Differences in Response of Tree Species at Different Succession Stages to Neighborhood Competition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Tree Selection
2.3. Sample Collection and Data Calculation
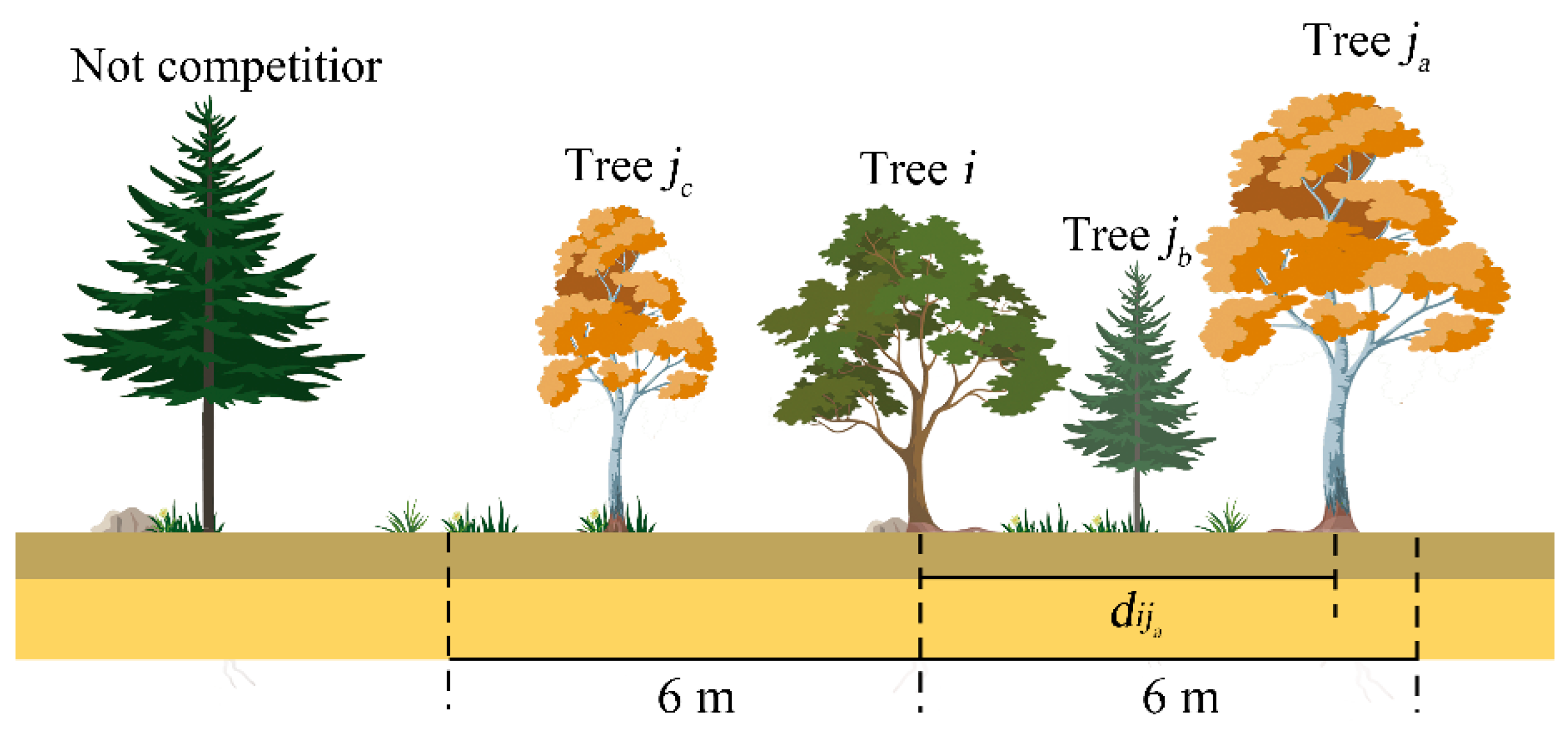
2.3.1. Competition Index
2.3.2. Annual Basal Area Increment
2.3.3. Architecture Indicator
2.3.4. Leaf Functional Traits Indicator
2.4. Statistical Analysis
3. Results
3.1. Tree Radial Growth
3.2. Tree Crown Architecture
3.3. Leaf Functional Traits
4. Discussion
4.1. Negative Correlation between Average Annual Growth of Basal Area and Competition Intensity
4.2. The Response of Tree Canopy Architecture to Neighboring Competition Varies among Different Succession Stages of Tree Species
4.3. Response of Leaf Functional Traits to Neighboring Competition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Yan, E.; Yan, X.; Wang, L. Analysis of degraded evergreen broad-leaved forest communities in Eastern China and issues in forest restoration. Acta Ecol. Sin. 2005, 25, 1796–1803. [Google Scholar]
- Enoki, T. Microtopography and distribution of canopy trees in a subtropical evergreen broad-leaved forest in the northern part of Okinawa Island, Japan. Ecol. Res. 2003, 18, 103–113. [Google Scholar] [CrossRef]
- Xue, J.H. Forest Ecology; China Forestry Publishing House: Beijing, China, 2006. [Google Scholar]
- Buma, B.; Bisbing, S.M.; Wiles, G.; Bidlack, A.L. 100yr of primary succession highlights stochasticity and competition driving community establishment and stability. Ecology 2019, 100, e02885. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D. Community Diversity and Succession: The Roles of Competition, Dispersal, and Habitat Modification. Ecol. Stud. 1993, 99, 327–344. [Google Scholar]
- Uriarte, M.; Canham, C.D.; Thompson, J.; Zimmerman, J.K. A neighborhood analysis of tree growth and survival in a hurricane-driven tropical forest. Ecol. Monogr. 2004, 74, 591–614. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, J. Spatial pattern and competitive relationships of moso bamboo in a native subtropical rainforest community. Forests 2018, 9, 774. [Google Scholar] [CrossRef]
- Song, M.H.; Hu, Q.W.; Tian, Y.Q.; Ouyang, H. Seasonal patterns of root and shoot interactions in an alpine meadow on the Tibetan Plateau. J. Plant Ecol. 2012, 5, 182–190. [Google Scholar] [CrossRef]
- Yang, X.Z.; Zhang, W.H.; He, Q.Y. Effects of intraspecific competition on growth, architecture and biomass allocation of Quercus Liaotungensis. J. Plant Interact. 2019, 14, 284–294. [Google Scholar] [CrossRef]
- Yang, Q.; Fu, F.; Zhang, L.; Liang, Y.; Tang, P.; Liu, Z.; Guo, X. A study on the neighborhood interrerence index in Toona ciliates var. pubescens community. Acta Agric. Univ. Jiangxiensis 2013, 35, 748–754. [Google Scholar]
- Coomes, D.A.; Allen, R.B. Effects of size, competition and altitude on tree growth. J. Ecol. 2007, 95, 1084–1097. [Google Scholar] [CrossRef]
- Masaki, T.; Mori, S.; Kajimoto, T.; Hitsuma, G.; Sawata, S.; Osumi, K.; Sakurai, S.; Seki, T. Long-term growth analyses of Japanese cedar trees in a plantation: Neighborhood competition and persistence of initial growth deviations. J. For. Res. 2006, 2006, 217–225. [Google Scholar] [CrossRef]
- Ding, Y.; Zang, R.; Huang, J.; Xu, Y.; Lu, X.; Guo, Z.; Ren, W. Intraspecific trait variation and neighborhood competition drive community dynamics in an old-growth spruce forest in northwest China. Sci. Total Environ. 2019, 678, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Fortunel, C.; Valencia, R.; Wright, S.J.; Garwood, N.C.; Kraft, N.J. Functional trait differences influence neighbourhood interactions in a hyperdiverse Amazonian forest. Ecol. Lett. 2016, 19, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Szwagrzyk, J.; Szewczyk, J.; Maciejewski, Z. Shade-tolerant tree species from temperate forests differ in their competitive abilities: A case study from Roztocze, south-eastern Poland. For. Ecol. Manag. 2012, 2012, 28–35. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Y.; Lian, J.; Ye, W. Difference in survival response of tree species to neighborhood crowding in a lower subtropical evergreen broad-leaved forest of Dinghushan. Biodivers. Sci. 2018, 26, 535. [Google Scholar] [CrossRef]
- Hubbell, S.P.; Ahumada, J.A.; Condit, R.; Foster, R.B. Local neighborhood effects on long-term survival of individual trees in a neotropical forest. Ecol. Res. 2001, 16, 859–875. [Google Scholar] [CrossRef]
- Chen, L.X.; Xiang, W.H.; Wu, H.L.; Lei, P.F.; Li, S.; Ouyang, S.; Deng, X.W.; Fang, X. Tree growth traits and social status affect the wood density of pioneer species in secondary subtropical forest. Ecol. Evol. 2017, 2017, 5366–5377. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, D.; Shier, A. Wood specific gravity and its radial variations: The many ways to make a tree. Trees 2002, 16, 437–443. [Google Scholar] [CrossRef]
- Anten, N.P.R.; Schieving, F. The role of wood mass density and mechanical constraints in the economy of tree architecture. Am. Nat. 2010, 175, 250–260. [Google Scholar] [CrossRef]
- Barbeito, I.; Collet, C.; Ningre, F.O. Crown responses to neighbor density and species identity in a young mixed deciduous stand. Trees 2014, 28, 1751–1765. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Linsenmair, K.E.; Davis, A.J.; Fiala, B.; Speight, M.R. Tree architecture in a Bornean lowland rain forest: Intraspecific and interspecific patterns. Springer Neth. 2001, 153, 279–292. [Google Scholar]
- Schröter, M.; Härdtle, W.; Oheimb, G.V. Crown plasticity and neighborhood interactions of European beech (Fagus sylvatica L.) in an old-growth forest. Eur. J. For. Res. 2012, 131, 787–798. [Google Scholar] [CrossRef]
- Thorpe, H.C.; Astrup, R.; Trowbridge, A.; Coates, K.D. Competition and tree crowns: A neighborhood analysis of three boreal tree species. For. Ecol. Manag. 2010, 259, 1586–1596. [Google Scholar] [CrossRef]
- Seidel, D.; Leuschner, C.; Müller, A.; Krause, B. Crown plasticity in mixed forests—Quantifying asymmetry as a measure of competition using terrestrial laser scanning. For. Ecol. Manag. 2011, 261, 2123–2132. [Google Scholar] [CrossRef]
- Kunz, M.; Fichtner, A.; Härdtle, W.; Raumonen, P.; Bruelheide, H.; von Oheimb, G. Neighbour species richness and local structural variability modulate aboveground allocation patterns and crown morphology of individual trees. Ecol. Lett. 2019, 22, 2130–2140. [Google Scholar] [CrossRef] [PubMed]
- Lintunen, A.; Kaitaniemi, P. Responses of crown architecture in Betula pendula to competition are dependent on the species of neighbouring trees. Trees 2010, 24, 411–424. [Google Scholar] [CrossRef]
- Nikinmaa, E.; Ilomki, S.; Mkel, A. Crown rise due to competition drives biomass allocation in silver birch. Can. J. For. Res. 2003, 33, 2395–2404. [Google Scholar]
- Poorter, L.; Bongers, F.; Sterck, F.J.; Wöll, H. Architecture of 53 rain forest tree species differing in adult stature and shade tolerance. Ecology 2003, 84, 602–608. [Google Scholar] [CrossRef]
- Kitajima, K. Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia 1994, 98, 419–428. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.; Falster, D.S.; Groom, P.K.; Hikosaka, K.; Lee, W.; Lusk, C.H.; Niinemets, Ü.; Oleksyn, J. Modulation of leaf economic traits and trait relationships by climate. Glob. Ecol. Biogeogr. 2005, 14, 411–421. [Google Scholar] [CrossRef]
- Ran, L.X.; Jing, L.Q.; Zhe, C.; Qing, M.Z. Specific leaf area and leaf area index of conifer plantaions in Qianyanzhou station of subtropical China. J. Plant Ecol. 2007, 31, 93. [Google Scholar] [CrossRef]
- Yu, G.; Song, X.; Wang, Q.; Liu, Y.; Guan, D.; Yan, J.; Sun, X.; Zhang, L.; Wen, X. Water-use efficiency of forest ecosystems in eastern China and its relations to climatic variables. New Phytol. 2008, 177, 927–937. [Google Scholar] [CrossRef]
- Briggs, L.J.; Shantz, H.L. The Water Requirement of Plants; US Government Printing Office: Washington, DC, USA, 1913.
- Le Roux, X.; Bariac, T.; Sinoquet, H.; Genty, B.; Piel, C.; Mariotti, A.; Girardin, C.; Richard, P. Spatial distribution of leaf water-use efficiency and carbon isotope discrimination within an isolated tree crown. Plant Cell Environ. 2001, 24, 1021–1032. [Google Scholar] [CrossRef]
- Zimmerman, J.K.; Ehleringer, J.R. Carbon isotope ratios are correlated with irradiance levels in the Panamanian orchid Catasetum viridiflavum. Oecologia 1990, 83, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.L.; Zou, F.; Lai, H. Study on the Diversity of Rare and Endangered Plants in Guanshan Natural Reserve of Jiangxi Province. J. Anhui Agric. Sci. 2012, 40, 4. [Google Scholar]
- State Soil Survey Service of China. China Soil; China Agricultural Press: Beijing, China, 1998. [Google Scholar]
- Liu, X.; Heping, W. Scientific Survey and Study on the Guanshan Nature Reserve in Jiangxi Province; China Forestry Publishing House: London, UK, 2005. [Google Scholar]
- Condit, R. Research in large, long-term tropical forest plots. Trends Ecol. Evol. 1995, 10, 18–22. [Google Scholar] [CrossRef]
- Duan, R.Y.; Wang, X.A. Intraspecific and interspecific competition in Larix chinensis. Chin. J. Plant Ecol. 2005, 29, 242. [Google Scholar]
- Hegyi, F. A Simulation Model for Managing Jack Pine Stands, in “Growth for Trees and Stand Simulation” IUFRO. Proc. Work. Party. S 1974, 4, 1–4. [Google Scholar]
- He, H. Measurement of tree-ring width with Win-DENERO and crossdating methods. J. Chongqing Norm. Univ. 2005, 22, 39–44. [Google Scholar]
- Lu, R.K. Methods of Soil and Agrochemical Analysis; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Farquhar, G.D.; O’Leary, M.H.; Berry, J.A. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Funct. Plant Biol. 1982, 9, 121–137. [Google Scholar] [CrossRef]
- Huang, Z.; Ran, S.; Fu, Y.; Wan, X.; Song, X.; Chen, Y.; Yu, Z. Functionally dissimilar neighbours increase tree water use efficiency through enhancement of leaf phosphorus concentration. J. Ecol. 2022, 110, 2179–2189. [Google Scholar] [CrossRef]
- Feng, X. Long-term ci/ca response of trees in western North America to atmospheric CO2 concentration derived from carbon isotope chronologies. Oecologia 1998, 117, 19–25. [Google Scholar] [CrossRef]
- Qi, C.J.T.; Guo, G. Dendrology; China Forestry Publishing House: Beijing, China, 2005. [Google Scholar]
- Chen, J.; Zhao, C.Z.; Wang, J.W.; Zhao, L.C. Canopy structure and radiation interception of Salix matsudana: Stand density dependent relationships. Chin. J. Plant Ecol. 2017, 41, 661–669. [Google Scholar]
- Osunkoya, O.O.; Omar-Ali, K.; Amit, N.; Dayan, J.; Daud, D.S.; Sheng, T.K. Comparative height–crown allometry and mechanical design in 22 tree species of Kuala Belalong rainforest, Brunei, Borneo. Am. J. Bot. 2007, 94, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.W.; Sillett, S.C.; Jennings, G.M.; Davis, S.D. The limits to tree height. Nature 2004, 428, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Fotis, A.T.; Morin, T.H.; Fahey, R.T.; Hardiman, B.S.; Bohrer, G.; Curtis, P.S. Forest structure in space and time: Biotic and abiotic determinants of canopy complexity and their effects on net primary productivity. Agric. For. Meteorol. 2018, 250, 181–191. [Google Scholar] [CrossRef]
- Falster, D.S.; Westoby, M. Plant height and evolutionary games. Trends Ecol. Evol. 2003, 18, 337–343. [Google Scholar] [CrossRef]
- De Schepper, V.; Van Dusschoten, D.; Copini, P.; Jahnke, S.; Steppe, K. MRI links stem water content to stem diameter variations in transpiring trees. J. Exp. Bot. 2012, 63, 2645–2653. [Google Scholar] [CrossRef] [PubMed]
- Gommers, C.M.; Visser, E.J.; St Onge, K.R.; Voesenek, L.A.; Pierik, R. Shade tolerance: When growing tall is not an option. Trends Plant Sci. 2013, 18, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Tomlinson, K.W.; Li, J. Strong intraspecific trait variation in a tropical dominant tree species along an elevational gradient. Plant Divers. 2020, 42, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.Z.; Xia, Z.W.; Fu, L.J.; Ming, Z.J. The Variation Characteristics of Plant Functional Traits among 16 Woody Plants in Subtropical Broad-leaved Forest at Dagang Mountain. J. Fujian Norm. Univ. 2019, 12, e8680. [Google Scholar]
- Firn, J.; McGree, J.M.; Harvey, E.; Flores-Moreno, H.; Schütz, M.; Buckley, Y.M.; Borer, E.T.; Seabloom, E.W.; La Pierre, K.J.; MacDougall, A.M. Leaf nutrients, not specific leaf area, are consistent indicators of elevated nutrient inputs. Nat. Ecol. Evol. 2019, 3, 400–406. [Google Scholar] [CrossRef]
- Gonzalez de Andres, E.; Camarero, J.J.; Blanco, J.A.; Imbert, J.B.; Lo, Y.H.; Sangüesa-Barreda, G.; Castillo, F.J. Tree-to-tree competition in mixed European beech–Scots pine forests has different impacts on growth and water-use efficiency depending on site conditions. J. Ecol. 2018, 106, 59–75. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S.v.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Will, R.E.; Wilson, S.M.; Zou, C.B.; Hennessey, T.C. Increased vapor pressure deficit due to higher temperature leads to greater transpiration and faster mortality during drought for tree seedlings common to the forest–grassland ecotone. New Phytol. 2013, 200, 366–374. [Google Scholar] [CrossRef]
- Qi, Z.S.; Lun, S. Research progress on water use efficiency of plant. Agric. Res. Arid. Areas 2002, 20, 1–5. [Google Scholar]
- Driscoll, A.W.; Bitter, N.Q.; Ehleringer, J.R. Interactions among intrinsic water-use efficiency and climate influence growth and flowering in a common desert shrub. Oecologia 2021, 197, 1027–1038. [Google Scholar] [CrossRef]
- Ducrey, M.; Huc, R.; Ladjal, M.; Guehl, J.-M. Variability in growth, carbon isotope composition, leaf gas exchange and hydraulic traits in the eastern Mediterranean cedars Cedrus libani and C. brevifolia. Tree Physiol. 2008, 28, 689–701. [Google Scholar] [CrossRef]




| Index | CL | PM | AF | CA | ED | CC |
|---|---|---|---|---|---|---|
| LDW | −0.698 ** | −0.702 ** | −0.386 ** | −0.571 ** | −0.136 | −0.318 ** |
| LA | −0.204 ** | 0.285 ** | 0.314 ** | −0.004 | 0.536 ** | 0.186 ** |
| SLA | 0.292 ** | 0.909 ** | 0.890 ** | 0.785 ** | 0.641 ** | 0.569 ** |
| TC | −0.660 ** | 0.054 | −0.410 | −0.227 | −0.247 | −0.105 |
| TN | 0.466 * | −0.161 | 0.169 | 0.513 * | −0.107 | 0.194 |
| TP | 0.438 * | −0.303 | 0.004 | −0.044 | −0.409 | −0.117 |
| C/N | −0.576 ** | 0.205 | −0.245 | −0.472 | 0.049 | −0.199 |
| C/P | −0.556 ** | 0.408 | −0.076 | 0.022 | 0.494 * | 0.041 |
| N/P | −0.144 | 0.286 | 0.208 | 0.360 | 0.383 | 0.134 |
| WUE | −0.527 | −0.118 | −0.845 ** | −0.447 | 0.266 | 0.645 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Li, J.; Peng, Q.; Gong, C.; Ran, H.; Xie, T.; Liao, T.; Zhou, T.; Huang, D.; Song, Q.; et al. Differences in Response of Tree Species at Different Succession Stages to Neighborhood Competition. Forests 2024, 15, 435. https://doi.org/10.3390/f15030435
Zeng X, Li J, Peng Q, Gong C, Ran H, Xie T, Liao T, Zhou T, Huang D, Song Q, et al. Differences in Response of Tree Species at Different Succession Stages to Neighborhood Competition. Forests. 2024; 15(3):435. https://doi.org/10.3390/f15030435
Chicago/Turabian StyleZeng, Xiaoxia, Jiejun Li, Qiaohua Peng, Chao Gong, Huan Ran, Tingting Xie, Ting Liao, Tianling Zhou, Dongmei Huang, Qingni Song, and et al. 2024. "Differences in Response of Tree Species at Different Succession Stages to Neighborhood Competition" Forests 15, no. 3: 435. https://doi.org/10.3390/f15030435
APA StyleZeng, X., Li, J., Peng, Q., Gong, C., Ran, H., Xie, T., Liao, T., Zhou, T., Huang, D., Song, Q., Liu, J., & Yang, Q. (2024). Differences in Response of Tree Species at Different Succession Stages to Neighborhood Competition. Forests, 15(3), 435. https://doi.org/10.3390/f15030435







