Effect of Forest Thinning on Soil Phosphorus Stocks and Dynamics on a Global Scale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Data Analysis
3. Results
3.1. Overview of the Dataset
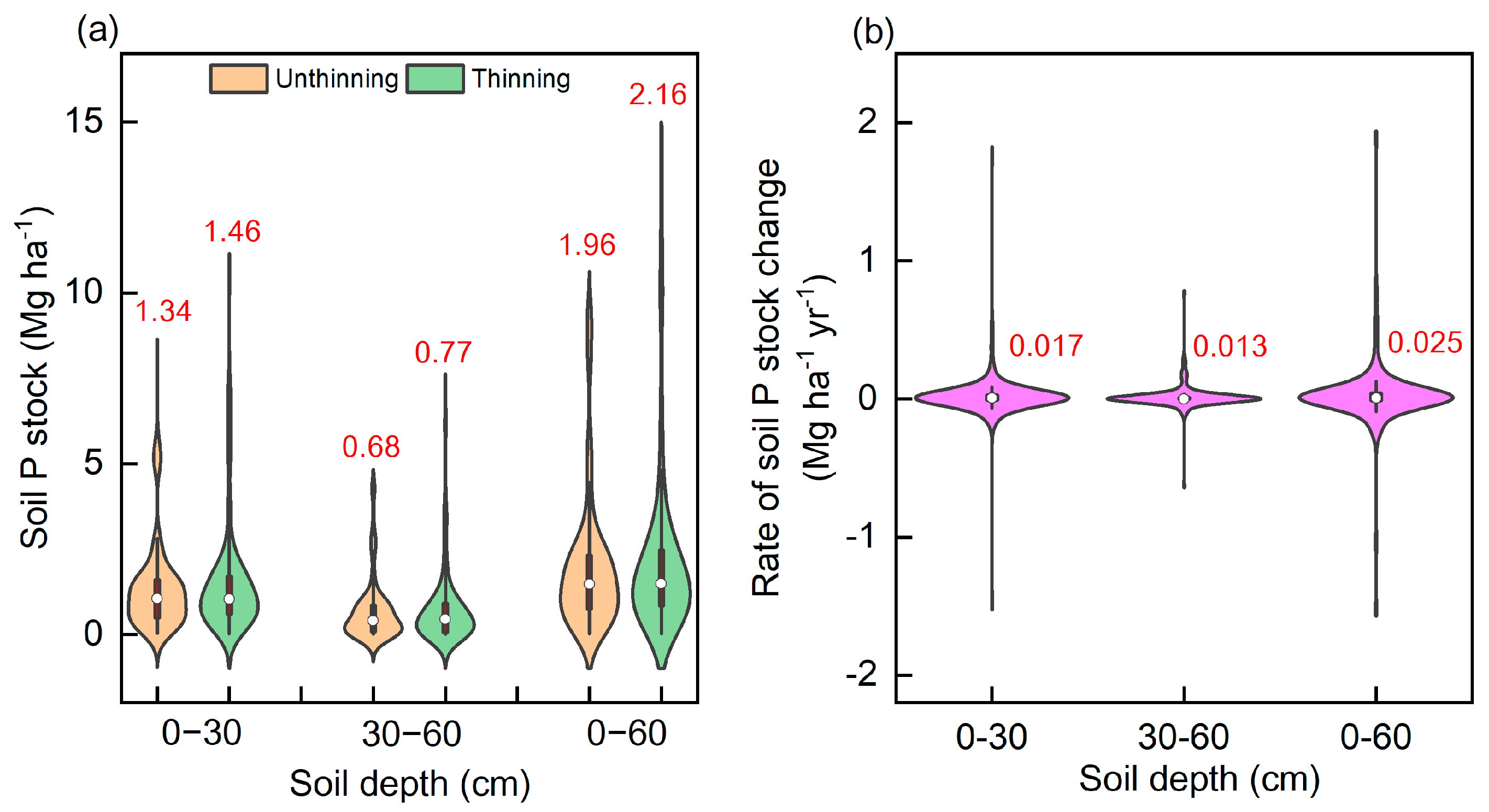
3.2. Responses of Soil P Stock to Forest Thinning
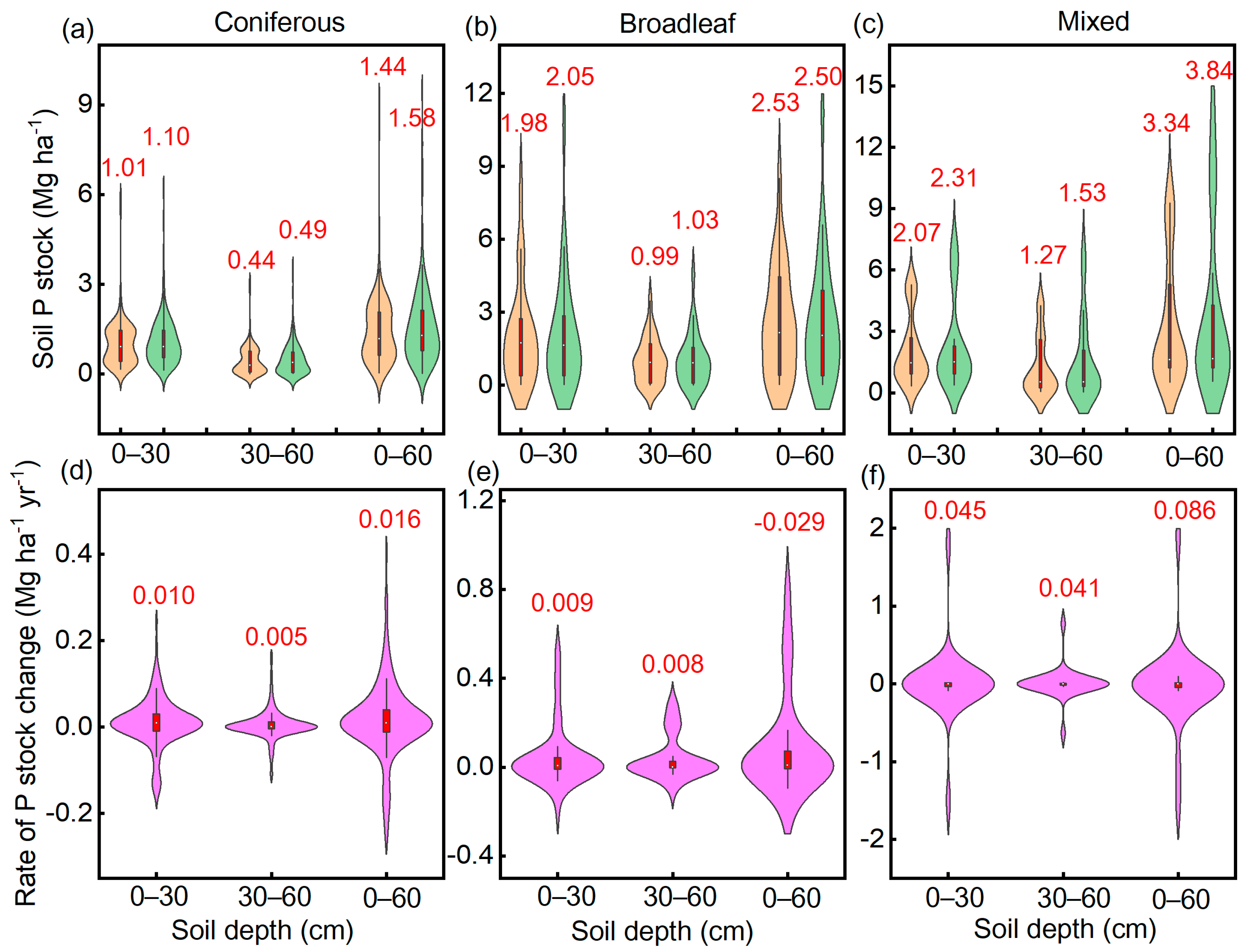
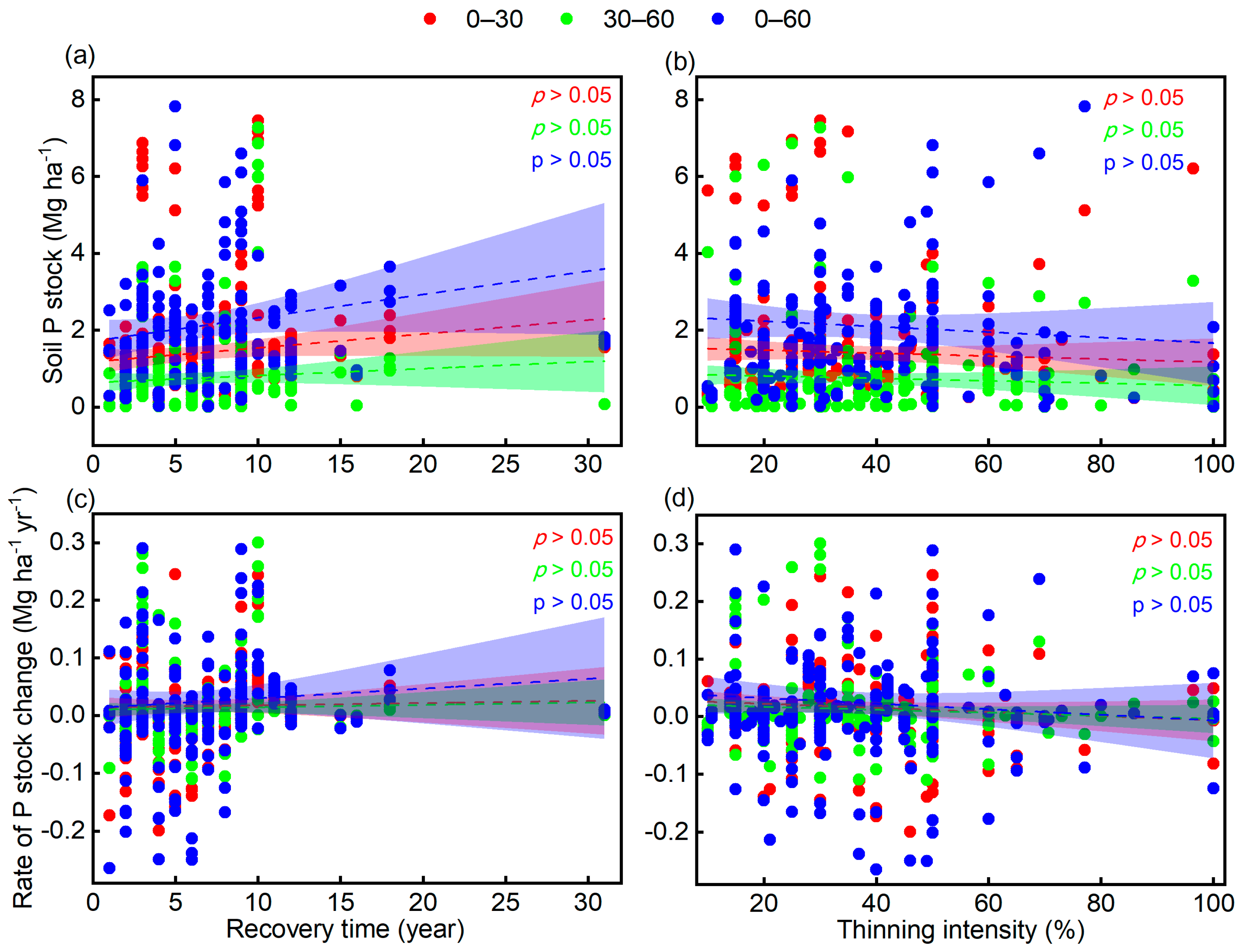
3.3. Factors Affecting Responses of Soil P Stock to Thinning
4. Discussion
4.1. Effects of Forest Thinning on Soil P Accumulation
4.2. Effects of Forest Types on Soil P Accumulation
4.3. Effects of Recovery Time and Thinning Intensity on Soil P Accumulation
4.4. Effects of MAP and Soil Depths on Soil P Accumulation
4.5. Limitations and Uncertainties
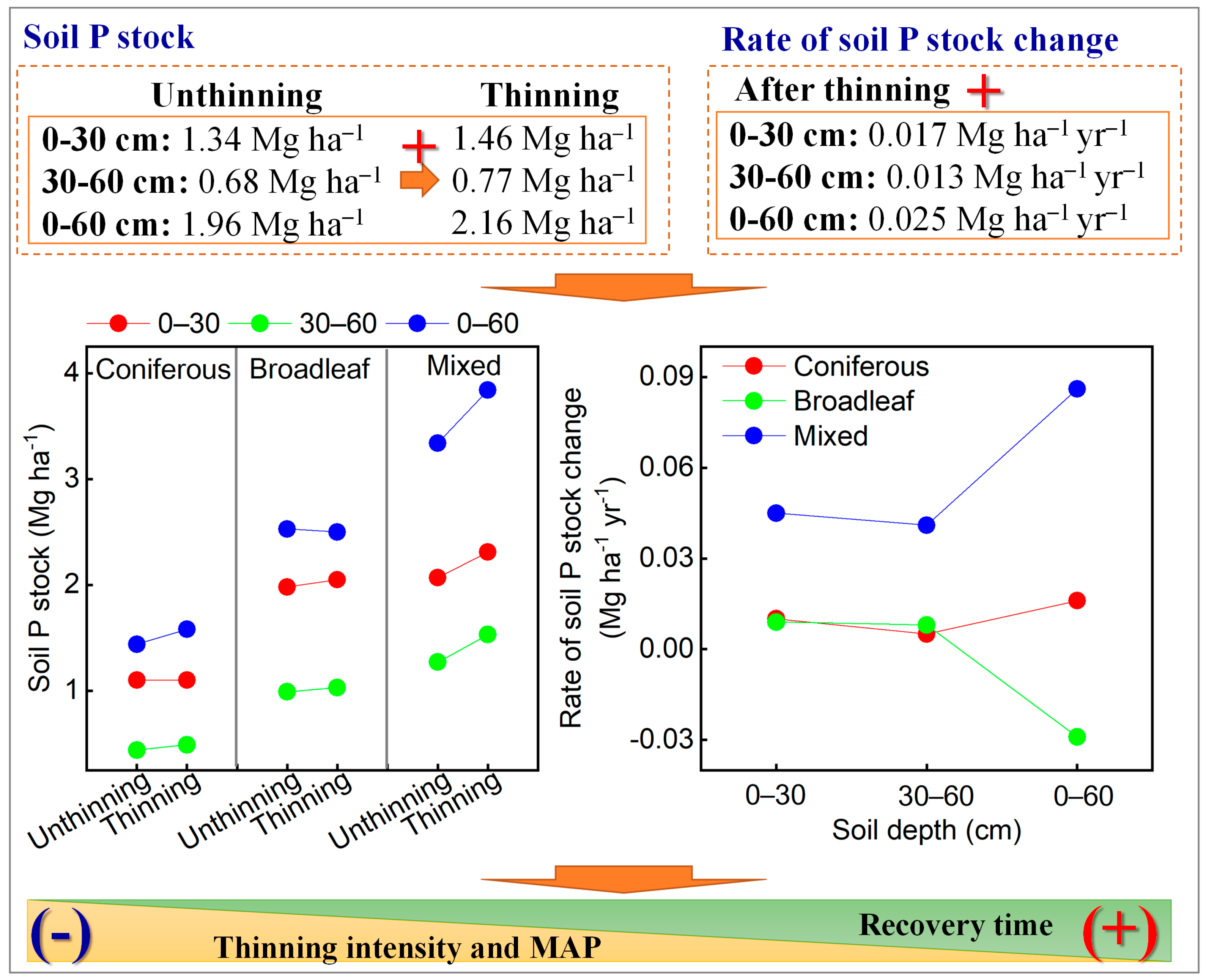
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, S.; Kim, C.; Lee, S.T.; Son, Y. Multi-site assessment of soil nitrogen stocks across temperate forests under different thinning intensities, recovery times, and site conditions. Sci. Total Environ. 2023, 894, 164996. [Google Scholar] [CrossRef] [PubMed]
- Vesterdal, L.; Dalsgaard, M.; Felby, C.; Raulundrasmussen, K.; Jorgensen, B. Effects of thinning and soil properties on accumulation of carbon, nitrogen and phosphorus in the forest floor of Norway spruce stands. For. Ecol. Manag. 1995, 77, 1–10. [Google Scholar] [CrossRef]
- Ring, E.; Lofgren, S.; Hogbom, L.; Ostlund, M.; Wiklund-McKie, M.L.; McKie, B.G. Long-term effects on water chemistry and macroinvertebrates of selective thinning along small boreal forest streams. For. Ecol. Manag. 2023, 549, 121459. [Google Scholar] [CrossRef]
- Alvarenga, L.C.B.R.; da Costa, M.G.; Gama-Rodrigues, A.C.; Aleixo, S.; Gama-Rodrigues, E.F.; Goncalves, J.L.D.M. Soil organic phosphorus in Eucalyptus plantations, Brazil: Extraction methods. Sci. Agric. 2024, 81, e20220131. [Google Scholar] [CrossRef]
- Xu, S.; Gu, C.; Rodrigues, J.L.M.; Li, C.; Bohannan, B.; Nuesslein, K.; Margenot, A. Soil phosphorus cycling across a 100-year deforestation chronosequence in the Amazon rainforest. Glob. Chang. Biol. 2024, 30, e17077. [Google Scholar] [CrossRef]
- Rui, Y.; Wang, Y.; Chen, C.; Zhou, X.; Wang, S.; Xu, Z.; Duan, J.; Kang, X.; Lu, S.; Luo, C. Warming and grazing increase mineralization of organic P in an alpine meadow ecosystem of Qinghai-Tibet Plateau, China. Plant Soil 2012, 357, 73–87. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Xie, X.; Chen, Y.; Lang, M.; Chen, X. Long-term moderate fertilization increases the complexity of soil microbial community and promotes regulation of phosphorus cycling genes to improve the availability of phosphorus in acid soil. Appl. Soil Ecol. 2024, 194, 105178. [Google Scholar] [CrossRef]
- Geng, X.; Zuo, J.; Meng, Y.; Zhuge, Y.; Zhu, P.; Wu, N.; Bai, X.; Ni, G.; Hou, Y. Changes in nitrogen and phosphorus availability driven by secondary succession in temperate forests shape soil fungal communities and function. Ecol. Evol. 2023, 13, e10593. [Google Scholar] [CrossRef]
- Baena, C.; Andrés-Abellán, M.; Lucas-Borja, M.; Martínez-García, E.; García-Morote, F.; Rubio, E.; López-Serrano, F. Thinning and recovery effects on soil properties in two sites of a Mediterranean forest, in Cuenca Mountain (South-eastern of Spain). For. Ecol. Manag. 2013, 308, 223–230. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, C.; Zhou, Z. Thinning promotes the nitrogen and phosphorous cycling in forest soils. Agric. For. Meteorol. 2021, 311, 108665. [Google Scholar] [CrossRef]
- Zhao, Q.; Zeng, D. Phosphorus cycling in terrestrial ecosystems and its controlling factors. Chin. J. Appl. Ecol. 2005, 29, 153–163, (In Chinese with English Abstract). [Google Scholar]
- Wang, M.; Zhang, C.; Chen, S.; Zhang, Y.; Yu, T.; Xue, X.; Wu, L.; Zhou, W.; Yun, X.; Yan, R.; et al. Moderate grazing increased carbon, nitrogen and phosphorus storage in plants and soil in the Eurasian meadow steppe ecosystem. Sci. Total Environ. 2024, 914, 169864. [Google Scholar] [CrossRef]
- Iida, S.; Noguchi, S.; Levia, D.F.; Araki, M.; Nitta, K.; Wada, S.; Narita, Y.; Tamura, H.; Abe, T.; Kaneko, T. Effects of forest thinning on sap flow dynamics and transpiration in a Japanese cedar forest. Sci. Total Environ. 2023, 912, 169060. [Google Scholar] [CrossRef] [PubMed]
- Ogaya, R.; Escola, A.; Liu, D.; Barbeta, A.; Penuelas, J. Effects of thinning in a water-limited holm oak forest. J. Sustain Forest. 2020, 39, 365–378. [Google Scholar] [CrossRef]
- Zeng, L.; Xiao, W.; Liu, C.; Lei, L.; Jian, Z.; Shen, Y.; Li, M. Effects of thinning and understorey removal on soil extracellular enzyme activity vary over time during forest recovery after treatment. Plant Soil 2023, 492, 457–469. [Google Scholar] [CrossRef]
- Kuo, C.; Wei, C.; Chen, J.; Chen, C.; Hsieh, Y. Effects of thinning intensities on litterfall characteristics and decomposition in the natural secondary lowland forests of Southeastern Taiwan. Scan. J. For. Res. 2023, 38, 166–173. [Google Scholar]
- Lindroth, A.; Holst, J.; Heliasz, M.; Vestin, P.; Lagergren, F.; Biermann, T.; Cai, Z.; Mölder, M. Effects of low thinning on carbon dioxide fluxes in a mixed hemiboreal forest. Agric. For. Meteorol. 2018, 262, 59–70. [Google Scholar] [CrossRef]
- Xu, H.; Wang, M.; You, C.; Tan, B.; Xu, L.; Li, H.; Zhang, L.; Wang, L.; Liu, S.; Hou, G.; et al. Warming effects on C:N:P stoichiometry and nutrient limitation in terrestrial ecosystems. Soil Tillage Res. 2024, 235, 105896. [Google Scholar] [CrossRef]
- Xu, H.; Qu, Q.; Li, G.; Liu, G.; Geissen, V.; Ritsema, C.J.; Xue, S. Impact of nitrogen addition on plant-soil-enzyme C-N-P stoichiometry and microbial nutrient limitation. Soil Biol. Biochem. 2022, 170, 108714. [Google Scholar] [CrossRef]
- Zhou, S.; Butenschoen, O.; Barantal, S.; Handa, I.T.; Makkonen, M.; Vos, V.; Aerts, R.; Berg, M.P.; McKie, B.; Van Ruijven, J.; et al. Decomposition of leaf litter mixtures across biomes: The role of litter identity, diversity and soil fauna. J. Ecol. 2020, 108, 2283–2297. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Q.; Sun, X.; Chen, D.; Insam, H.; Koide, R.T.; Zhang, S. Effects of mixed-species litter on bacterial and fungal lignocellulose degradation functions during litter decomposition. Soil Biol. Biochem. 2020, 141, 107690. [Google Scholar] [CrossRef]
- Yang, Y.; Mohammat, A.; Feng, J.; Zhou, R.; Fang, J. Storage, patterns and environmental controls of soil organic carbon in China. Biogeochemistry 2007, 84, 131–141. [Google Scholar] [CrossRef]
- Gong, C.; Tan, Q.; Liu, G.; Xu, M. Forest thinning increases soil carbon stocks in China. For. Ecol. Manag. 2021, 482, 118812. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, C.; Zhou, Z. Impacts of forest thinning on soil microbial community structure and extracellular enzyme activities: A global meta-analysis. Soil Biol. Biochem. 2020, 149, 107915. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhang, J.; Peng, S.; Chen, Y.; Cao, Y. The effects of forest thinning on understory diversity in China: A meta-analysis. Land Degrad. Dev. 2020, 31, 1225–1240. [Google Scholar] [CrossRef]
- Xu, M.; Liu, H.; Zhang, Q.; Zhang, Z.; Ren, C.; Feng, Y.; Yang, G.; Han, X.; Zhang, W. Effect of forest thinning on soil organic carbon stocks from the perspective of carbon-degrading enzymes. Catena 2022, 218, 106560. [Google Scholar] [CrossRef]
- Banerjee, T. Impacts of forest thinning on wildland fire behavior. Forests 2020, 11, 918. [Google Scholar] [CrossRef]
- Makineci, E. Long term effects of thinning on soil and forest floor in a sessile oak (Quercus petrea (Matlusch) Lieb.) forest in Turkey. J. Environ. Biol. 2005, 26, 257–263. [Google Scholar] [PubMed]
- Sohn, J.A.; Hartig, F.; Kohler, M.; Huss, J.; Bauhus, J. Heavy and frequent thinning promotes drought adaptation in Pinus sylvestris forests. Ecol. Appl. 2016, 26, 2190–2205. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.; Zhang, W.; Zhang, Q.; Lu, D.; Zhang, Y.; Zheng, X.; Xu, S.; Wang, G. Litter decomposition and nutrient release from monospecific and mixed litters: Comparisons of litter quality, fauna and decomposition site effects. J. Ecol. 2022, 110, 1673–1686. [Google Scholar] [CrossRef]
- Don, A.; Schumacher, J.; Freibauer, A. Impact of tropical land-use change on soil organic carbon stocks–A meta-analysis. Glob. Chang. Biol. 2011, 17, 1658–1670. [Google Scholar] [CrossRef]
- Jobbagy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Deng, L.; Liu, G.B.; Shangguan, Z.P. Land-use conversion and changing soil carbon stocks in China’s “Grain-for-Green” Program: A synthesis. Glob. Chang. Biol. 2014, 20, 3544–3556. [Google Scholar] [CrossRef]
- Li, D.; Niu, S.; Luo, Y. Global patterns of the dynamics of soil carbon and nitrogen stocks following afforestation: A meta-analysis. New Phytol. 2012, 195, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F.; He, Z.B.; Zhao, W.Z.; Ma, L.; Liu, S.E.; Zhao, L.W.; Yang, S.P.; Feng, X.Y. The long-term effects of thinning on soil respiration vary with season in subalpine spruce plantations. Agric. For. Meteorol. 2023, 342, 109756. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Y.; Wang, G.; Cheng, F.; Xia, F. Short-term effects of thinning on the understory natural environment of mixed broadleaf-conifer forest in Changbai Mountain area, Northeast China. PeerJ 2019, 7, e7400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, L.; Wang, Y.; Jiang, P.; Hu, Y.; Ouyang, S.; Wu, H.; Lei, P.; Kuzyakov, Y.; Xiang, W. Plantations thinning: A meta-analysis of consequences for soil properties and microbial functions. Sci. Total Environ. 2023, 877, 162894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Q.; Wei, L.M.; Sun, Q.Y.; Zeng, D.H. Tree roots exert greater impacts on phosphorus fractions than aboveground litter in mineral soils under a Pinus sylvestris var. mongolica plantation. For. Ecol. Manag. 2023, 545, 121242. [Google Scholar] [CrossRef]
- Saunders, M.; Tobin, B.; Black, K.; Gioria, M.; Nieuwenhuis, M.; Osborne, B.A. Thinning effects on the net ecosystem carbon exchange of a Sitka spruce forest are temperature-dependent. Agric. For. Meteorol. 2012, 157, 1–10. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, X.; Tian, H.; Liu, L.; Han, H. Effects of thinning and litter manipulation on soil phosphorus dynamicsin a Larixprincipis-rupprechtii plantation. Acta Ecol. Sin. 2019, 39, 7686–7696, (In Chinese with English Abstract). [Google Scholar]
- He, Y.; Zhang, Q.; Wang, S.; Jiang, C.; Lan, Y.; Zhang, H.; Ye, S. Mixed plantations induce more soil macroaggregate formation and facilitate soil nitrogen accumulation. Forests 2023, 14, 735. [Google Scholar] [CrossRef]
- Sohn, J.A.; Saha, S.; Bauhus, J. Potential of forest thinning to mitigate drought stress: A meta-analysis. For. Ecol. Manag. 2016, 380, 261–273. [Google Scholar] [CrossRef]
- Tian, D.; Chen, Y.; Shi, Y.; Lian, S.; Bian, L.; Tang, L. Effects of thinning and fertilization on soil microbial characteristics in a near-mature Chinese fir plantation. J. Forest. Environ. 2023, 43, 569–578, (In Chinese with English Abstract). [Google Scholar]
- Nazari, M.; Pausch, J.; Bickel, S.; Bilyera, N.; Rashtbari, M.; Razavi, B.; Zamanian, K.; Sharififar, A.; Shi, L.; Dippold, M.; et al. Keeping thinning-derived deadwood logs on forest floor improves soil organic carbon, microbial biomass, and enzyme activity in a temperate spruce forest. Eur. J. For. Res. 2022, 142, 287–300. [Google Scholar] [CrossRef]
- Xu, H.; Qu, Q.; Wang, M.; Li, P.; Li, Y.; Xue, S.; Liu, G. Soil organic carbon sequestration and its stability after vegetation restoration in the Loess Hilly Region, China. Land Degrad. Dev. 2020, 31, 568–580. [Google Scholar] [CrossRef]
- Bashir, M.A.; Rehim, A.; Liu, J.; Imran, M.; Liu, H.; Suleman, M.; Naveed, S. Soil survey techniques determine nutrient status in soil profile and metal retention by calcium carbonate. Catena 2019, 173, 141–149. [Google Scholar] [CrossRef]
- Tian, L.; Zhao, L.; Wu, X.; Fang, H.; Zhao, Y.; Yue, G.; Liu, G.M.; Chen, H. Vertical patterns and controls of soil nutrients in alpine grassland: Implications for nutrient uptake. Sci. Total Environ. 2017, 607, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.B.; Hutchison, K.J.; Hesterberg, D.L.; Grabow, G.L.; Huffman, R.L.; Hardy, D.H.; Parsons, J.T. Leaching of Nutrients and Trace Elements from Stockpiled Turkey Litter into Soil. J. Environ. Qual. 2009, 38, 1053–1065. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Dong, Q.; Yang, J.; Tan, B.; Xu, Z.; Wu, Q.; Xu, H. Effect of Forest Thinning on Soil Phosphorus Stocks and Dynamics on a Global Scale. Forests 2024, 15, 362. https://doi.org/10.3390/f15020362
Yang Y, Dong Q, Yang J, Tan B, Xu Z, Wu Q, Xu H. Effect of Forest Thinning on Soil Phosphorus Stocks and Dynamics on a Global Scale. Forests. 2024; 15(2):362. https://doi.org/10.3390/f15020362
Chicago/Turabian StyleYang, Yulian, Qing Dong, Jiaping Yang, Bo Tan, Zhenfeng Xu, Qinggui Wu, and Hongwei Xu. 2024. "Effect of Forest Thinning on Soil Phosphorus Stocks and Dynamics on a Global Scale" Forests 15, no. 2: 362. https://doi.org/10.3390/f15020362




