Construction of Riboswitches for Screening Antibacterial Agents from Forest Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Strains, and Culture Conditions
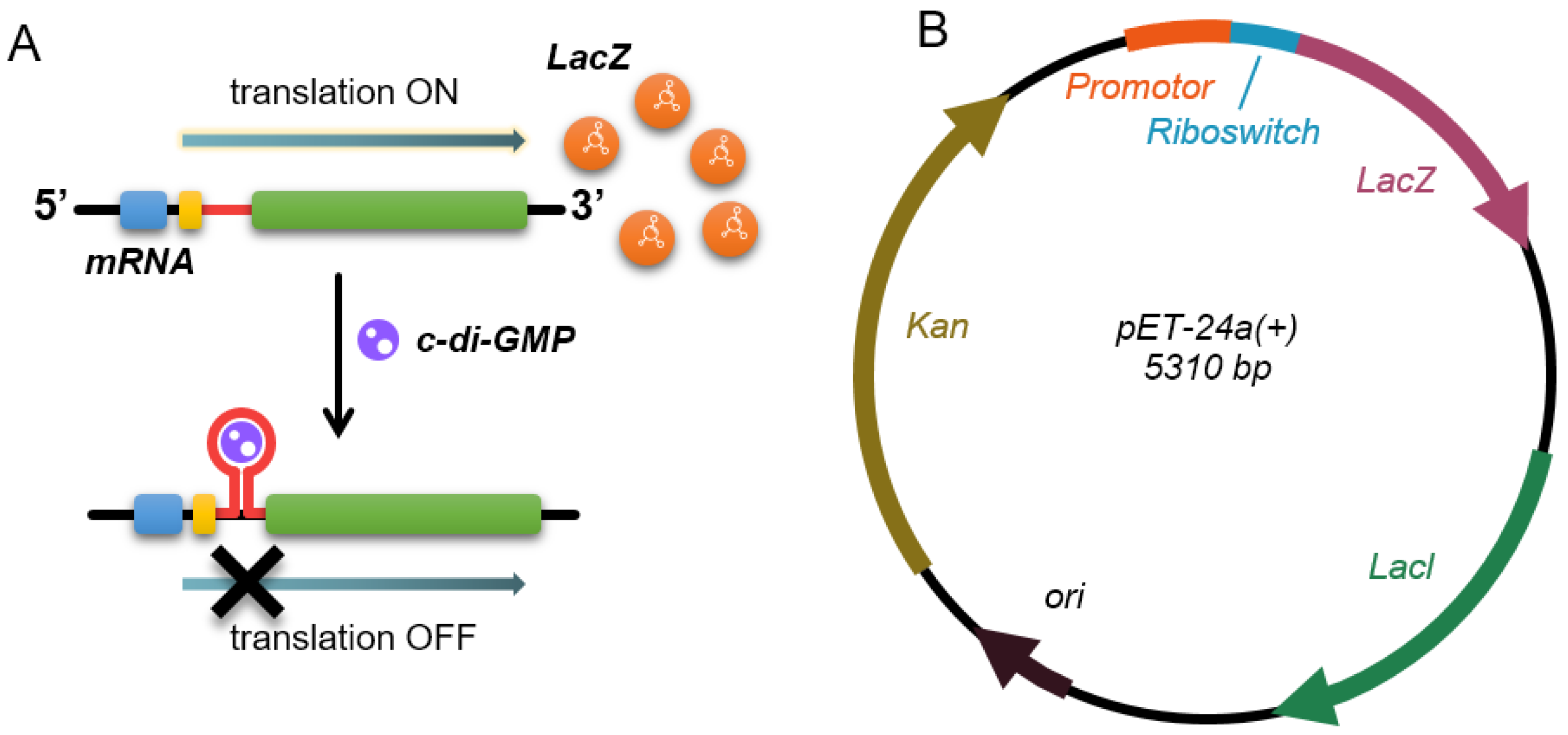
2.2. Plasmid Construction
2.3. Plasmid Transformation
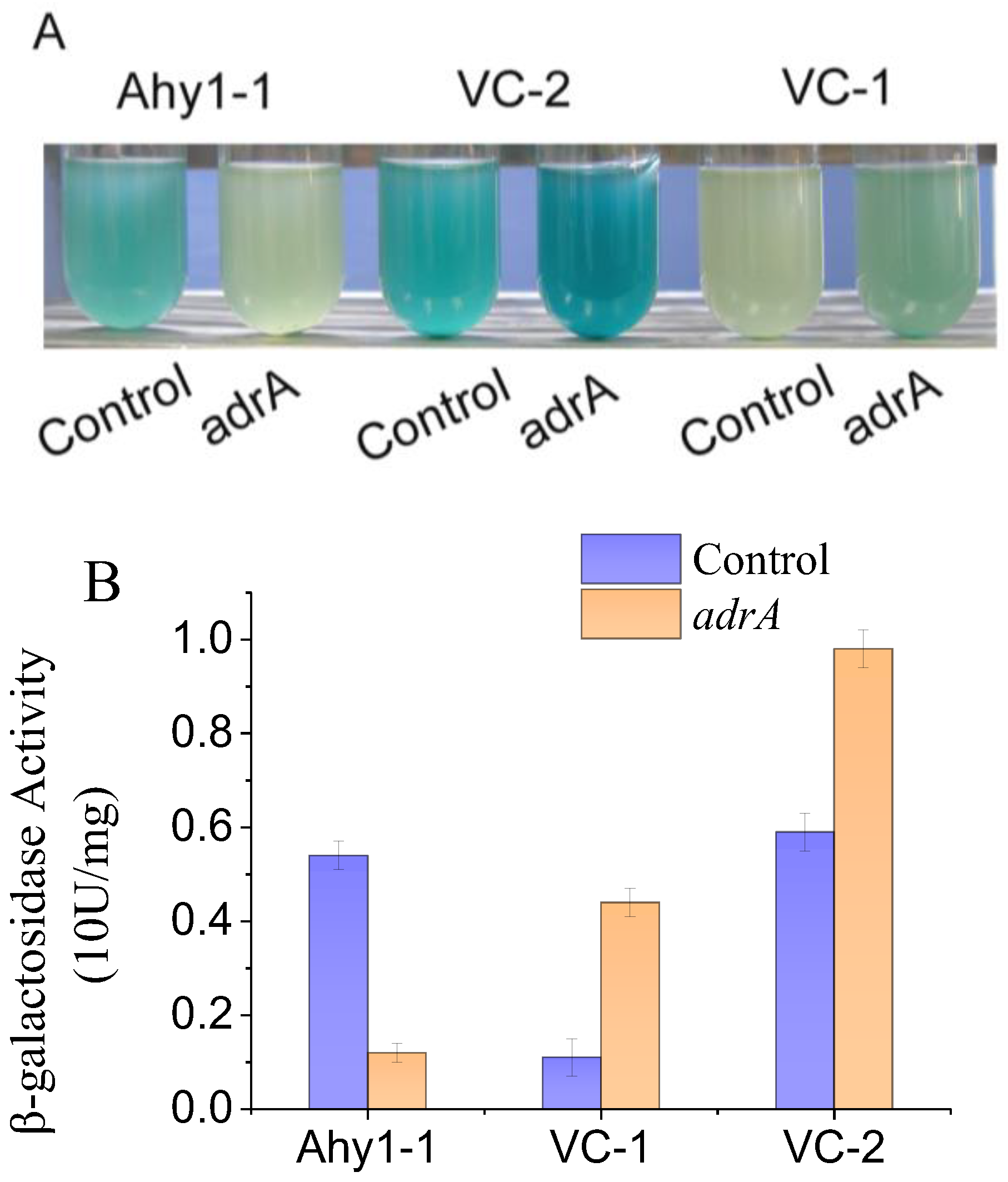
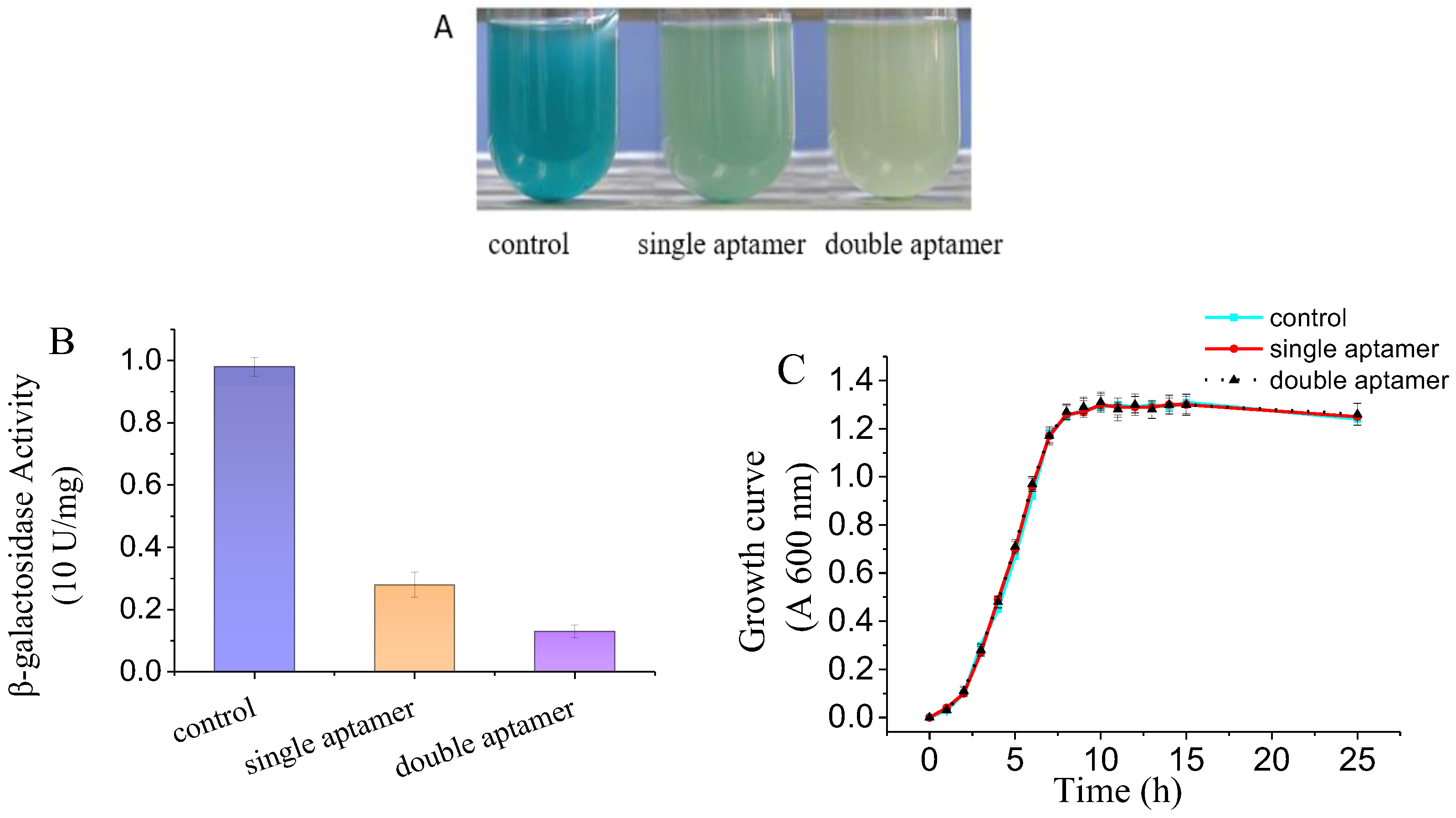
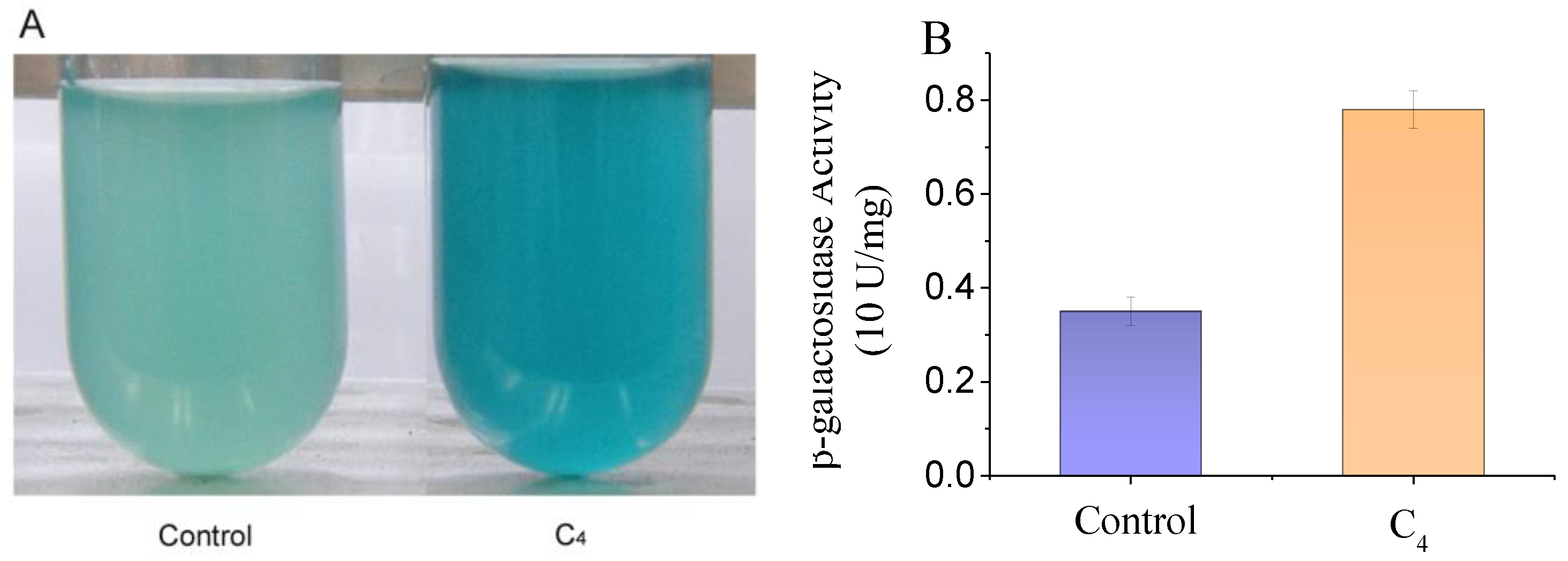
2.4. β-Galactosidase Assays
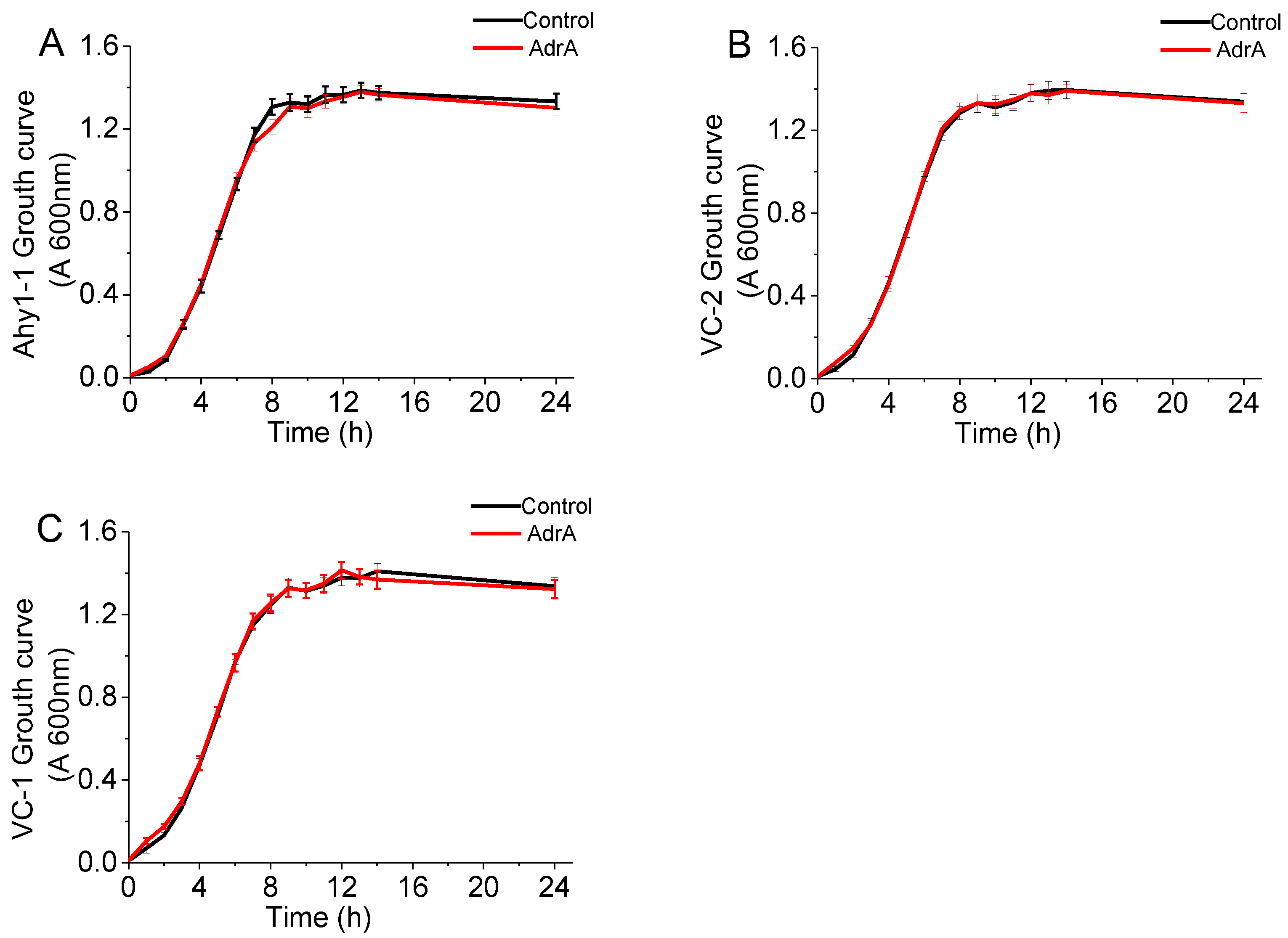
2.5. Quantitative Analysis of adrA
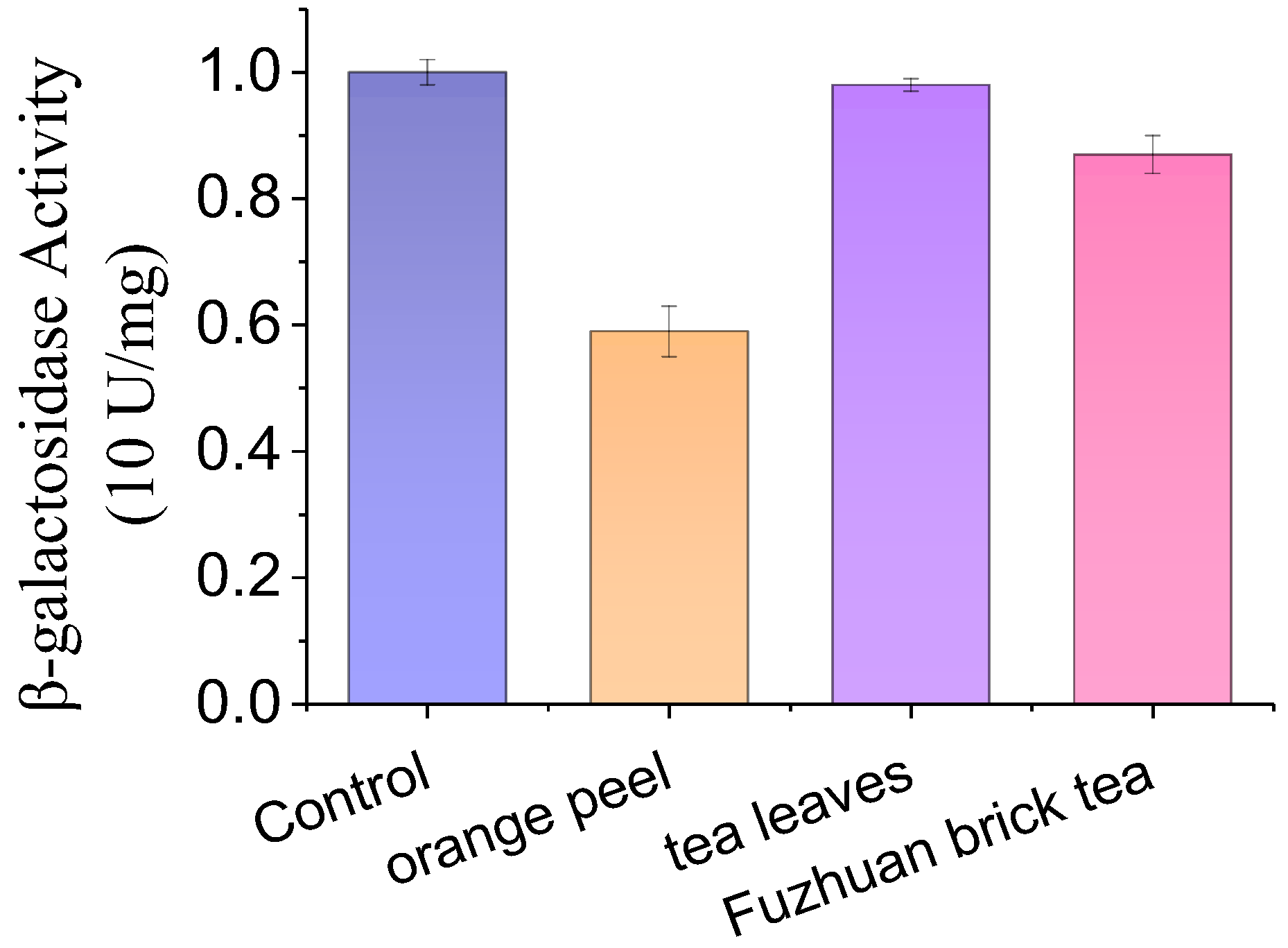
2.6. Screening c-di-GMP Biosynthesis Regulator from Orange Peel and Tea
2.7. Statistical Analysis
3. Results
3.1. Construction of Intragenic Synthetic c-di-GMP Riboswitches
3.2. Screening c-di-GMP Biosynthesis Regulators in Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, X.; Liu, R.; Liu, Y. Forest Change in China: A Review. Chin. Geogr. Sci. 2023, 33, 489–502. [Google Scholar] [CrossRef]
- Xie, Y.; Cheng, C.; Zhang, T.; Wu, X.; Wang, P. Donor-side valuation of forest ecosystem services in China during 1990–2020. Energy Ecol. Environ. 2023, 8, 503–521. [Google Scholar] [CrossRef]
- Li, C.; Tang, Y.; Wang, Y.; Yuan, X.; Zhang, B.; Wu, Z.; Tian, H. A novel environment-friendly adhesive based on recycling of broussonetia papyrifera leaf forestry waste protein. Forests 2022, 13, e291. [Google Scholar] [CrossRef]
- Xiao, L.; Yang, C.; Zhang, X.; Wang, Y.; Li, Z.; Chen, Y.; Liu, Z.; Zhu, M.; Xiao, Y. Effects of solid-state fermentation with Bacillus subtilis LK-1 on the volatile profile, catechins composition and antioxidant activity of dark teas. Food Chem. X 2023, 19, e100811. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Le, T.; Wang, W.; Yu, L.; Yang, L.; Jiang, H. Effects of Key Components on the Antioxidant Activity of Black Tea. Foods 2023, 12, e3134. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Vitale, E.; Guerretti, V.; Palumbo, G.; De Clemente, I.; Vitale, L.; Arena, C.; De Maio, A. Antioxidant Characterization of Six Tomato Cultivars and Derived Products Destined for Human Consumption. Antioxidants 2023, 12, e761. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, M.; Wang, X.; Guo, Q.; Zhu, C.; Lin, S.; Xu, C.; Jiang, Y.; Li, Y.; Jiang, J.; et al. Antiviral enantiomers of a bisindole alkaloid with a new carbon skeleton from the roots of Isatis indigotica. Chin. Chem. Lett. 2015, 26, 931–936. [Google Scholar] [CrossRef]
- Bocci, G.; Di Paolo, A.; Danesi, R. The pharmacological bases of the antiangiogenic activity of paclitaxel. Angiogenesis 2013, 16, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Zhang, X.; Li, B.; Liu, Z.; Wang, M.; Liu, S. Anti-tumour and immunomodulatory activities of oligosaccharides isolated from Panax ginseng CA Meyer. Int. J. Biol. Macromol. 2014, 64, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Tan, Y.; Wang, K.; Cai, Y.; Li, J.; Sonne, C.; Li, C. Reviewing wood-based solar-driven interfacial evaporators for desalination. Water Res. 2022, 223, e119011. [Google Scholar] [CrossRef]
- Liu, S.; Li, T.; Yu, S.; Zhou, X.; Liu, Z.; Zhang, X.; Cai, H.; Hu, Z. Analysis of bacterial community structure of Fuzhuan tea with different processing techniques. Open Life Sci. 2023, 18, e20220573. [Google Scholar] [CrossRef]
- Stickel, S.; Gomes, N.; Frederick, B.; Raben, D.; Su, T. Bouvardin is a Radiation Modulator with a Novel Mechanism of Action. Radiat. Res. 2015, 184, 392–403. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, e2041. [Google Scholar] [CrossRef]
- Kokoska, L.; Polesny, Z.; Rada, V.; Nepovim, A.; Vanek, T. Screening of some Siberian medicinal plants for antimicrobial activity. J. Ethnopharmacol. 2002, 82, 51–53. [Google Scholar] [CrossRef]
- Ding, D.; Wang, B.; Zhang, X.; Zhang, J.; Zhang, H.; Liu, X.; Gao, Z.; Yu, Z. The spread of antibiotic resistance to humans and potential protection strategies. Ecotox. Environ. Safe. 2023, 254, e114734. [Google Scholar] [CrossRef]
- Blount, K.; Breaker, R. Riboswitches as antibacterial drug targets. Nature Biotechnol. 2006, 24, 1558–1564. [Google Scholar] [CrossRef]
- Pavlova, N.; Penchovsky, R. Genome-wide bioinformatics analysis of FMN, SAM-I, glmS, TPP, lysine, purine, cobalamin, and SAH riboswitches for their applications as allosteric antibacterial drug targets in human pathogenic bacteria. Expert Opin. Ther. Tar. 2019, 23, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Jia, J.; Yao, P.; Majumder, M.; Hatzoglou, M.; Fox, P. A stress-responsive RNA switch regulates VEGFA expression. Nature 2009, 457, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, X.; Jiang, P.; Yu, W. Riboswitches: New Targets for Drug Discovery. J. Wuhan Univ. 2010, 56, 313–319. [Google Scholar]
- Aghdam, E.; Hejazi, M.; Barzegar, A. Riboswitches: From living biosensors to novel targets of antibiotics. Gene 2016, 592, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Sinumvayo, J.; Zhao, C.; Tuyishime, P. Recent advances and future trends of riboswitches: Attractive regulatory tools. World J. Microb. Biot. 2018, 34, e171. [Google Scholar] [CrossRef]
- Silva-Rohwer, A.; Held, K.; Sagawa, J.; Fernandez, N.; Waters, C.; Vadyvaloo, V. CsrA Enhances Cyclic-di-GMP Biosynthesis and Yersinia pestis Biofilm Blockage of the Flea Foregut by Alleviating Hfq-Dependent Repression of the hmsT mRNA. mBio 2021, 12, e01358-21. [Google Scholar] [CrossRef]
- Zhao, X.; Koestler, B.; Waters, C.; Hammer, B. Post-transcriptional activation of a diguanylate cyclase by quorum sensing small RNAs promotes biofilm formation in Vibrio cholera. Mol. Microbiol. 2013, 89, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Camilli, A.; Bassler, B. Bacterial small-molecule signaling pathways. Science 2006, 311, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Lipchock, S.; Ames, T.; Wang, J.; Breaker, R.; Strobel, S. Structural basis of ligand binding by a c-di-GMP riboswitch. Nat. Struct. Mol. Boil. 2009, 16, 1218–1223. [Google Scholar] [CrossRef]
- NaLiuhvi, A.; Sudarsan, N.; Ebert, M.; Zou, X.; Brown, K.; Breaker, R. Genetic control by a metabolite binding mRNA. Chem. Boil. 2009, 9, e1043. [Google Scholar]
- Beisel, C.; Smolke, C. Design principles for riboswitch function. PLoS Comput. Boil. 2009, 5, e1000363. [Google Scholar] [CrossRef] [PubMed]
- Welz, R.; RBreaker, R. Ligand binding and gene control characteristics of tandem riboswitches in Bacillus anthracis. RNA 2007, 13, 573–582. [Google Scholar] [CrossRef]
- Babina, A.; Lea, N.; Meyer, M. in vivo Behavior of the Tandem Glycine Riboswitch in Bacillus subtilis. mBio 2017, 8, e01602-17. [Google Scholar] [CrossRef]
- Blouin, S.; Mulhbacher, J.; Penedo, J.; Lafontaine, D. Riboswitches: Ancient and promising genetic regulators. ChemBioChem 2009, 10, 400–416. [Google Scholar] [CrossRef]
- Giarimoglou, N.; Kouvela, A.; Maniatis, A.; Papakyriakou, A.; Zhang, J.; Stamatopoulou, V.; Stathopoulos, C. A Riboswitch-Driven Era of New Antibacterials. Antibiotics 2022, 11, e1243. [Google Scholar] [CrossRef] [PubMed]
- Waters, C.; Lu, W.; Rabinowitz, J.; Bassler, B. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repression of vpsT. J. Bacterial. 2008, 190, 2527–2536. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiao, H.; Meng, J.; Qiao, M.; Du, H.; He, M.; Ming, K.; Liu, J.; Wang, D.; Wu, Y. Baicalin Inhibits Biofilm Formation and the Quorum-Sensing System by Regulating the MsrA Drug Efflux Pump in Staphylococcus saprophyticus. Front. Microbiol. 2020, 10, e2800. [Google Scholar] [CrossRef] [PubMed]
- Kovacikova, G.; Lin, W.; Skorupski, K. Dual regulation of genes involved in acetoin biosynthesis and motility/biofilm formation by the virulence activator AphA and the acetate-responsive LysR-type regulator AlsR in Vibrio cholera. Mol. Microbial. 2005, 57, 420–433. [Google Scholar] [CrossRef]
- Pyrzynska, K. Hesperidin: A Review on Extraction Methods, Stability and Biological Activities. Nutrients 2022, 14, 2387. [Google Scholar] [CrossRef]
- Li, Q.; Jin, Y.; Jiang, R.; Xu, Y.; Zhang, Y.; Luo, Y.; Huang, J.; Wang, K.; Liu, Z. Dynamic changes in the metabolite profile and taste characteristics of Fu brick tea during the manufacturing process. Food Chem. 2021, 344, 128576. [Google Scholar] [CrossRef]

| Gene | Primers (5′ to 3′) |
|---|---|
| Ahy1-1 (Forward) | TCTAGACAGTGAGCCAACGCACATTAC |
| Ahy1-1 (Reverse) | GGATCCGGCAGCCAAAGCCACCAGC |
| VC-2 (Forward) | TCTAGAATAACGCCTATATTTGAAAGC |
| VC-2 (Reverse) | GGATCCGTTTAATACTGGTTTATCCATGC |
| VC-1 (Forward) | TCTAGAAAGCGTGAGAGCTTGATTCCA |
| VC-1 (Reverse) | GGATCCGGTCATTTTAGGTTGTTTTTTCA |
| BC-1 (Forward) | TCTAGAATAAATACCCGAAGAAATCC |
| BC-1 (Reverse) | GGATCCGAACGATAACTTATGCCAATA |
| BC-2 (Forward) | TCTAGAACTAAGCCCCGAGTTAAGAG |
| BC-2 (Reverse) | GGATCCGCTTTTAGTACTTTTCATTTGC |
| adrA (Forward) | CATATGTTCCCAAAAATAATGAATGATG |
| adrA (Reverse) | GGATCCTCAGGCCGCCACTTCGGTG |
| LacZ (Forward) | GGATCCACCATGATTACGGATTCAC |
| LacZ (Reverse) | GAATTCTCAGTTGCACCACAGATGAAAC |
| Gene | Forward Primers | Reverse Primers |
|---|---|---|
| adrA | 5′ AAAACGAACATCAGCGGTCC 3′ | 5′ GCGTTGAAGCAATCGGTAAGA 3′ |
| 16S | 5′ GCGCAACCCTTGTCCTTAGTT 3′ | 5′ TGTCACCGGCAGTCTCCTTAG 3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Li, T.; Zhang, X.; Liu, S.; Hu, Z.; Yu, S.; Zhou, X. Construction of Riboswitches for Screening Antibacterial Agents from Forest Plants. Forests 2024, 15, 367. https://doi.org/10.3390/f15020367
Liu Z, Li T, Zhang X, Liu S, Hu Z, Yu S, Zhou X. Construction of Riboswitches for Screening Antibacterial Agents from Forest Plants. Forests. 2024; 15(2):367. https://doi.org/10.3390/f15020367
Chicago/Turabian StyleLiu, Zhanjun, Taotao Li, Xingyu Zhang, Shiquan Liu, Zhiyuan Hu, Songlin Yu, and Xiaohong Zhou. 2024. "Construction of Riboswitches for Screening Antibacterial Agents from Forest Plants" Forests 15, no. 2: 367. https://doi.org/10.3390/f15020367
APA StyleLiu, Z., Li, T., Zhang, X., Liu, S., Hu, Z., Yu, S., & Zhou, X. (2024). Construction of Riboswitches for Screening Antibacterial Agents from Forest Plants. Forests, 15(2), 367. https://doi.org/10.3390/f15020367






