Patterns in Tree Cavities (Hollows) in Euphrates Poplar (Populus euphratica, Salicaceae) along the Tarim River in NW China
Abstract
:1. Introduction
2. Materials and Methods
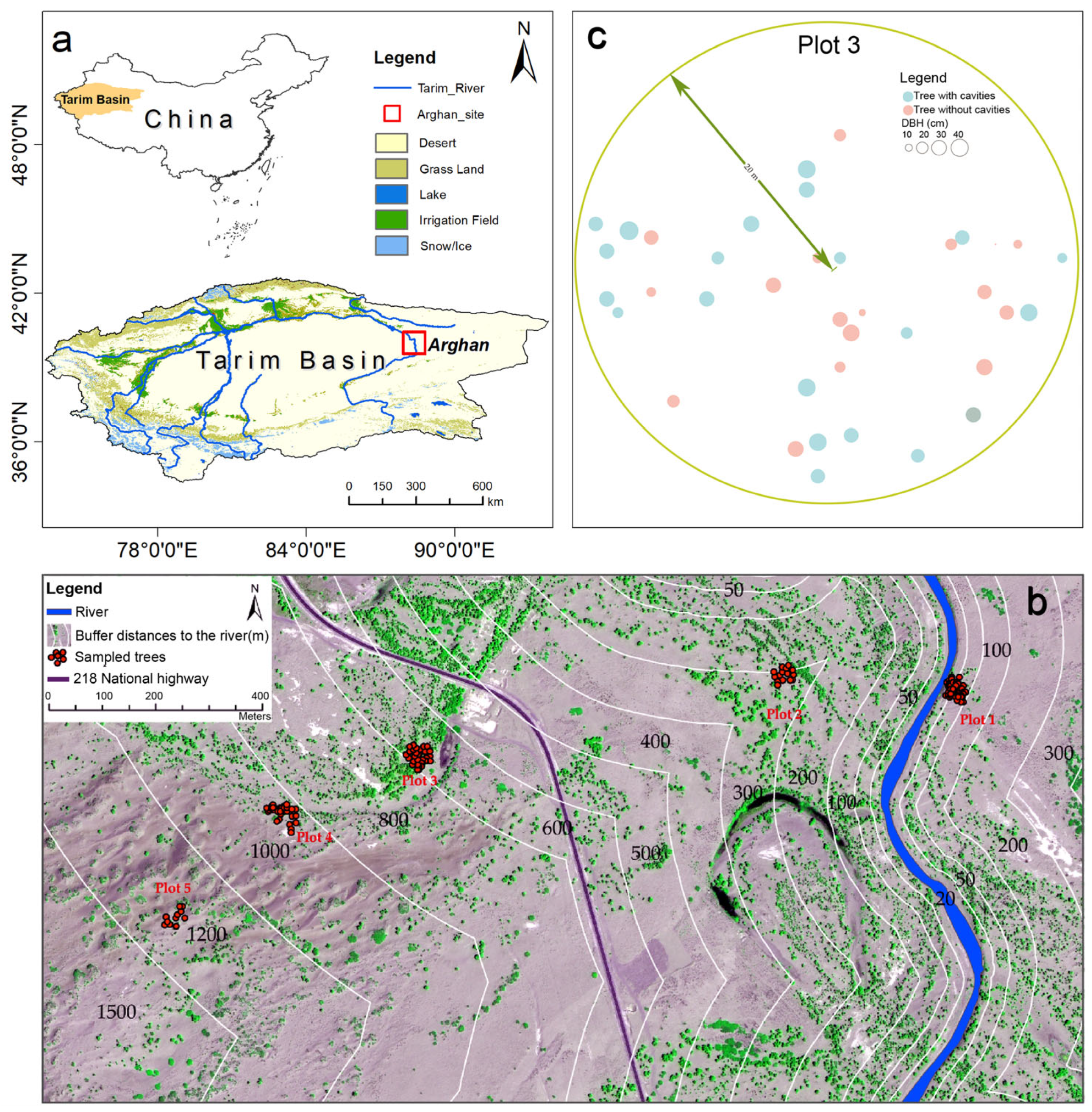
2.1. Study Site
2.2. Data Collection and Processing
2.2.1. Plot Design and Measurements
2.2.2. Data Processing and Analysis
3. Results
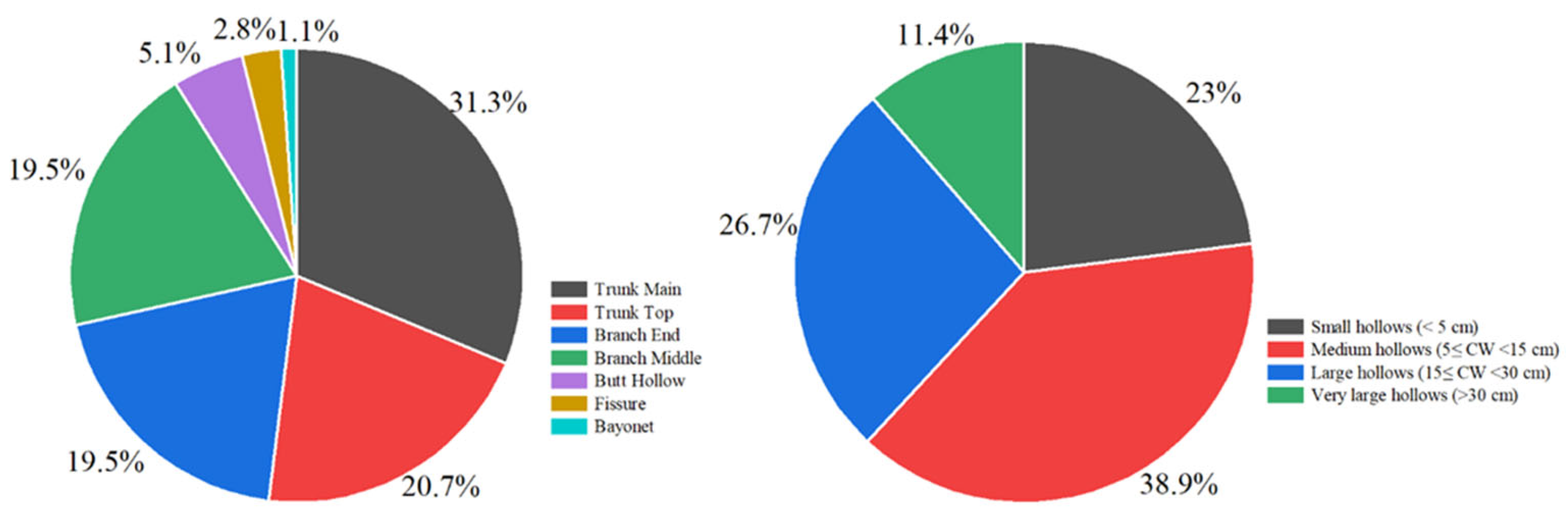
3.1. Cavity Types and Distribution Patterns
3.2. Architectural Traits of Trees with and without Hollows
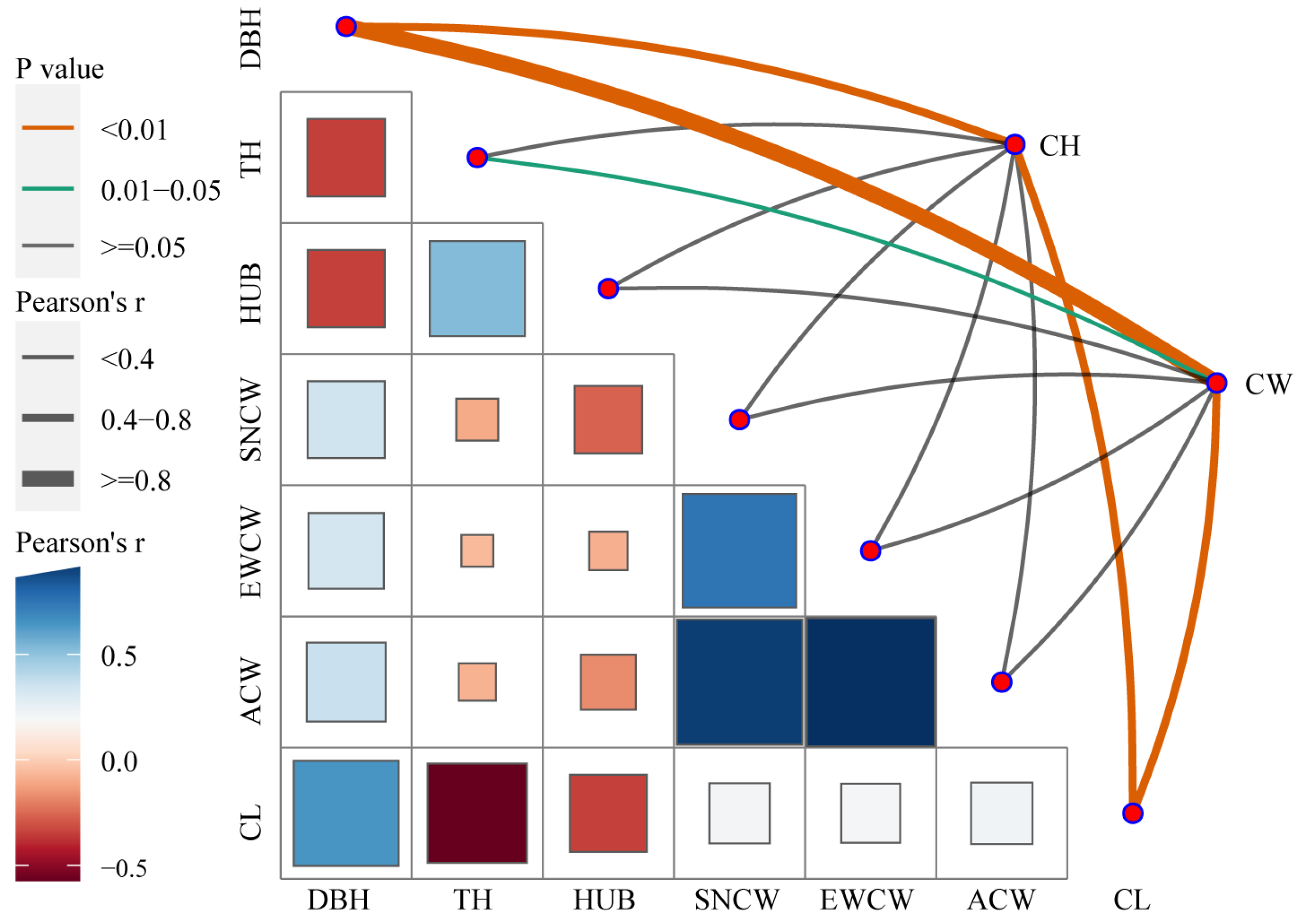
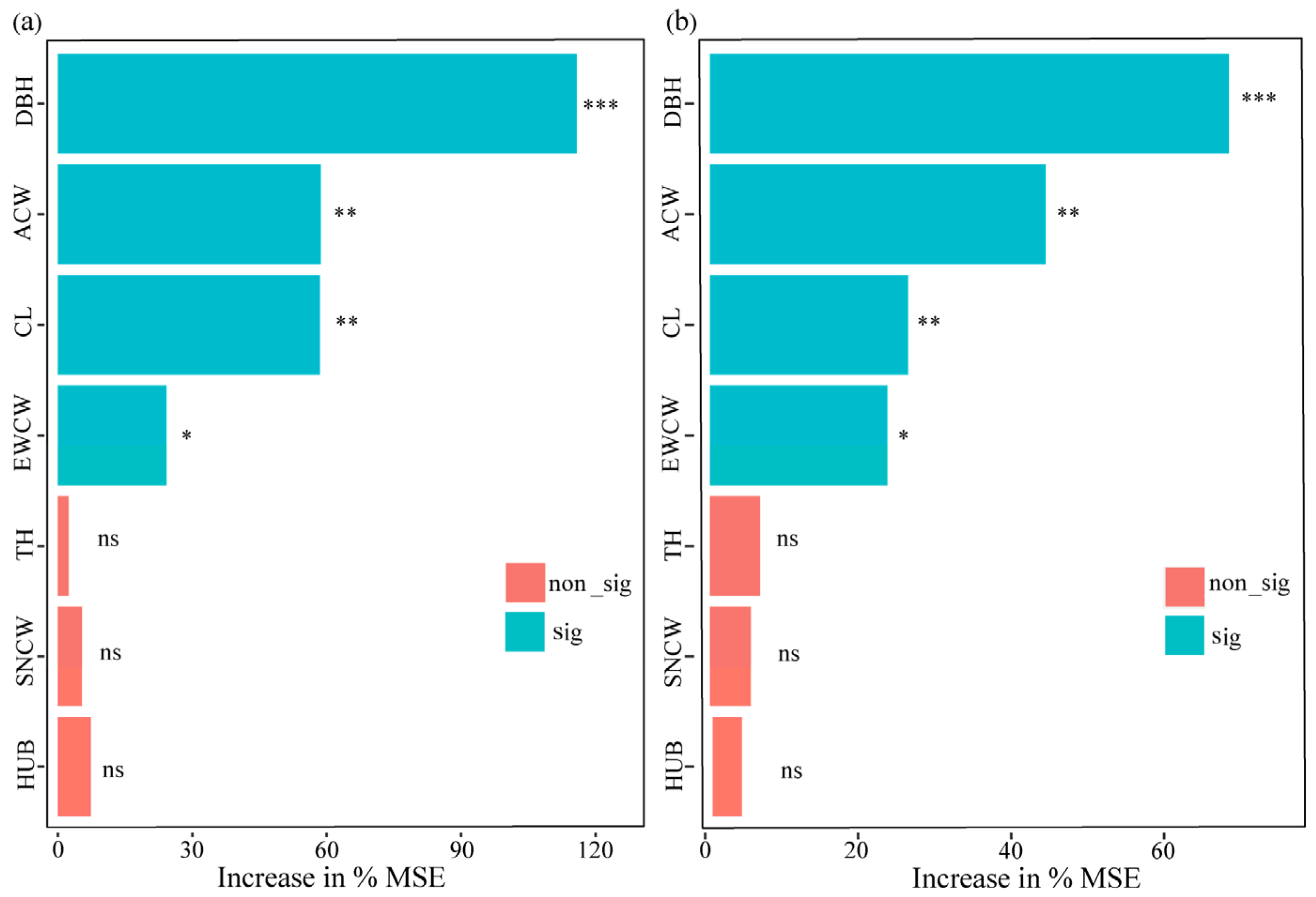
3.3. Relationship between Tree Attributes and Cavity Parameters
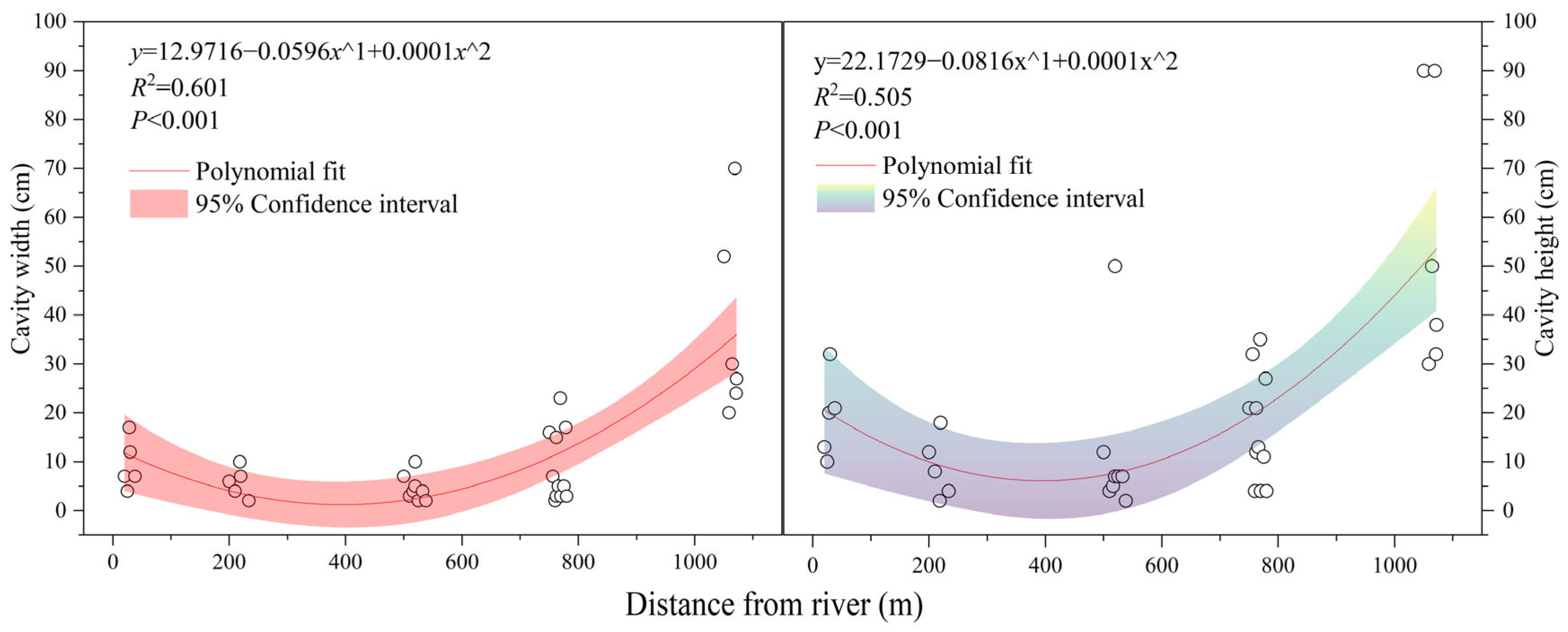
3.4. Relationship between Water Availability and Cavity Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsutomu, U.; Masato, S.; Akio, K.; Hiroyuki, T.; JooYoung, C. Both stem and crown mass affect tree resistance to uprooting. J. For. Res. 2012, 17, 65–71. [Google Scholar]
- Wei, Z.C.; Halik, U.; Aishan, T.; Abliz, A.; Welp, M. Spatial distribution patterns of trunk internal decay of Euphrates poplar riparian forest along the Tarim River, northwest China. For. Ecol. Manag. 2022, 522, 120434. [Google Scholar] [CrossRef]
- Marra, R.E.; Brazee, N.J.; Fraver, S. Estimating carbon loss due to internal decay in living trees using tomography: Implications for forest carbon budgets. Environ. Res. Lett. 2018, 13, 105004. [Google Scholar] [CrossRef]
- Niringiyimana, A.; Nzarora, A.; Twahirwa, J.C.; van der Hoek, Y. Density and characteristics of tree cavities inside and outside Volcanoes National Park, Rwanda. Ecol. Evol. 2022, 12, e9461. [Google Scholar] [CrossRef] [PubMed]
- LaMontagne, J.M.; Kilgour, R.J.; Anderson, E.C.; Magle, S. Tree cavity availability across forest, park, and residential habitats in a highly urban area. Urban Ecosyst. 2015, 18, 151–167. [Google Scholar] [CrossRef]
- Harper, M.J.; McCarthy, M.A.; van der Ree, R. The abundance of hollow-bearing trees in urban dry sclerophyll forest and the effect of wind on hollow development. Biol. Conserv. 2005, 122, 181–192. [Google Scholar] [CrossRef]
- Fan, Z.F.; Shifley, S.R.; Spetich, M.A.; Thompson, F.R.; Larsen, D.R. Distribution of cavity trees in midwestern old-growth and second-growth forests. Can. J. For. Res. 2003, 33, 1481–1494. [Google Scholar] [CrossRef]
- Liu, J.Y.; Zheng, Z.; Xu, X.; Dong, T.F.; Chen, S.C. Abundance and distribution of cavity trees and the effect of topography on cavity presence in a tropical rainforest, southwestern China. Can. J. For. Res. 2018, 48, 1058–1066. [Google Scholar] [CrossRef]
- Takashima, A.; Nakanishi, A.; Morishita, M.; Abe, S.; Saito, K.; Kotaka, N. Tree-cavity formation in the mature subtropical forests of Yambaru, Okinawa Island. J. For. Res.-Jpn. 2021, 26, 410–418. [Google Scholar] [CrossRef]
- Soge, A.O.; Popoola, O.I.; Adetoyinbo, A.A. Detection of wood decay and cavities in living trees: A review. Can. J. For. Res. 2021, 51, 937–947. [Google Scholar] [CrossRef]
- Jauregui, A.; Rodriguez, S.A.; Garcia, L.N.G.; Gonzalez, E.; Segura, L.N. Wood density and tree size used as cues to locate and excavate cavities in two Colaptes woodpeckers inhabiting a threatened southern temperate forest of Argentina. For. Ecol. Manag. 2021, 502, 119723. [Google Scholar] [CrossRef]
- Dudinszky, N.; Ippi, S.; Kitzberger, T.; Ceron, G.; Ojeda, V. Tree size and crown structure explain the presence of cavities required by wildlife in cool-temperate forests of South America. For. Ecol. Manag. 2021, 494, 119295. [Google Scholar] [CrossRef]
- Peng, Y.; He, G.J.; Wang, G.Z. Spatial-temporal analysis of the changes in Populus euphratica distribution in the Tarim National Nature Reserve over the past 60 years. Int. J. Appl. Earth Obs. Geoinf. 2022, 113, 103000. [Google Scholar]
- Aishan, T. Degraded Tugai Forests under Rehabilitation in the Tarim Riparian Ecosystem, Northwest China: Monitoring, Assessing and Modelling. Ph.D. Thesis, Katholische Universitat Eichstatt-Ingolstadt, Eichstätt, Germany, 2016; 152p. [Google Scholar]
- Ling, H.B.; Guo, B.; Zhang, G.P.; Xu, H.L.; Deng, X.Y. Evaluation of the ecological protective effect of the “large basin“ comprehensive management system in the Tarim River basin, China. Sci. Total Environ. 2019, 650, 1696–1706. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.B.; Zhang, P.; Xu, H.L.; Zhao, X.F. How to Regenerate and Protect Desert Riparian Populus euphratica Forest in Arid Areas. Sci. Rep. 2015, 5, 15418. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Li, W.H.; Yuan, F.; Gong, W.C.; Tian, Z.P. Ecological adaptation characteristics of Populus euphratica and Tamarix ramosissima leaf microstructures in the lower reaches of Tarim River. Acta Ecol. Sin. 2010, 30, 62–66. [Google Scholar] [CrossRef]
- Li, D.; Si, J.H.; Zhang, X.Y.; Gao, Y.Y.; Luo, H.; Qin, J.; Ren, L.X. Ecological adaptation of Populus euphratica to drought stress. J. Desert Res. 2020, 40, 17–23. (In Chinese) [Google Scholar]
- Zhao, R.; Chen, S.L. The salt-stress signaling network involved in the regulation of ionic and ROS homeostasis in poplar. Sci. Sinica Vitae 2020, 50, 167–175. (In Chinese) [Google Scholar]
- Eusemann, P.; Petzold, A.; Thevs, N.; Schnittler, M. Growth patterns and genetic structure of Populus euphratica Oliv. (Salicaceae) forests in NW China–Implications for conservation and management. Forest Ecol. Manag. 2013, 297, 27–36. [Google Scholar] [CrossRef]
- Renton, K.; Salinas-Melgoza, A.; Rueda-Hernandez, R.; Vazquez-Reyes, L.D. Differential resilience to extreme climate events of tree phenology and cavity resources in tropical dry forest: Cascading effects on a threatened species. Forest Ecol. Manag. 2018, 426, 164–175. [Google Scholar] [CrossRef]
- Vazquez, L.; Renton, K. High density of tree-cavities and snags in tropical dry forest of western Mexico raises questions for a latitudinal gradient. PLoS ONE 2015, 10, e0116745. [Google Scholar] [CrossRef] [PubMed]
- Onodera, K.; Tokuda, S.; Abe, T.; Nagasaka, A. Occurrence probabilities of tree cavities classified by entrance width and internal dimensions in hardwood forests in Hokkaido, Japan. J. Forest Res.-Jpn. 2013, 18, 101–110. [Google Scholar] [CrossRef]
- Mumin, R.; Aishan, T.; Halik, U. Hollow-bearing characteristics of Populus euphratica in the lower reaches of Tarim River, China. Chin. J. Appl. Ecol. 2020, 31, 1933–1940. (In Chinese) [Google Scholar]
- Cheng, Q.; Aishan, T.; Halik, U.; Wang, X.Y. Hollow tree characteristics of different aged Populus euphratica forests in the middle reaches of the Tarim River. Arid Zone Res. 2023, 40, 247–256. (In Chinese) [Google Scholar]
- Li, Z.W.; Yu, G.A.; Brierley, G.J.; Wang, Z.Y.; Jia, Y.H. Migration and cutoff of meanders in the hyperarid environment of the middle Tarim River, northwestern China. Geomorphology 2017, 276, 116–124. [Google Scholar] [CrossRef]
- Lindenmayer, D.B. Cavity sizes and types in Australian eucalypts from wet and dry forest types—A simple of rule of thumb for estimating size and number of cavities. Forest Ecol. Manag. 2000, 137, 139–150. [Google Scholar] [CrossRef]
- Wang, S.J.; Chen, B.H.; Li, H.Q. Populus euphratica Forest; China Environmental Science Press: Beijing, China, 1995; pp. 57–59. (In Chinese) [Google Scholar]
- Remm, J.; Lõhmus, A.; Remm, K. Tree cavities in riverine forests: What determines their occurrence and use by hole-nesting passerines? Forest Ecol. Manag. 2006, 221, 267–277. [Google Scholar] [CrossRef]
- Wang, Y.T.; Xu, H.D.; Wang, L.H.; Li, F.R.; Sun, H. Field investigation of decay rate of Korean pine standing trees in natural forests in Lesser Xing’an Mountains. J. Beijing For. Univ. 2015, 37, 97–104. (In Chinese) [Google Scholar]
- Brandeis, T.J.; Newton, M.; Filip, G.M. Cavity-nester habitat development in artificially made Douglas-fir snags. J. Wildl. Manag. 2002, 66, 625–633. [Google Scholar] [CrossRef]
- Boyle, W.A.; Ganong, C.N.; Clark, D.B. Density, distribution, and attributes of tree cavities in an old-growth tropical rain forest. Biotropica 2008, 40, 241–245. [Google Scholar] [CrossRef]
- Gruebler, M.U.; Schaller, S.; Keil, H.; Naef-Daenzer, B. The occurrence of cavities in fruit trees: Effects of tree age and management on biodiversity in traditional European orchards. Biodivers. Conserv. 2013, 22, 3233–3246. [Google Scholar] [CrossRef]
- Ibarra, J.T.; Novoa, F.J.; Jaillard, H.; Altamirano, T.A. Large trees and decay: Suppliers of a keystone resource for cavity-using wildlife in old-growth and secondary Andean temperate forests. Austral Ecol. 2020, 45, 1135–1144. [Google Scholar] [CrossRef]
- Thomas, F.M. Ecology of phreatophytes. Prog. Bot. 2014, 75, 335–375. [Google Scholar]
- Thomas, F.M.; Jeschke, M.; Zhang, X.; Lang, P. Stand structure and productivity of Populus euphratica along a gradient of groundwater distances at the Tarim River (NW China). J. Plant Ecol. 2017, 10, 753–764. [Google Scholar]
- Si, J.H.; Feng, Q.; Cao, S.K.; Yu, T.F.; Zhao, C.Y. Water use sources of desert riparian Populus euphratica forests. Environ. Monit. Assess 2014, 186, 5469–5477. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.L.; Rahim, R.A.; Rahiman, M.H.F.; Talib, M.T.M.; Tee, Z.C. Sensing wood decay in standing trees: A review. Sensor Actuat. A Phys. 2018, 269, 276–282. [Google Scholar] [CrossRef]
- Li, H.B.; Zhang, X.W.; Li, Z.Q.; Wen, J.; Tan, X. A Review of Research on Tree Risk Assessment Methods. Forests 2022, 13, 1556. [Google Scholar] [CrossRef]
- Okun, A.; Brazee, N.J.; Clark, J.R.; Cunningham-Minnick, M.J.; Burcham, D.C.; Kane, B. Assessing the Likelihood of Failure Due to Stem Decay Using Different Assessment Techniques. Forests 2023, 14, 1043. [Google Scholar] [CrossRef]



| Attributes at Tree and Cavity Levels | Sample Plot | ||||
|---|---|---|---|---|---|
| Plot 1 | Plot 2 | Plot 3 | Plot 4 | Plot 5 | |
| Number of trees (n) | 42 | 26 | 41 | 55 | 11 |
| Distance from the river (DR) (m) | DR ≤ 20 m | 20 < DR ≤ 200 m | 200 < DR ≤ 500 m | 500 < DR ≤ 750 m | 750 < DR ≤ 1050 m |
| Number of trees with cavities (n) | 11 | 14 | 21 | 41 | 11 |
| Proportion of hollow trees (%) | 26.20 | 53.80 | 51.20 | 74.50 | 100 |
| Tree density (n/ha) | 334 | 207 | 326 | 438 | 88 |
| Mean tree height (m) | 8.20 ± 2.0 | 7.10 ± 1.41 | 7.52 ± 2.13 | 5.54 ± 2.12 | 4.30 ± 1.33 |
| Mean DBH (cm) | 28.50 ± 8.90 | 35.42 ± 18.80 | 25.93 ± 8.51 | 29.91 ± 14.93 | 74.90 ± 30.20 |
| Mean crown width (m) | 7.20 ± 1.90 | 8.50 ± 2.00 | 7.13 ± 1.81 | 6.60 ± 1.73 | 6.30 ± 2.31 |
| Mean crown loss (%) | 12.3 ± 12.0 | 29.2 ± 0.2 | 26.0 ± 0.1 | 33.0 ± 0.2 | 67.0 ± 0.1 |
| Number of butt hollows (n) | 0 | 2 | 3 | 8 | 5 |
| Number of fissures (n) | 0 | 3 | 0 | 3 | 4 |
| Number of trunk main holes (n) | 4 | 14 | 11 | 42 | 36 |
| Number of branch middle holes (n) | 6 | 9 | 12 | 8 | 34 |
| Number of branch end holes (n) | 1 | 13 | 3 | 20 | 33 |
| Number of bayonet holes (n) | 0 | 1 | 1 | 1 | 1 |
| Number of trunk top holes (n) | 5 | 6 | 6 | 39 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aishan, T.; Mumin, R.; Halik, Ü.; Jiang, W.; Sun, Y.; Yusup, A.; Chen, T. Patterns in Tree Cavities (Hollows) in Euphrates Poplar (Populus euphratica, Salicaceae) along the Tarim River in NW China. Forests 2024, 15, 421. https://doi.org/10.3390/f15030421
Aishan T, Mumin R, Halik Ü, Jiang W, Sun Y, Yusup A, Chen T. Patterns in Tree Cavities (Hollows) in Euphrates Poplar (Populus euphratica, Salicaceae) along the Tarim River in NW China. Forests. 2024; 15(3):421. https://doi.org/10.3390/f15030421
Chicago/Turabian StyleAishan, Tayierjiang, Reyila Mumin, Ümüt Halik, Wen Jiang, Yaxin Sun, Asadilla Yusup, and Tongyu Chen. 2024. "Patterns in Tree Cavities (Hollows) in Euphrates Poplar (Populus euphratica, Salicaceae) along the Tarim River in NW China" Forests 15, no. 3: 421. https://doi.org/10.3390/f15030421








