of N:P in P. massoniana seedlings, were significantly influenced by substrate P treatment and component types (Table 2, p < 0.05). Additionally, component P concentration and N:P ratio were further affected by the interaction between substrate P treatment and component types (Table 2, p < 0.01). The P concentration of fine roots followed an increasing–decreasing trend, with the highest concentration observed in the 2 AP treatment (Figure 2). Comparing the nutrient concentrations of aboveground and belowground parts, the component N and P concentrations of aboveground parts were significantly higher than those of belowground parts (Figure 2, p < 0.05). The P concentration of components in aboveground parts initially decreased and then increased with increasing substrate P content, with the AP treatment showing the lowest P concentration in components (Figure 2).
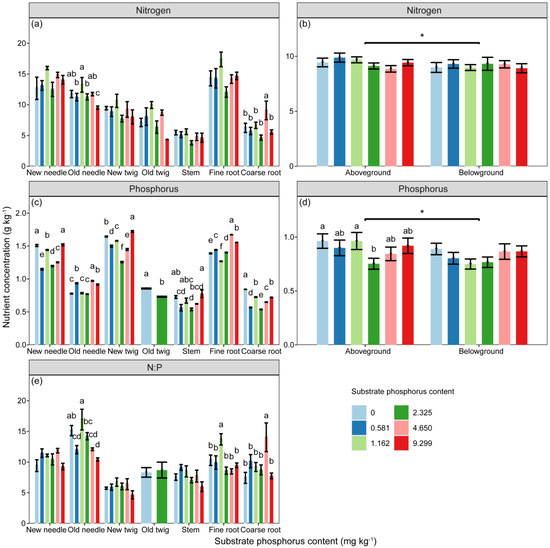
Figure 2.
The concentrations of (a,b) nitrogen (N, g·kg−1), and (c,d) phosphorus (P, g·kg−1) in components, as well as the stoichiometric ratio of (e) N:P, vary with substrate phosphorus treatment. The differences in component N, P concentrations and N:P among substrate phosphorus treatments are examined using the LSD method, and significant differences at the 0.05 level are indicated by lowercase letters. The significance of differences in nutrient concentrations between aboveground and belowground parts is denoted by asterisks (p < 0.05, *).
The differences in N:P ratio due to substrate P treatment were only significant in old needles and roots, while the differences in other components did not reach a significant level (Figure 2). The order of N:P ratio from highest to lowest was as follows: old needles (10.423–16.922) > new needles (9.268–11.858) > fine roots (8.485–13.705) > coarse roots (7.427–14.037) > old twigs (8.324–8.697) > stem (5.991–9.123) > new twigs (4.667–6.739).
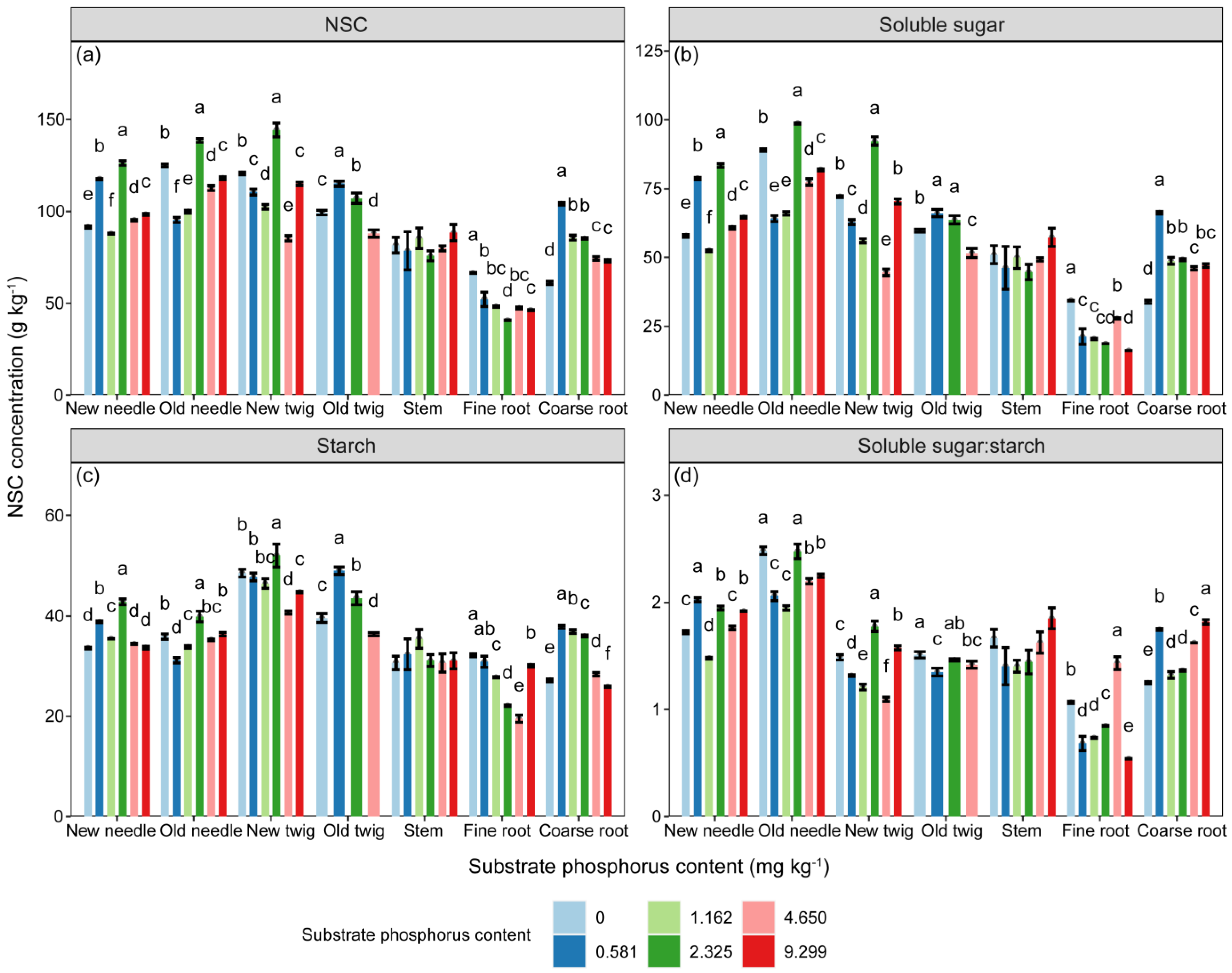
The concentrations of NSC, soluble sugar and starch, and the ratio of soluble sugar to starch in P. massoniana seedlings were significantly influenced by the substrate P treatment, component types, and their interaction (Table 2, p < 0.001). However, as shown in Figure 3, there was no significant differences in the NSC, soluble sugar, and starch concentrations of stem among different substrate P treatments. The concentrations of NSC and soluble sugar in needles, as well as the concentration of starch in old needles, increased with increasing substrate P content, but those in new twigs and roots showed the opposite trend. The concentrations of NSC and its components in aboveground parts were higher than those of belowground parts (Figure S1, p < 0.001). Only the starch concentration of aboveground parts decreased and then increased with increasing substrate P content, reaching its lowest point in the AP treatment (Figure S1). The soluble sugar to starch ratio in new needles increased but in fine roots decreased with increasing substrate P content.
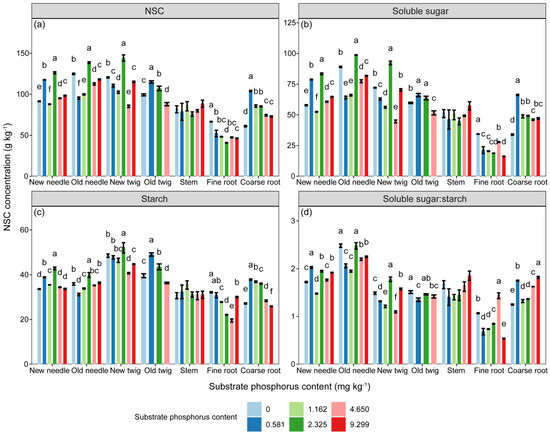
Figure 3.
The variations in (a) NSC (g·kg−1), (b) soluble sugar (g·kg−1), and (c) starch (g·kg−1) concentrations and (d) ratio of soluble sugar to starch in different components among different substrate phosphorus treatments are examined. The differences in NSC, soluble sugar, and starch concentrations among substrate phosphorus treatments are tested using the LSD method, and significant differences at the 0.05 level are indicated by lowercase letters.
3.3. Changes in Reserve Size of Nutrient and NSC with Substrate Phosphorus Treatment
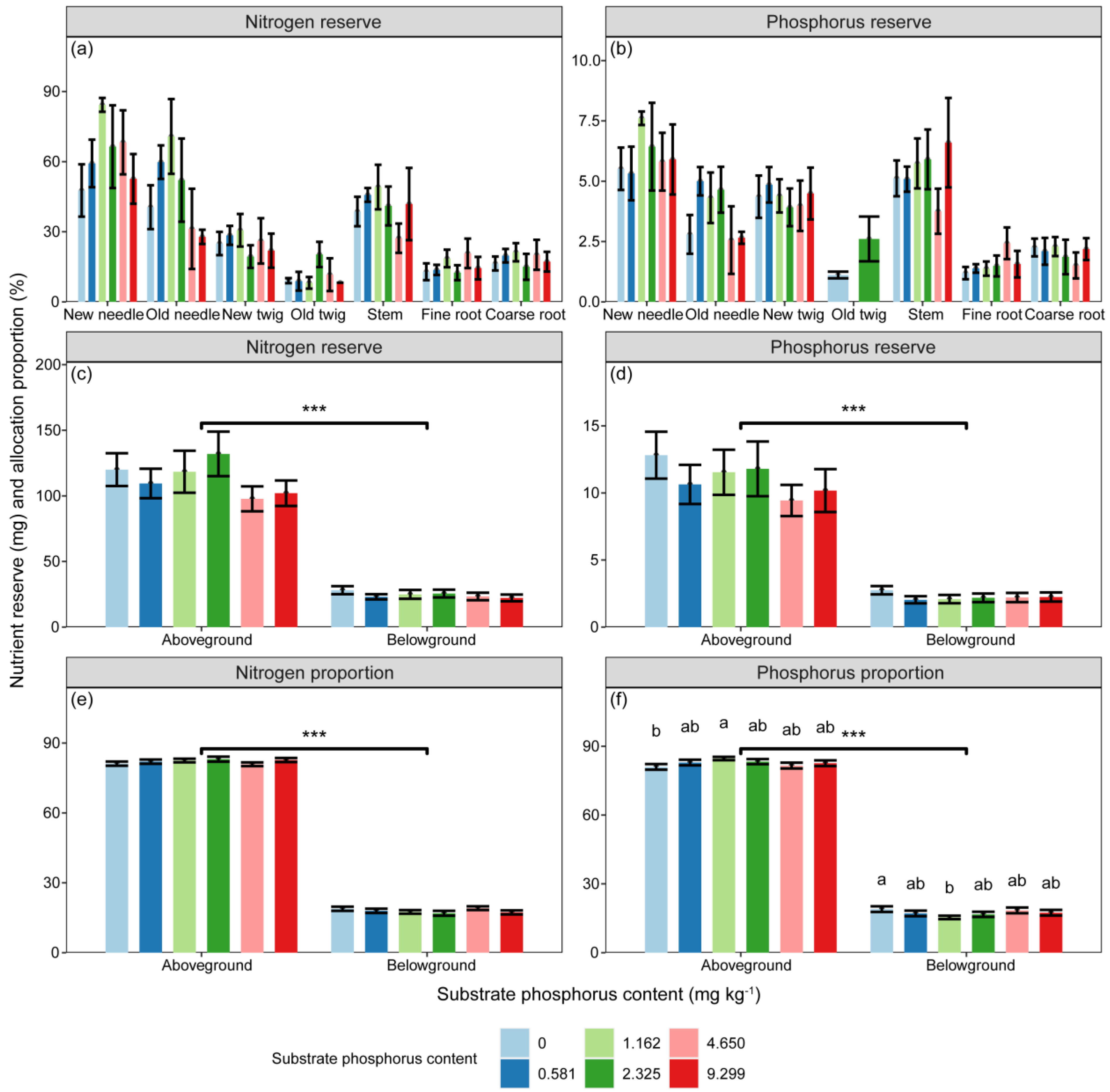
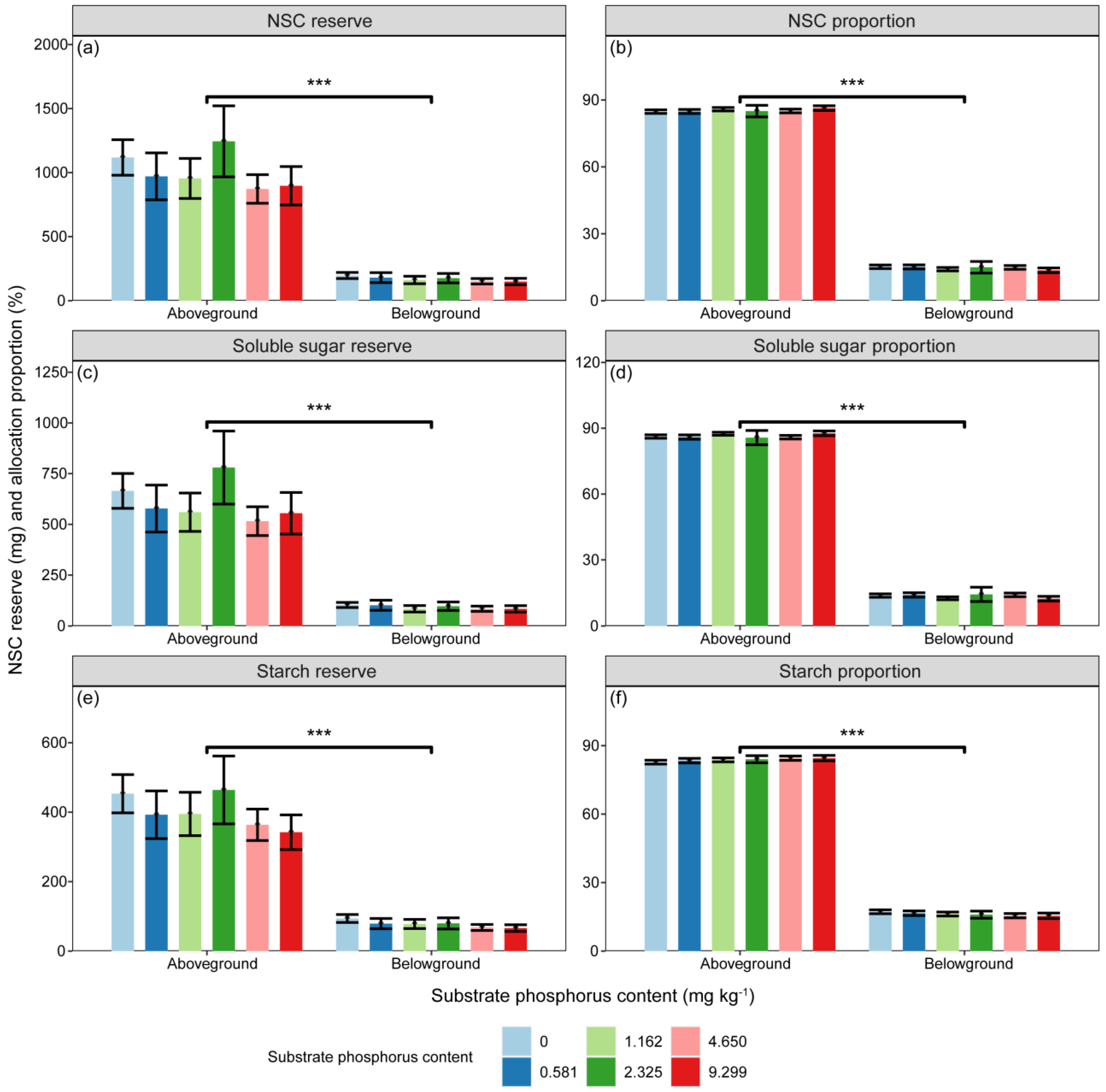
The reserves of N, NSC, soluble sugar, and starch were significantly influenced by substrate P treatment and component types (Table 2, p < 0.05). However, the P reserve was only significantly affected by component types (Table 2, p < 0.001). The differences in N and P reserves among different substrate P treatments did not reach a significant level. There were significant differences in the proportions of N, P, NSC, soluble sugar, and starch reserves between aboveground and belowground (Figure 4 and Figure 5, p < 0.001).
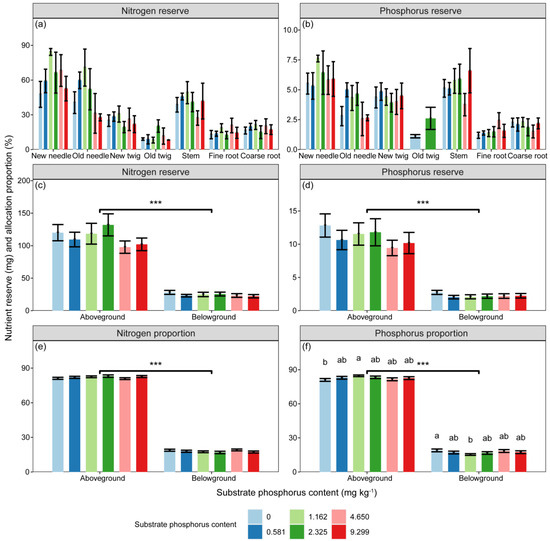
Figure 4.
The variations in (a,c) N (mg) and (b,d) P (mg) reserves, as well as the (e,f) proportions of nutrient reserves (%) in different components, as well as aboveground and belowground parts, with different substrate phosphorus treatments are examined. The differences in N and P reserves and nutrient reserve proportions among substrate phosphorus treatments are tested using the LSD method, and significant differences at the 0.05 level are indicated by lowercase letters. The significance of differences in nutrient reserves between aboveground and belowground parts is denoted by asterisks (p < 0.001, ***).
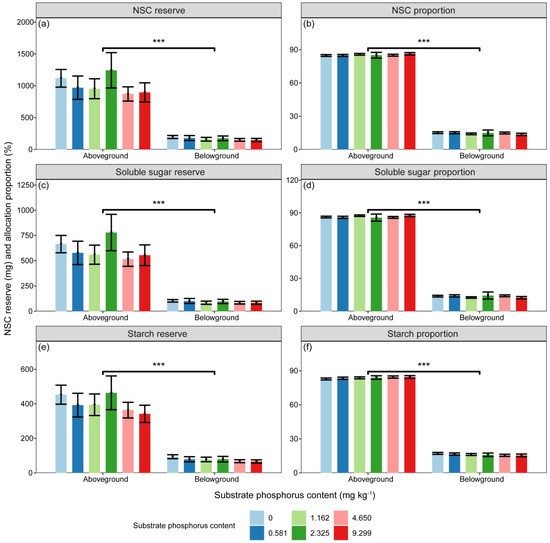
Figure 5.
The variations in (a) NSC (mg), (c) soluble sugar (mg), (e) and starch (mg) reserves, as well as the proportions of (b) NSC (%), (d) soluble sugar (%), and (f) starch (%) reserves in aboveground and belowground parts with different substrate phosphorus treatments are examined. The differences in NSC, soluble sugar, starch reserves, and NSC reserve proportions among substrate phosphorus treatments are tested using the LSD method, but did not reach a significant level. The significance of differences in substance reserves between aboveground and belowground parts is denoted by asterisks (p < 0.001, ***).
The proportions of P reserves in relation to the total P reserves of seedlings significantly varied under different substrate P treatments in both aboveground and belowground parts. The proportions of P reserve in aboveground part initially increased and then decreased with increasing substrate P content, reaching the maximum values under the 1/2 AP treatment (Figure 4). In contrast, the proportions of P reserve in belowground part showed the opposite trend, with the minimum values observed under the 1/2 AP treatment (Figure 4).
3.4. Nutrient Resorption Efficiency of Nitrogen and Phosphorus in Needles
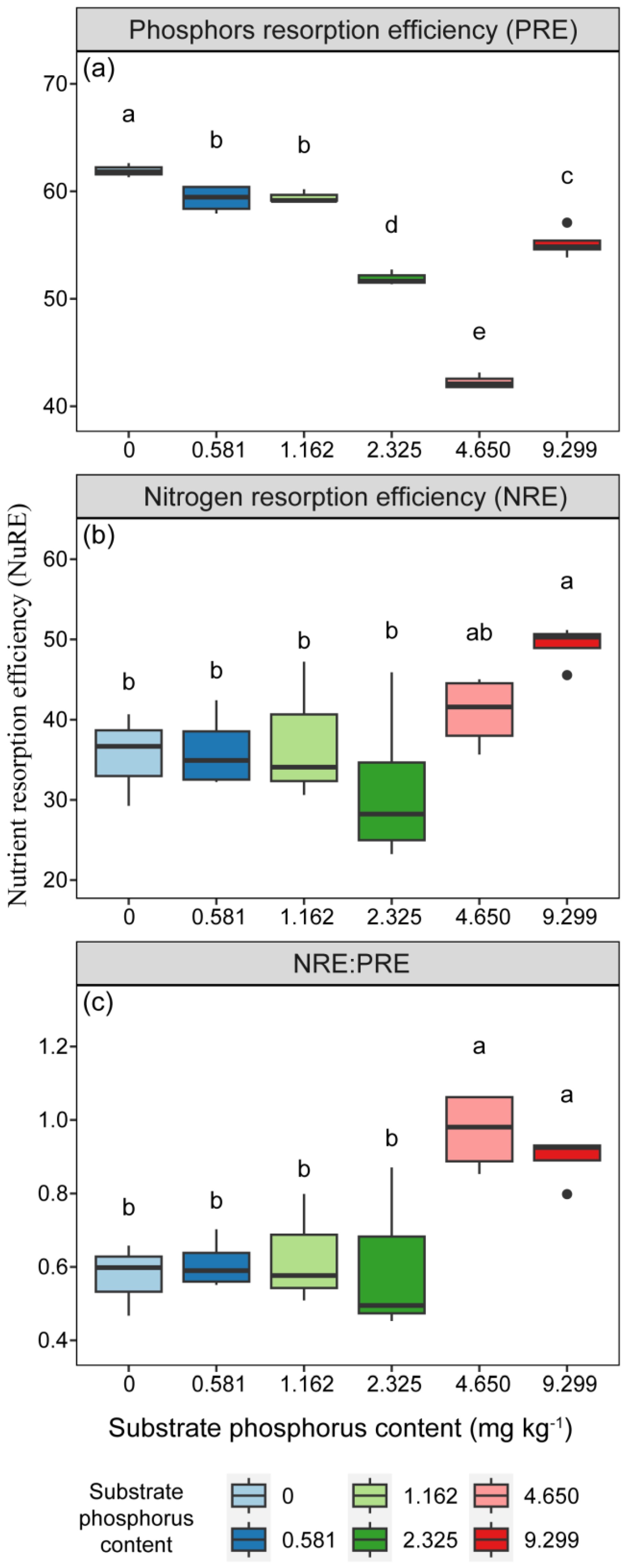
The resorption efficiencies of N and P in needles were significantly influenced by P treatment (Table 2, p < 0.05). The PRE exhibited a decreasing trend with increasing substrate P content, except for the 4 AP treatment, which showed an increase with P addition (Figure 6). The PRE of the 0 AP treatment was higher than that of the other treatments (Figure 6, p < 0.05). The NRE of needles, increased with increasing substrate P content, was lower than the PRE. The NRE under the 4 AP treatment was significantly higher than that of the other treatments (Figure 6, p < 0.05). The NRE to PRE ratios under P addition treatments (2 AP, 4 AP) were significantly higher than that under treatments with lower or equal to actual mean soil P content (Figure 6, p < 0.05).
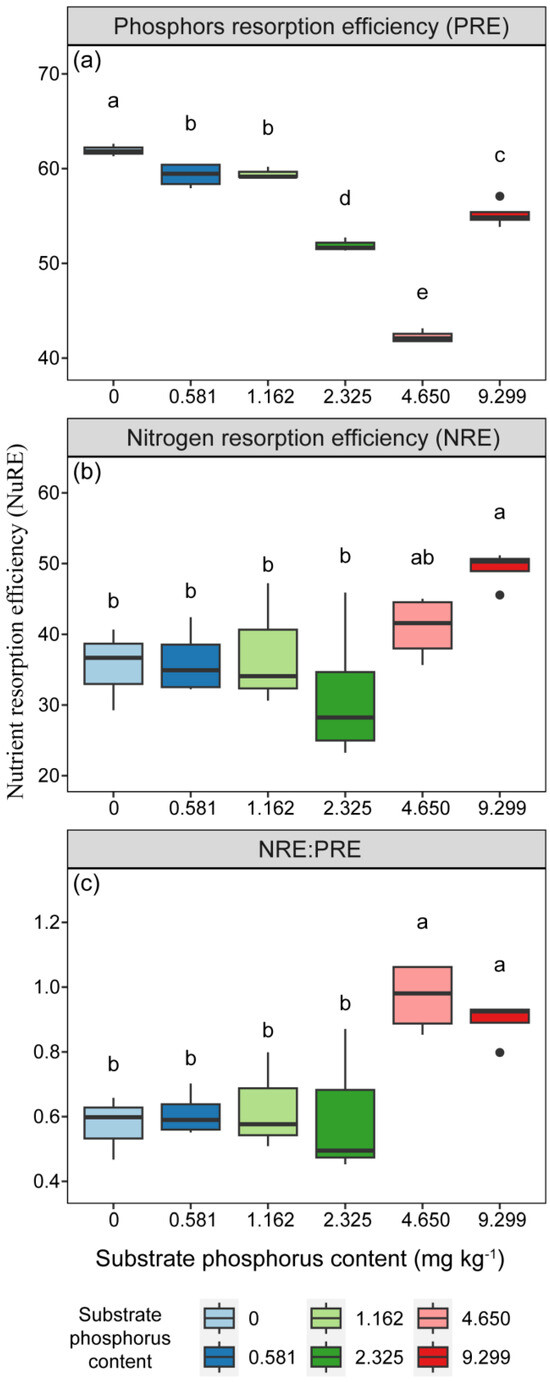
Figure 6.
The variations in (a) phosphorus and (b) nitrogen resorption efficiencies and (c) the ratio of nitrogen resorption efficiency to phosphorus resorption efficiency of needles with different substrate phosphorus treatments are examined. The differences in nutrient resorption efficiencies among different treatments are tested using the LSD method. Lowercase letters are used to indicate significant differences among treatments at the 0.05 level. PRE is short for phosphorus resorption efficiency, and NRE is short for nitrogen resorption efficiency.
3.5. Correlation between Substrate Phosphorus Treatment and Component Nutrient, NSC, and Resorption Efficiencies
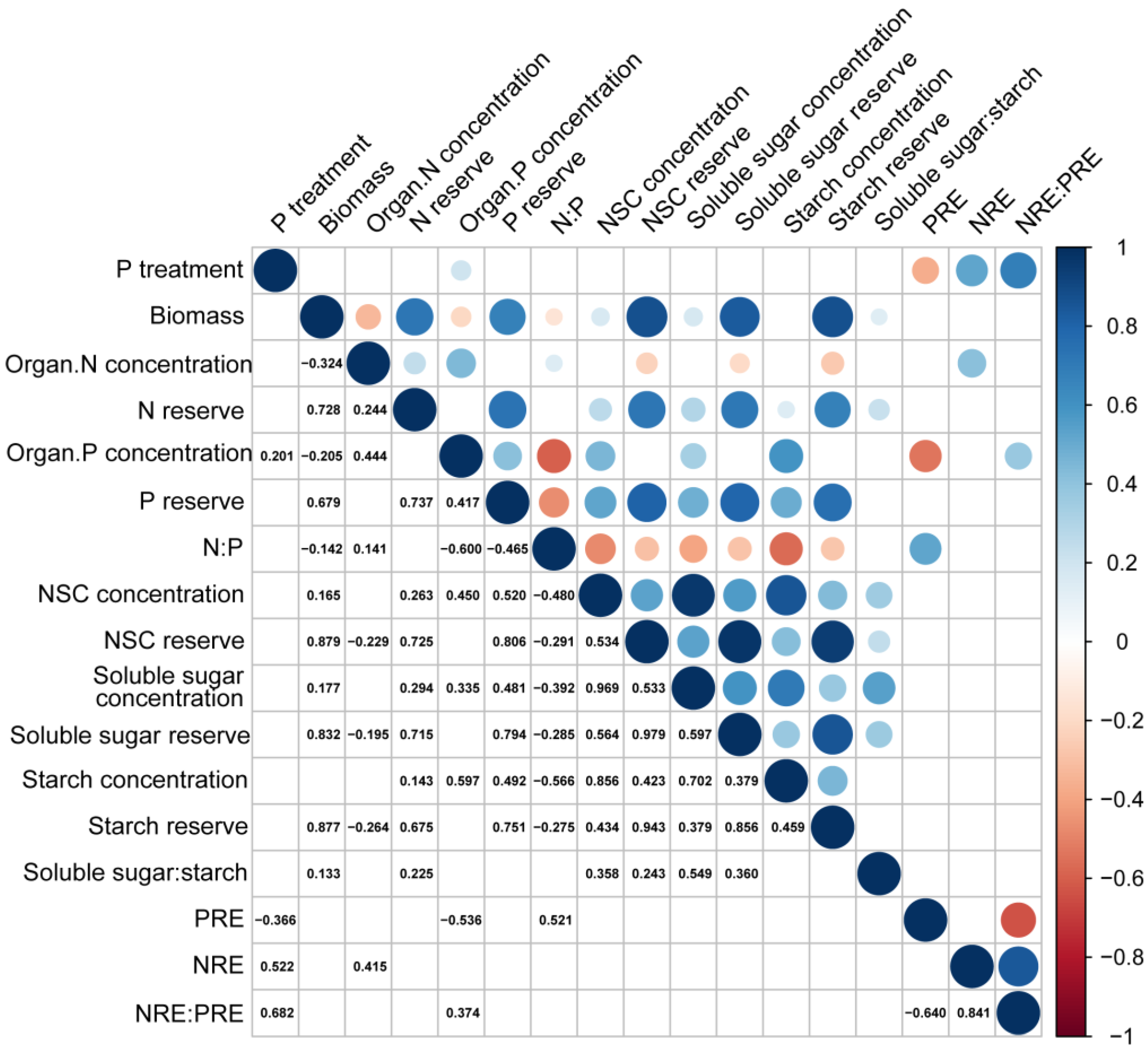
Component P concentration and the NRE were positively correlated with substrate P treatment (Figure 7, p < 0.05). Seedling biomass was positively correlated with NSC and its component concentrations but exhibited a negative correlation with N and P concentration of seedlings (Figure 7, p < 0.001). The N:P ratio was negatively correlated with NSC and its component concentrations (Figure 7, p < 0.001). PRE was negatively correlated with substrate P treatment and component P concentrations, but positively correlated with N:P ratio (Figure 7, p < 0.001). In addition, a strong positive correlation existed between the NRE: PRE ratio and substrate P treatment (Figure 7, p < 0.001).
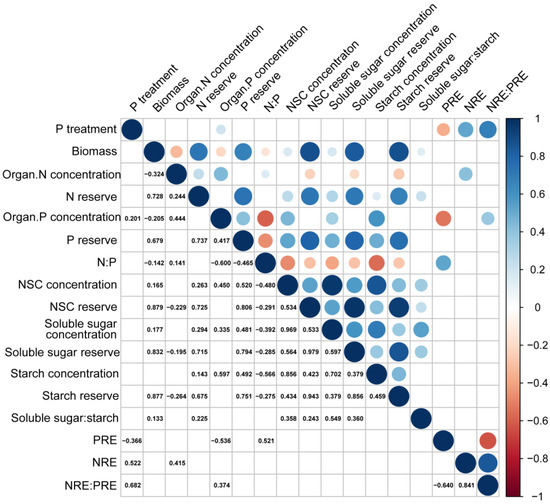
Figure 7.
Correlation among substrate phosphorus treatment, biomass, nutrient concentration, NSC concentration, and nutrient resorption efficiency of P. massoniana seedlings. In the figure, significant correlations are depicted as circles in the upper triangle. The size of each circle corresponds to the magnitude of the relationship coefficient, with red indicating a negative correlation and blue indicating a positive correlation (p < 0.05). The lower triangle displays the significant relationship coefficients.
4. Discussion
4.1. Effect of Low Phosphorus on Biomass Allocation in Different Components
In our study, the biomass allocation proportions showed significant differences among substrate P treatments, and with increasing substrate P content, the allocation of aboveground biomass tended to increase (Table 2, Figure 1). This was consistent with our previous study results, which found that P. massoniana seedlings tended to allocate more biomass proportion to fine roots under low P conditions, although biomass did not exhibit significant differences along the P gradient [40]. Although the allocation of plant biomass to underground increased as substrate P decreased, the overall biomass of seedlings under the condition of P deficiency was smaller than that in the P-rich environment. The increase in the allocation and the decrease in biomass weakened the difference of biomass change. The insignificant change may be caused by the accumulation of biomass [41] and the decline of root/shoot ratio [42] due to increased soil P content often occurring simultaneously.
We also found that the allocation of biomass was more of a priority to new needles than old needles (Figure 1), suggesting that P. massoniana seedlings would allocate resources to high-active components to increase individual growth. The overall biomass of needles and whole seedling reached its maximum in the AP treatment. However, an inhibition phenomenon in biomass was observed when the P treatment exceeded the realistic forest soil content, consistent with similar biomass inhibition under high P conditions, as reported by Mariotte [43]. In general, plants tend to grow in the optimal environment with the increase in available resources, but when exceeding the optimal conditions, the growth enters a stagnant period or decline. The detailed reason for this needed to be further study. Our results suggest that the current nutrient conditions may represent the optimal growth conditions for P. massoniana seedlings as biomass production has reached its maximum.
4.2. Effect of Low Phosphorus on Nutrient Concentation and Its Reserve
Nutrient concentration is a widely employed indicator of plant nutrient status, with leaves and roots, as vital organs for photosynthesis and nutrient absorption, being closely associated with plant productivity [22,44]. In our study, the mean concentrations of N (13.873 g·kg−1) and P (1.345 g·kg−1) in new needles were higher than those in the old needles, with values of 11.392 g·kg−1 and 0.866 g·kg−1, respectively, demonstrating that the P. massoniana seedling obtained nutrients mainly for new needles, which supported the nutrient concentration control strategy [45]. The concentrations of N and P in needles were also similar with the value (12.13 g·kg−1 and 1.37 g·kg−1) reported by Tian [21], which conducted a statistical analysis of nutrient concentration in global coniferous species, but higher than the values of Chinese coniferous species [20]. In fact, there were large differences among individuals, especially in P concentration, which could differ by more than 100-fold, and N concentration could vary by up to 30-fold [21]. In our previous study, the soil P content in the major distribution region of the P. massoniana plantation in China was classified as an “extremely deficient” level [30]. The low soil P content may be an important factor to the lower leaf P concentration in Chinese conifers compared to the global average.
The root system plays a key role in the absorption of nutrient sources from the soil, serving as both a source and reservoir for plants, especially for fine roots with a diameter ≤ 2 mm [46]. The previous studies reported that the global average of N and P concentrations in fine roots ranged from 10.2 to 11.0 g·kg−1 and 0.83 to 0.9 g·kg−1 [22,47], but the mean value of Chinese plants was 9.2 g·kg−1 and 1.0 g·kg−1 [23], respectively. In our study, the concentrations of N and P in fine roots, ranging from 12.074 to 17.386 g·kg−1 and 1.268 to 1.672 g·kg−1, respectively, were higher than the value of global and Chinese plants. Furthermore, the nutrient concentrations in coarse roots were also relatively higher than that of Chinese plants [23]. This may be because broad-leaved trees have a lower nutrient content in the roots than coniferous trees, owing to their larger leaf area obtaining natural light energy to support their growth.
Nutrient translocation between organs is an important strategy in solving nutrient deficiency [48,49,50,51]. Variations in nutrient concentration between new and old components (Figure 2 and Figure 4), that is, active and inactive components, indicated nutrient translocation in P. massoniana seedlings. Scarce elements can also be transferred from other components to roots that need to expand rapidly to increase their ability to absorb nutrients. In this study, there was no consistent pattern observed among different treatments and components, especially when examining the aboveground and belowground element concentrations (Figure 2). While aboveground nutrient concentration was significantly higher than belowground (p < 0.05), nutrient concentrations in both aboveground and belowground components still were lightly impacted by substrate P treatment, resulting in relatively stable nutrient shifting. This suggests that P. massoniana has the ability to maintain a relatively stable nutrient composition in a changing environment, which was in accordance with the Stoichiometric Homeostasis Theory [17]. Our findings were consistent with the results of Guo [51], which indicated a homeostatic adjustment in P concentration in P. massoniana.
In addition, there was a certain similarity in the changes in nutrient concentration and reserve observed among different treatments (Figure 2 and Figure 4). For example, in our study, the concentrations of N and P in needles and roots (Figure 2), as well as needle reserves (Figure 4), increased and then decreased as substrate P content increased, reaching their maximum values nearly at 1/2 AP or AP treatments. This suggests that from a nutrient concentration perspective, the current soil nutrient conditions in the distribution region of P. massoniana may be the optimal conditions for our study species. In addition, our findings indicated that the needles of our study species would maximize the storage nutrient resources to ensure the individual growth needs. However, different from the changes in the needle reserve, the root P reserve sightly decreased and then increased with increasing substrate P content (Figure 4). The possible reasons for the ability of P. massoniana seedling roots to maintain a higher P reserve under 0 AP, 2 AP, and 4 AP treatment conditions could be as follows: In a low P environment, plants tend to prioritize nutrient allocation to the most demanding components and perform energy-intensive activities related to P. Although nutrient concentration may not necessarily change significantly, accumulation over time may lead to an increase in the size of the P reserve. Conversely, in a P sufficient environment, other components allocated fewer nutrients to the root system, and the root system relied more on its own absorption capacity to accumulate a higher nutrient reserve.
4.3. The N:P Stoichiometric Characteristics under Low Phosphorus Conditions
The N:P ratio is also commonly used to indicate whether plants are limited by N or P [16]. However, an increasing number of studies have questioned the universality of N:P thresholds in determining nutrient limitation [52,53,54], as different vegetation types and growth stages may have different N:P thresholds for nutrient limitation [19,55,56]. In this study, the average N:P ratios in new needles, old needles, fine roots, and coarse roots were 10.588, 13.277, 10.081, and 9.548, respectively, which were lower than the mean value of global and Chinese conifers [20,21,22,23]. According to the 14/16 threshold, at the community level, leaf N:P ratios greater than 16 indicate P limitation, N:P ratios less than 14 indicate N limitation, and 14–16 indicate N-P co-limitation [16]. If we follow this hypothesis, in our study, P. massoniana was almost limited by N, not P. However, we also found that the N concentrations of needles and roots were higher than the average level in all treatments (Figure 2). This contradictory result may indicate that the 14/16 threshold is not suitable for characterizing nutrient limitation for P. massoniana seedlings. However, this study was unable to provide an appropriate threshold for determination, and it is necessary to redefine the applicable threshold for P. massoniana based on more research.
Additionally, the study results will provide a new insight that categorizing leaves of different ages into uniform groups may lead to errors in determination, particularly in studies concerning nutrient limitation. Plants experiencing nutrient stress undergo nutrient transfer, with nutrients being transferred from senescent tissues to actively growing parts [48,49,50,51]. It is necessary to assess the rate and timing of nutrient transfer, especially in studies of nutrient adaptation mechanisms. Leaf samples used for nutrient determination are typically well-developed upper leaves, mainly newly grown leaves of the current year; however, elder leaves are expected to respond to nutrients more rapidly [57]. Different components of plants and different ages of the same component can affect the nutrient concentration and stoichiometric characteristics of plants [48,49,50]. Therefore, taking into account the root system, leaf sampling should also be more finely divided for accurate diagnosis.
As leaves are the actively growing tissues, their metabolic functions rely on a relatively stable nutrient composition [51,57]. Therefore, when the external nutrient environment changes, leaves exhibit greater internal stability compared to other organs [57,58], especially in evergreen tree species that experience long-term growth in relatively nutrient-poor habitats [59]. They may develop certain nutrient adaptation mechanisms, resulting in less sensitivity to fertilization effects [52,59]. The internal stability of plant ecological stoichiometry is an important mechanism for maintaining ecosystem structure, function, and stability [60]. The high internal stability of evergreen tree species [61] may also be a reason for the insignificant N:P changes in this study species.
Surprisingly, in our study, although there were no significant changes in the N:P ratio of needles, there were noticeable differences in the ratio of NRE to PRE (Table 2, Figure 6). We thought that P addition alleviated the limitation of P in P. massoniana seedlings, resulting in a decrease in PRE of needles. P addition may lead to relative inhibition of N [6,7], leading to an increase in NRE in our study (Figure 6). Our findings aligned with the “nutrient limitation control” strategy, which suggested that the resorption capacity of plants depends on their nutrient limitation status [26,62]. Specifically, the results of NRE: PRE revealed significant variations between treatments with P addition (2 AP, 4 AP) and other treatments (Figure 6), indicating a rapid decline in the demand for P resorption as the P content in the substrate increased. NuRE differences reflected alterations in nutrient utilization strategies in P. massoniana. Therefore, the lack of P limitation characteristics in the elemental stoichiometric ratio of N:P suggests that NRE: PRE may be more sensitive in representing soil nutrient content than the N:P ratio. Although few studies have investigated this phenomenon in low P environments, some related results can be found in certain studies. Chen (2021) demonstrated that the N:P ratio of leaves in the P-limited system (non-karst forest, N:P = 20.89) was 1.89 times higher than that of the high P system (karst forest, N:P = 11.06) [26]. Furthermore, the NRE: PRE of the high P system (1.2) was 2.4 times higher than that of the P-limited system (0.5) [26]. In the study conducted by Zhang (2022), changes in the NRE: PRE of the community were observed in response to variations in community P concentration, and these changes were even more significant than those observed in the N:P ratio [63]. These findings provided additional support for our findings.
4.4. Effect of Low Phosphorus on NSC Allocation in Different Components
NSC plays a crucial role in carbon assimilation and consumption during plant growth processes [64,65], but plants also provide NSCs, a pivotal photosynthetic product to flexibly adjust their strategies in response to resource limitation [66]. In our study, the response of NSC to P was mainly manifested in components with vigorous physiological activities. For example, the NSC concentration and its components in different components varied among P treatments, but that in the stems and twigs were relatively stable (Figure 3 and Figure 5). This is also in agreement with the vitality characteristics of leaves and roots as plant growth energy-obtaining organs [9,40,58]. In addition, the NSC concentration of needles increased with the substrate P addition, and that of roots in high substrate P treatments was higher than in low substrate P treatments (Figure 3 and Figure S1). We suggested that substrate P promoted the accumulation of substances and enhanced photosynthetic absorption [14]. However, the decrease in root substance in high substrate P conditions may attributed to decreased demand for active absorption from nutrient-rich environments and more substances are kept in other components for storage or other elevated activities.
The ratio of soluble sugar to starch in needles was significantly higher than that in roots (Figure 3), indicating clear differences in the utilization and storage of NSCs between these two components. The NSC was primarily present in needles in the form of soluble sugar, which can directly provide energy substrates for photosynthesis. In contrast, the NSC was predominantly stored in the form of starch in the roots. Starch molecules are larger and can store more carbon, providing sufficient energy resources for potential nutrient deficiencies in the future [40]. These differences may be attributed to variations in organ functions, leading to divergent adaptive strategies of different plant organs in response to P limitations.
5. Conclusions
In this study, the biomass, nutrient concentrations, NSC and its components, and nutrient resorption efficiency of Pinus massoniana seedlings were significantly affected by substrate phosphorus treatment. Owing to the adaptation and regulation mechanisms of plants to low P environments, the nutrient concentration and its reserve remained relatively stable, indicating the presence of homeostatic adjustment in P. massoniana seedlings. NSCs existed more in the form of soluble sugar in needles and starch in roots to cope with limited P conditions, while physiologically inactive stems and twigs also maintained relative stability. Apart from nutrient transfer in old needles, the components of P. massoniana seedlings were capable of maintaining higher nutrient concentrations to ensure their survival. In addition, we hold the opinion that the N:P ratio was not sufficiently sensitive to determine nutrient limitation in P. massoniana seedlings, whereas the NRE: PRE ratio served as a better indicator.
Overall, our findings highlight the remarkable adaptive strategies employed by P. massoniana seedlings in response to low P conditions. This understanding can be helpful in making informed decisions regarding nutrient management and conservation strategies to support the growth and survival of this important species. Hopefully, our study can provide valuable insights into the physiological responses of plants to P limitation in subtropical areas and stimulate further research in this field.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15020351/s1, Figure S1: The variations in (a) NSC (g·kg−1), (b) soluble sugar (g·kg−1), and (c) starch (g·kg−1) concentrations in aboveground and belowground parts with different substrate phosphorus treatments are examined. The differences in NSC, soluble sugar, and starch concentrations among substrate phosphorus treatments are tested using the LSD method, and significant differences at the 0.05 level are indicated by lowercase letters. The significance of differences in substance concentrations between aboveground and belowground parts is denoted by asterisks (p < 0.05, *; p < 0.01, **; p < 0.001, ***).
Author Contributions
Conceptualization, J.X., Y.N. and W.X.; Methodology, J.X. and X.D.; Software, J.X. and Z.J.; Validation, J.X., Y.N. and W.X.; Formal Analysis, J.X. and Y.Z.; Investigation, J.X., Z.J. and X.D.; Data Curation, J.X. and Y.Z.; Writing—Original Draft Preparation, J.X.; Writing—Review and Editing, Y.N., Z.J. and W.X.; Visualization, J.X. and Z.J.; Supervision, W.X. and L.Z.; Funding Acquisition, L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Chinese Academy of Forestry [grant numbers: CAFYBB2021QD002].
Data Availability Statement
The data presented in this study are contained within the article.
Acknowledgments
We are grateful to the Zigui Forest Ecosystem Research Station for providing the experimental site and experimental supports.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vance, C.P.; Uhde-Stone, C.; Alle, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, Q.W.; Li, K.H.; Gong, Y.M.; Liu, Y.Y.; Han, W.X. Patterns of nitrogen and phosphorus stoichiometry among leaf, stem and root of desert plants and responses to climate and soil factors in Xinjiang, China. Catena 2021, 199, 105100. [Google Scholar] [CrossRef]
- Liu, X.; Sheng, H.; Jiang, S.Y.; Yuan, Z.W.; Zhang, C.S.; Elser, J.J. Intensification of phosphorus cycling in China since the 1600s. Proc. Natl. Acad. Sci. USA 2016, 113, 2609–2614. [Google Scholar] [CrossRef]
- Gérard, F. Clay minerals, iron/aluminum oxides, and their contribution to phosphate sorption in soils—A myth revisited. Geoderma 2016, 262, 213–226. [Google Scholar] [CrossRef]
- Peñuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; van der Velde, M.; Bopp, L.; Boucher, O.; Godderi, Y.; Hinsinger, P.; Llusia, J.; et al. Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013, 4, 2934. [Google Scholar] [CrossRef]
- Yu, Z.P.; Wang, M.H.; Huang, Z.Q.; Lin, T.C.; Vadeboncoeur, M.A.; Searle, E.B.; Chen, H.Y.H. Temporal changes in soil C-N-P stoichiometry over the past 60 years across subtropical China. Glob. Chang. Biol. 2018, 24, 1308–1320. [Google Scholar] [CrossRef] [PubMed]
- Prietzel, J.; Falk, W.; Reger, B.; Uhl, E.; Pretzsch, H.; Zimmermann, L. Half a century of Scots pine forest ecosystem monitoring reveals long-term effects of atmospheric deposition and climate change. Glob. Chang. Biol. 2020, 26, 5796–5815. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Hui, D.F.; Dennis, S.; Reddy, K.C. Responses of terrestrial ecosystem phosphorus cycling to nitrogen addition: A meta-analysis. Glob. Ecol. Biogeogr. 2017, 26, 713–728. [Google Scholar] [CrossRef]
- Wright, S.J.; Turner, B.L.; Yavitt, J.B.; Hams, K.E.; Kaspari, M.; Tanner, E.V.J.; Bujan, J.; Griffin, E.A.; Mayor, J.R.; Pasquini, S.C.; et al. Plant responses to fertilization experiments in lowland, species-rich, tropical forests. Ecology 2018, 99, 1129–1138. [Google Scholar] [CrossRef]
- Chapin, F.S.; Schulze, A.E.; Mooney, H.A. The ecology and economics of storage in plants. Annu. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
- Richardson, A.D.; Carbone, M.S.; Keenan, T.F.; Czimczik, C.I.; Hollinger, D.Y.; Murakami, P.; Schaberg, P.G.; Xu, X.M. Seasonal dynamic and age of stem wood nonstructural carbohydrates in temperate forest trees. New Phytol. 2013, 197, 850–861. [Google Scholar] [CrossRef]
- Liu, J.F.; Arend, M.; Yang, W.J.; Schaub, M.; Ni, Y.Y.; Gessler, A.; Jiang, Z.P.; Rigling, A.; Li, M.H. Effects of drought on leaf carbon source and growth of European beech are modulated by soil type. Sci. Rep. 2017, 7, 42462. [Google Scholar] [CrossRef]
- Liu, M.H.; Wang, Y.X.; Li, Q.; Xiao, W.F.; Song, X.Z. Photosynthesis, ecological stoichiometry, and non-structural carbohydrate response to simulated nitrogen deposition and phosphorus addition in Chinese Fir forests. Forests 2019, 10, 1068. [Google Scholar] [CrossRef]
- Yu, L.; Song, M.Y.; Xia, Z.C.; Korpelainen, H.; Li, C.Y. Plant-plant interactions and resource dynamics of Abies fabri and Picea brachytyla as affected by phosphorus fertilization. Environ. Exp. Bot. 2019, 168, 103893. [Google Scholar] [CrossRef]
- Deng, X.X.; Xiao, W.F.; Shi, Z.; Zeng, L.X.; Lei, L. Combined effects of drought and shading on growth and non-structural carbohydrates in Pinus massoniana Lamb. seedlings. Forests 2020, 11, 18. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F. The vegetation N: P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002; Available online: http://www.jstor.org/stable/j.ctt1jktrp3 (accessed on 9 February 2024).
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Han, W.X.; Tang, L.Y.; Chen, Y.H.; Fang, J.Y. Relationship between the relative limitation and resorption efficiency of nitrogen vs. phosphorus in woody plants. PLoS ONE 2013, 8, e83366. [Google Scholar] [CrossRef] [PubMed]
- Han, W.X.; Fang, J.Y.; Guo, D.L.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef]
- Tian, D.; Yan, Z.B.; Niklas, K.J.; Han, W.X.; Kattge, J.; Reich, P.B.; Luo, Y.K.; Chen, Y.H.; Tang, Z.Y.; Hu, H.F.; et al. Global leaf nitrogen and phosphorus stoichiometry and their scaling exponent. Natl. Sci. Rev. 2018, 5, 723–739. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H.; Reich, P.B. Global-scale latitudinal patterns of plant fine-root nitrogen and phosphorus. Nat. Commun. 2011, 2, 344. [Google Scholar] [CrossRef]
- Ma, Y.Z.; Zhong, Q.L.; Jin, B.J.; Lu, H.D.; Guo, B.Q.; Zheng, Y.; Li, M.; Cheng, D.L. Spatial changes and influencing factors of fine root carbon, nitrogen and phosphorus stoichiometry of plants in China. Chin. J. Plant Ecol. 2015, 39, 159–166. [Google Scholar] [CrossRef]
- Aerts, R. Nutrient Resorption from Senescing Leaves of Perennials: Are there General Patterns? J. Ecol. 1996, 84, 597–608. [Google Scholar] [CrossRef]
- Wang, K.; Wang, G.G.; Song, L.N.; Zhang, R.S.; Yan, T.; Li, Y.H. Linkages between nutrient resorption and ecological stoichiometry and homeostasis along a chronosequence of Mongolian pine plantations. Front. Plant Sci. 2021, 12, 692683. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Reed, S.C.; Lü, X.T.; Xiao, K.C.; Wang, K.L.; Li, D.J. Coexistence of multiple leaf nutrient resorption strategies in a single ecosystem. Sci. Total Environ. 2021, 772, 144951. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.J.; Ni, Y.Y.; Lei, L.; Xu, J.; Xiao, W.F.; Zeng, L.X. Phosphorus is the key soil indicator controlling productivity in planted Masson pine forests across subtropical China. Sci. Total Environ. 2022, 822, 153525. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, C.B.; Teng, M.J.; Zhou, Z.X.; Wang, P.C. Net primary productivity of Pinus massoniana dependence on climate, soil and forest characteristics. Forests 2020, 11, 404. [Google Scholar] [CrossRef]
- Ni, Y.Y.; Jian, Z.J.; Zeng, L.X.; Liu, J.F.; Lei, L.; Zhu, J.H.; Xu, J.; Xiao, W.F. Climate, soil nutrients, and stand characteristics jointly determine large-scale patterns of biomass growth rates and allocation in Pinus massoniana plantations. For. Ecol. Manag. 2022, 504, 119839. [Google Scholar] [CrossRef]
- Jian, Z.J.; Ni, Y.Y.; Xu, J.; Lei, L.; Zeng, L.X.; Xiao, W.F. Soil fertility in the Pinus massoniana forests of China. Acta Ecol. Sin. 2021, 41, 5279–5288. [Google Scholar] [CrossRef]
- Epstein, E. Mineral Nutrition of Plants: Principles and Perspectives; John Wiley and Sons: New York, NY, USA, 1972. [Google Scholar]
- Ames, B.N. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 1996, 8, 115–118. [Google Scholar]
- Seifter, S.; Dayton, S.; Novic, B.; Muntwyler, E. The Estimation of Glycogen with the Anthrone Reagent. Arch. Biochem. 1950, 25, 191–200. [Google Scholar]
- Schönbeck, L.; Gessler, A.; Hoch, G.; McDowell, N.G.; Rigling, A.; Schaub, M.; Li, M.H. Homeostatic levels of nonstructural carbohydrates after 13 yr of drought and irrigation in Pinus sylvestris. New Phytol. 2018, 219, 1314–1324. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; He, N.P.; Yu, G.R. Nitrogen storage in China’s terrestrial ecosystems. Sci. Total Environ. 2019, 709, 136201. [Google Scholar] [CrossRef] [PubMed]
- Cartaxana, P.; Catarino, F. Nitrogen resorption from senescing leaves of three salt marsh plant species. Plant Ecol. 2002, 159, 95–102. [Google Scholar] [CrossRef]
- Wright, I.J.; Westoby, M. Nutrient concentration, resorption and lifespan: Leaf traits of Australian sclerophyll species. Funct. Ecol. 2003, 17, 10–19. [Google Scholar] [CrossRef]
- Yan, L.; Wen, Y.G.; Zhou, X.G.; Li, H.Y.; Wu, W.X.; Sunoj, V.S.J.; Lambers, H.; Finnegan, P.M. Adding Castanopsis hystrix to a Pinus massoniana plantation changed leaf phosphorus and nitrogen investment and soil nitrogen concentrations. Plant Soil 2023. [Google Scholar] [CrossRef]
- Vergutz, L.; Manzoni, S.; Porporato, A.; Novais, R.F.; Jackson, R.B. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol. Monogr. 2012, 82, 205–220. [Google Scholar] [CrossRef]
- Xu, J.; Lei, L.; Zeng, L.X.; Ni, Y.Y.; Jian, Z.J.; Deng, X.X.; Xiao, W.F. The responses of C allocation of new needle and fine root affected the phosphorus adaptation of Pinus massoniana seedlings. J. Soil Sci. Plant Nutr. 2023. [Google Scholar] [CrossRef]
- Alvarez-Clare, S.; Mack, M.C.; Brooks, M.E. A direct test of nitrogen and phosphorus limitation to net primary productivity in a lowland tropical wet forest. Ecology 2013, 94, 1540–1551. [Google Scholar] [CrossRef]
- Gutiérrez, G.V.; Pérez-Aviles, D.; Raczka, N.; Pereira-Arias, D.; Tijerín-Triviño, J.; Pereira-Arias, L.D.; Medvigy, D.; Waring, B.G.; Morrisey, E.; Brzostek, E.; et al. Throughfall exclusion and fertilization effects on tropical dry forest tree plantations, a large-scale experiment. Biogeosciences 2023, 20, 2143–2160. [Google Scholar] [CrossRef]
- Mariotte, P.; Cresswell, T.; Johansen, M.P.; Harrison, J.J.; Keitel, C.; Dijkstra, F.A. Plant uptake of nitrogen and phosphorus among 671 grassland species affected by drought along a soil available phosphorus gradient. Plant Soil 2020, 448, 121–132. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Sun, X.B.; Li, D.J.; Lü, X.T.; Fang, Y.T.; Ma, Z.L.; Wang, Z.C.; Chu, C.J.; Li, M.M.; Chen, H. Widespread controls of leaf nutrient resorption by nutrient limitation and stoichiometry. Funct. Ecol. 2023, 37, 1653–1662. [Google Scholar] [CrossRef]
- Jackson, R.B.; Mooney, H.A.; Schulze, E.D. A global budget for fine root biomass, surface area, and nutrient contents. Proc. Natl. Acad. Sci. USA 1997, 94, 7362–7366. [Google Scholar] [CrossRef]
- Gordon, W.S.; Jackson, R.B. Nutrient concentrations in fine roots. Ecology 2000, 81, 275–280. [Google Scholar] [CrossRef]
- Hayes, P.; Turner, B.L.; Lambers, H.; Laliberté, E. Foliar nutrient concentrations and resorption efficiency in plants of contrasting nutrient-acquisition strategies along a 2-million-year dune chronosequence. J. Ecol. 2014, 102, 396–410. [Google Scholar] [CrossRef]
- Lang, F.; Krüger, J.; Amelung, W.; Willbold, S.; Frossard, E.; Bünemann, E.K.; Bauhus, J.; Nitschke, R.; Kandeler, E.; Marhan, S.; et al. Soil phosphorus supply controls P nutrition strategies of beech forest ecosystems in Central Europe. Biogeochemistry 2017, 136, 5–29. [Google Scholar] [CrossRef]
- Grau, O.; Peñuelas, J.; Ferry, B.; Freycon, V.; Blanc, L.; Desprez, M.; Baraloto, C.; Chave, J.; Descroix, L.; Dourdain, A.; et al. Nutrient-cycling mechanisms other than the direct absorption from soil may control forest structure and dynamics in poor Amazonian soils. Sci. Rep. 2017, 7, srep45017. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.Q.; Li, H.E.; Sun, X.G.; An, Z.F.; Ding, G.J. Patterns of needle nutrient resorption and ecological stoichiometry homeostasis along a chronosequence of Pinus massoniana plantations. Forests 2023, 14, 607. [Google Scholar] [CrossRef]
- Aerts, R.; Chapin, F.S., III. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res. 1999, 30, 1–67. [Google Scholar] [CrossRef]
- Craine, J.M.; Morrow, C.; Stock, W.D. Nutrient concentration ratios and co-limitation in South African grasslands. New Phytol. 2008, 179, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Hedin, L.O.; Li, M.X.; Xu, L.; Yan, P.; Dai, G.H.; He, N.P. Leaf N:P ratio does not predict productivity trends across natural terrestrial ecosystems. Ecology 2022, 103, e3789. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C:N:P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Selvaraj, S.; Duraisamy, V.; Huang, Z.J.; Guo, F.T.; Ma, X.Q. Influence of long-term successive rotations and stand age of Chinese fir (Cunninghamia lanceolata) plantations on soil properties. Geoderma 2017, 306, 127–134. [Google Scholar] [CrossRef]
- Schreeg, L.A.; Santiago, L.S.; Wright, S.J.; Turner, B.L. Stem, root, and older leaf N:P ratios are more responsive indicators of soil nutrient availability than new foliage. Ecology 2014, 95, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.B.; Guan, H.Y.; Han, W.X.; Han, T.S.; Guo, Y.L.; Fang, J.Y. Reproductive organ and young tissues show constrained elemental composition in Arabidopsis thaliana. Ann. Bot. 2016, 117, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, Q.S.; Elser, J.J.; He, N.P.; Wu, H.H.; Zhang, G.M.; Wu, J.G.; Bai, Y.F.; Han, X.G. Linking stoichiometric homoeostasis with ecosystem structure, functioning and stability. Ecol. Lett. 2010, 13, 1390–1399. [Google Scholar] [CrossRef]
- Sistla, S.A.; Appling, A.P.; Lewandowska, A.M.; Taylor, B.N.; Wolf, A.A. Stoichiometric flexibility in response to fertilization along gradients of environmental and organismal nutrient richness. Oikos 2015, 124, 949–959. [Google Scholar] [CrossRef]
- Güsewell, S. Nutrient resorption of wetland graminoids is related to the type of nutrient limitation. Funct. Ecol. 2005, 19, 344–354. [Google Scholar] [CrossRef]
- Zhang, P.; Lü, X.T.; Li, M.H.; Wu, T.G.; Jin, G.Z. N limitation increases along a temperate forest succession: Evidences from leaf stoichiometry and nutrient resorption. J. Plant Ecol. 2022, 15, 1021–1035. [Google Scholar] [CrossRef]
- Raessler, M.; Wissuwa, B.; Breul, A.; Unger, W.; Grimn, T. Chromatographic analysis of major non-structural carbohydrates in several wood species—An analytical approach for higher accuracy of data. Anal. Methods 2010, 2, 532–538. [Google Scholar] [CrossRef]
- Mei, L.; Xiong, Y.M.; Gu, J.C.; Wang, Z.Q.; Guo, D.L. Whole-tree dynamics of non-structural carbohydrate and nitrogen pools across different seasons and in response to girdling in two temperate trees. Oecologia 2015, 1177, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Freschet, G.T.; Roumet, C.; Comas, L.H.; Weemstra, M.; Bengough, A.G.; Rewald, B.; Bardgett, R.D.; De Deyn, G.B.; Johnson, D.; Klimešová, J.; et al. Root traits as drivers of plant and ecosystem functioning: Current understanding, pitfalls and future research needs. New Phytol. 2020, 232, 1123–1158. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).