Can Growth Increase of Small Trees after Drought Compensate for Large Trees’ Growth Loss?
Abstract
:1. Introduction
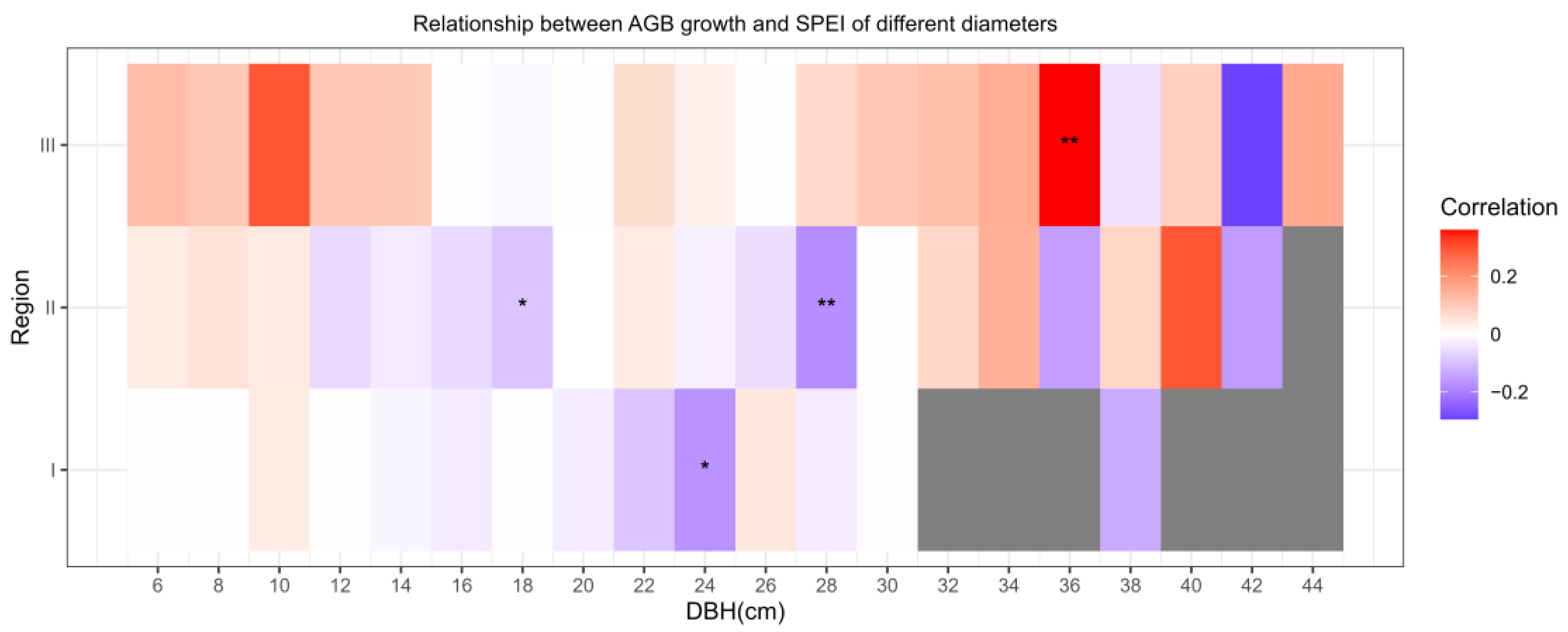
- In the southern part of the study area, droughts would suppress the growth of larches, while in the northern part, growth will be prompted by droughts.
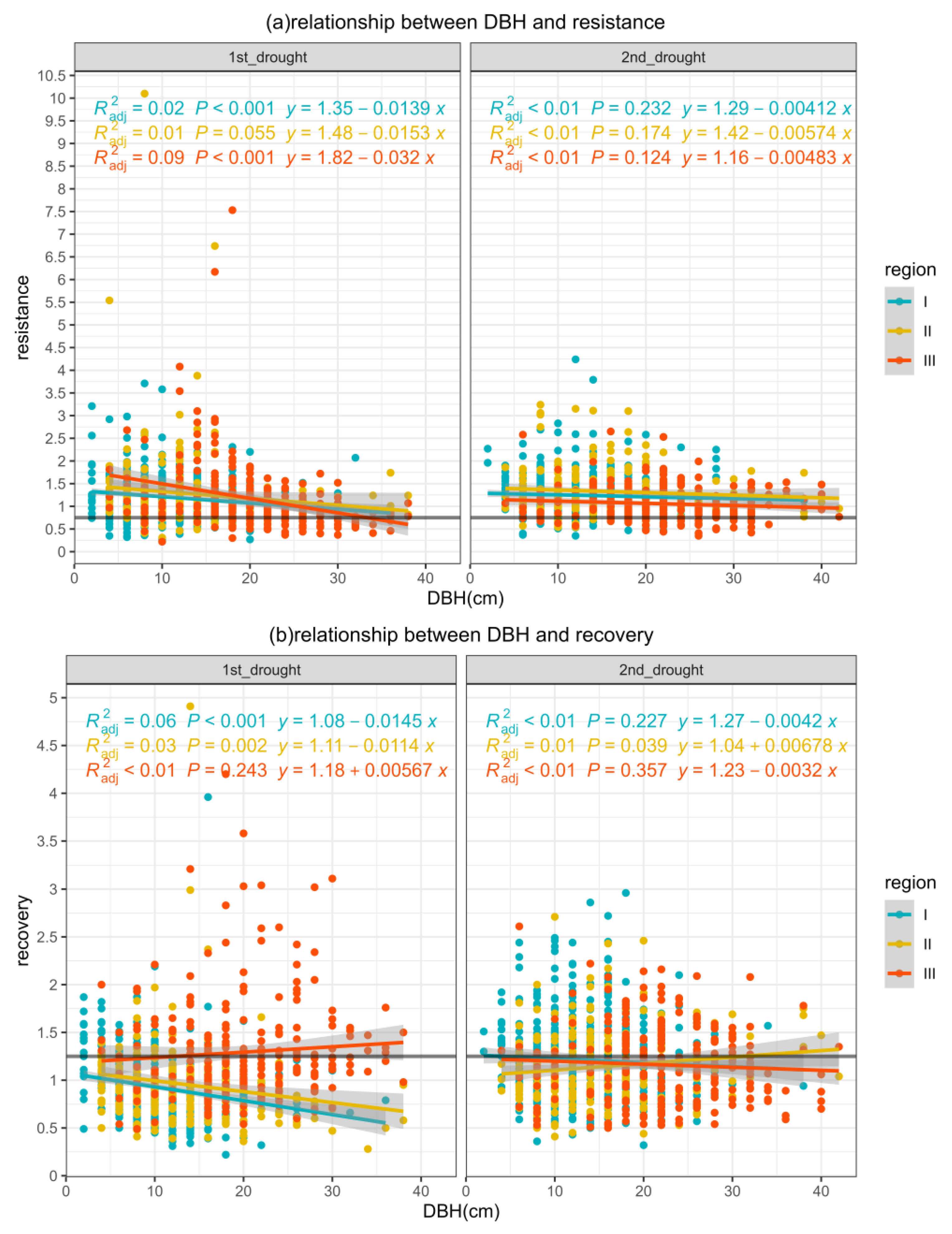
- Larger trees will suffer from droughts, but smaller trees could maintain stand growth after a drought event.
- Multiple droughts will lead to larch aboveground biomass growth reduction in the study area.
2. Materials and Methods
2.1. Study Area
2.2. Field and Laboratory Measurements
2.3. Statistical Analysis Methods
2.3.1. Drought Events Selection and Region Partition
2.3.2. Reconstruction of Historical AGB
2.3.3. Size-Different AGB Response to Drought Events
3. Results
3.1. Stand Age of the Study Area
3.2. Size-Different Relationship between Aboveground Biomass Growth and Drought Severity
3.2.1. Drought Characteristics of Each Region
3.2.2. Size Affects Relationships between AGB Growth and Local SPEI
3.3. Size Affects Tree Resilience to Drought Events
3.3.1. Resistance
3.3.2. Recovery
3.3.3. Resilience
3.4. Growth of Small Trees Cannot Compensate for Large Trees’ Growth Loss
4. Discussion
4.1. Response of Trees’ Growth in Different Sizes to Drought Events
4.2. Tree Resilience under Multiple Drought Events
4.3. Stand-Level AGB Growth Dynamics under Frequent Droughts
4.4. Prospects and Outlook
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Seidl, R.; Schelhaas, M.-J.; Rammer, W.; Verkerk, P.J. Increasing Forest Disturbances in Europe and Their Impact on Carbon Storage. Nat. Clim. Change 2014, 4, 806–810. [Google Scholar] [CrossRef]
- Fan, L.; Wigneron, J.-P.; Ciais, P.; Chave, J.; Brandt, M.; Sitch, S.; Yue, C.; Bastos, A.; Li, X.; Qin, Y.; et al. Siberian Carbon Sink Reduced by Forest Disturbances. Nat. Geosci. 2023, 16, 56–62. [Google Scholar] [CrossRef]
- Bennett, A.C.; McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.J. Larger Trees Suffer Most during Drought in Forests Worldwide. Nat. Plants 2015, 1, 15139. [Google Scholar] [CrossRef] [PubMed]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of Tree Resilience: Effects of Successive Low-Growth Episodes in Old Ponderosa Pine Forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Zhang, S.-G.; Lu, Y.-C.; Meng, J.-H.; Zeng, J.; Bai, S.-B. Instant response of individual size inequality indices to thinning regimes in plantation. Ying Yong Sheng Tai Xue Bao 2014, 25, 1645–1651. [Google Scholar] [PubMed]
- Ovenden, T.S.; Perks, M.P.; Clarke, T.-K.; Mencuccini, M.; Jump, A.S. Life after Recovery: Increased Resolution of Forest Resilience Assessment Sheds New Light on Post-Drought Compensatory Growth and Recovery Dynamics. J. Ecol. 2021, 109, 3157–3170. [Google Scholar] [CrossRef]
- Hubau, W.; De Mil, T.; Van den Bulcke, J.; Phillips, O.L.; Angoboy Ilondea, B.; Van Acker, J.; Sullivan, M.J.P.; Nsenga, L.; Toirambe, B.; Couralet, C.; et al. The Persistence of Carbon in the African Forest Understory. Nat. Plants 2019, 5, 133–140. [Google Scholar] [CrossRef]
- Chen, S.; Zang, S.; Sun, L. Characteristics of Permafrost Degradation in Northeast China and Its Ecological Effects: A Review. Sci. Cold Arid. Reg. 2020, 12, 1–11. [Google Scholar]
- Burrell, A.L.; Sun, Q.; Baxter, R.; Kukavskaya, E.A.; Zhila, S.; Shestakova, T.; Rogers, B.M.; Kaduk, J.; Barrett, K. Climate Change, Fire Return Intervals and the Growing Risk of Permanent Forest Loss in Boreal Eurasia. Sci. Total Environ. 2022, 831, 154885. [Google Scholar] [CrossRef] [PubMed]
- Huang, X. Relationship between Plant Functional Diversity and Productivity of Pinus Massoniana Plantations in Guangxi. Biodivers. Sci. 2018, 26, 690. [Google Scholar] [CrossRef]
- Sun, B.F.; Zhao, H.; Wang, X.K. Spatiotemporal Characteristics of Drought in Northeast China Based on SPEI. Ecol. Environ. Sci. 2015, 24, 22–28. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global Convergence in the Vulnerability of Forests to Drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhao, H.; Gao, F.; Zhu, H.; Qu, H.; Zhao, F. Climate Change Trend in Future and Its Influence on Wetlands in the Greater Khingan Mountains. J. Glaciol. Geocryol. 2016, 38, 47–56. [Google Scholar]
- Yiliang, Z. Vegetation Geography in Northeast China; Science Press: Beijing, China, 1997. [Google Scholar]
- Kajimoto, T.; Matsuura, Y.; Osawa, A.; Prokushkin, A.S.; Sofronov, M.A.; Abaimov, A.P. Root System Development of Larix Gmelinii Trees Affected by Micro-Scale Conditions of Permafrost Soils in Central Siberia. In Roots: The Dynamic Interface between Plants and the Earth: The 6th Symposium of the International Society of Root Research, 11–15 November 2001, Nagoya, Japan; Abe, J., Ed.; Developments in Plant and Soil Sciences; Springer Netherlands: Dordrecht, The Netherlands, 2003; pp. 281–292. ISBN 978-94-017-2923-9. [Google Scholar]
- Zhang, P.; Liu, B. Effect of Climate Change on Larix Gmelinii Growth in Different Latitudes. J. Northeast. For. Univ. 2015, 43, 10–13. [Google Scholar]
- Chang, Y.; Chen, Z.; Zhang, X.; Bai, X.; Zhao, X.; Li, J.; Lu, X. Responses of Radial Growth to Temperature in Larix Gmelinii of the Da Hinggan Ling under Climate Warming. Chin. J. Plant Ecol. 2017, 41, 279–289. [Google Scholar]
- Jiang, Y.; Zhang, J.; Han, S.; Chen, Z.; Setälä, H.; Yu, J.; Zheng, X.; Guo, Y.; Gu, Y. Radial Growth Response of Larix Gmelinii to Climate along a Latitudinal Gradient in the Greater Khingan Mountains, Northeastern China. Forests 2016, 7, 295. [Google Scholar] [CrossRef]
- Dapao, Y.; Shunzhong, W. Relationship between tree-ring chronology of Larix olgensis in Changbai Mountains and the climae change. Chin. J. Appl. Ecol. 2005, 16, 14. [Google Scholar]
- McDowell, N.G.; Allen, C.D. Darcy’s Law Predicts Widespread Forest Mortality under Climate Warming. Nat. Clim. Change 2015, 5, 669–672. [Google Scholar] [CrossRef]
- Yang, L.B.; Sui, X.; Wei, D.; Cui, F.X.; Zhu, D.G.; Ni, H.W. Fungal diversity in the brown coniferous forest soils of Daxing’anling Mountains, Northeast China. Chin. J. Appl. Ecol. 2019, 30, 3411–3418. [Google Scholar] [CrossRef]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 51–67. [Google Scholar]
- Yindelehei, B.A.; Xiang, B.A.O.; Mei, Z.; Pengwu, Z.; Liang, S.H.I.; Liangjie, H.A. Temporal and Spatial Variation Characteristics of Vegetation Cover on the Northern Slope of Daxing’anling Based on MODIS NDVI. For. Resour. Wanagement 2018, 6, 50. [Google Scholar] [CrossRef]
- National Climate Center; China Meteorological Administration Bureau of Forecasting and Network; China Meteorological Bureau Lanzhou Institute of Arid Meteorology Grades of Meteorological Drought 2018. Available online: https://www.nssi.org.cn/nssi/front/107243850.html (accessed on 26 September 2021).
- Pang, R.; Liu, M.; Gao, L.; Li, S.; Han, X. Dynamic Study on Probability Distribution Zone of Larix Gmelinii Forest Based on Species Distribution Model. J. Northwest For. Univ. 2023, 38, 1–9. [Google Scholar] [CrossRef]
- Guerrero-Salazar, P.L.A.; Yevjevich, V.M. Analysis of Drought Characteristics by the Theory of Runs. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 1975. [Google Scholar]
- State Forestry Administration of China. Tree biomass models and related parameters to carbon accounting for Larix 2016. Available online: https://www.nssi.org.cn/nssi/front/106651533.html (accessed on 12 October 2021).
- Qibing, Z.; Ouya, F.; Lixin, L. Dendroecological Studies on the Tibetan Plateau; Science Press: Beijing, China, 2019; ISBN 978-7-03-062181-8. [Google Scholar]
- Kassambara, A.; Kassambara, M.A. Package ‘Ggpubr’, R package version 0.1.; R Team: Viena, Austria, 2020; Volume 6. [Google Scholar]
- Ali, A.; Wang, L.-Q. Big-Sized Trees and Forest Functioning: Current Knowledge and Future Perspectives. Ecol. Indic. 2021, 127, 107760. [Google Scholar] [CrossRef]
- Baltzer, J.L.; Veness, T.; Chasmer, L.E.; Sniderhan, A.E.; Quinton, W.L. Forests on Thawing Permafrost: Fragmentation, Edge Effects, and Net Forest Loss. Glob. Change Biol. 2014, 20, 824–834. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Team: Viena, Austria, 2013. [Google Scholar]
- Binkley, D. A Hypothesis about the Interaction of Tree Dominance and Stand Production through Stand Development. For. Ecol. Manag. 2004, 190, 265–271. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G.; Biber, P. Drought Can Favour the Growth of Small in Relation to Tall Trees in Mature Stands of Norway Spruce and European Beech. For. Ecosyst. 2018, 5, 20. [Google Scholar] [CrossRef]
- Bréda, N.; Granier, A.; Aussenac, G. Effects of Thinning on Soil and Tree Water Relations, Transpiration and Growth in an Oak Forest (Quercus petraea (Matt.) Liebl.). Tree Physiol. 1995, 15, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Hessburg, P.F.; Charnley, S.; Wendel, K.; White, E.M.; Singleton, P.H.; Peterson, D.W.; Halofsky, J.E.; Gray, A.N.; Spies, T.A.; Flitcrof, R.L. The 1994 Eastside Screens Large-Tree Harvest Limit: Review of Science Relevant to Forest Planning 25 Years Later; United States Department of Agriculture, Forest Service Pacific Northwest: Portland, OR, USA, 2020. [Google Scholar]
- Pretzsch, H.; Biber, P. Size-Symmetric versus Size-Asymmetric Competition and Growth Partitioning among Trees in Forest Stands along an Ecological Gradient in Central Europe. Can. J. For. Res. 2010, 40, 370–384. [Google Scholar] [CrossRef]
- Lucash, M.S.; Scheller, R.M.; Sturtevant, B.R.; Gustafson, E.J.; Kretchun, A.M.; Foster, J.R. More than the Sum of Its Parts: How Disturbance Interactions Shape Forest Dynamics under Climate Change. Ecosphere 2018, 9, e02293. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.-B.; Fang, O.; Mu, Y.-M.; Jia, H.; Lyu, L. Recovery Time of Juniper Trees Is Longer in Wet than Dry Conditions on the Tibetan Plateau in the Past Two Centuries. For. Ecol. Manag. 2021, 497, 119514. [Google Scholar] [CrossRef]
- An, Y.-Y.; Liang, Z.-S. Staged strategy of plants in response to drought stress. Chin. J. Appl. Ecol. 2012, 23, 2907–2915. [Google Scholar] [CrossRef]
- Sánchez-Pinillos, M.; D’Orangeville, L.; Boulanger, Y.; Comeau, P.; Wang, J.; Taylor, A.R.; Kneeshaw, D. Sequential Droughts: A Silent Trigger of Boreal Forest Mortality. Glob. Change Biol. 2022, 28, 542–556. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Ali, A.; Sanaei, A.; Ruiz-Benito, P.; Jucker, T.; Fang, L.; Bai, E.; Ye, J.; Lin, F.; Fang, S.; et al. Few Large Trees, Rather than Plant Diversity and Composition, Drive the above-Ground Biomass Stock and Dynamics of Temperate Forests in Northeast China. For. Ecol. Manag. 2021, 481, 118698. [Google Scholar] [CrossRef]
- Bose, A.K.; Rohner, B.; Bottero, A.; Ferretti, M.; Forrester, D.I. Did the 2018 Megadrought Change the Partitioning of Growth between Tree Sizes and Species? A Swiss Case-Study. Plant Biol. 2022, 24, 1146–1156. [Google Scholar] [CrossRef]
- Ni, M.; Vellend, M. Space-for-Time Inferences about Range-Edge Dynamics of Tree Species Can Be Influenced by Sampling Biases. Glob. Change Biol. 2021, 27, 2102–2112. [Google Scholar] [CrossRef]
- Wang, F.; Ye, D.-M.; Zang, C.-F.; Zhang, Q.-L. Tree Biomass Distribution of Larix Gmelinii in Daxing’anling Mountains of Inner Mongolia. J. Northwest For. Univ. 2017, 32, 23–28. [Google Scholar] [CrossRef]
- Jin, Y.; Li, J.; Bai, X.; Zhao, Y.; Cui, D.; Chen, Z. High Temperatures Constrain Latewood Formation in Larix Gmelinii Xylem in Boreal Forests. Glob. Ecol. Conserv. 2021, 30, e01767. [Google Scholar] [CrossRef]
- Hu, H.Q.; Luo, B.Z.; Wei, S.J.; Wei, S.W.; Wen, Z.M.; Sun, L.; Luo, S.; Wang, L.M.; Ma, H.B. Estimating Biological Carbon Storage of Five Typical Forest Types in the Daxing’anling Mountains, Heilongjiang, China. Acta Ecol. Sin. 2015, 35, 5745–5760. [Google Scholar]

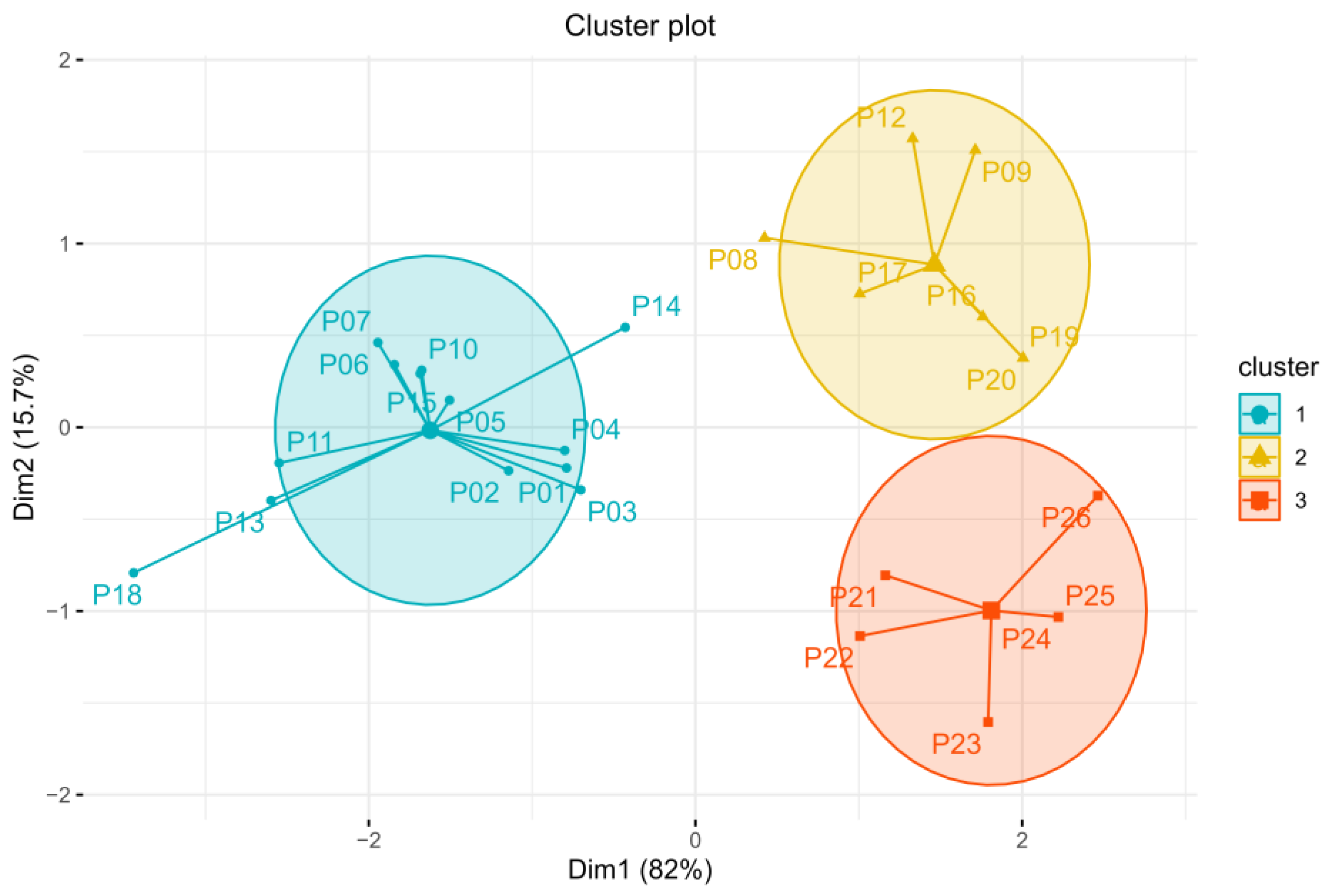



| Plots | Longitude (E) | Latitude (N) | Elevation/m | Slope/° | Slope Direction | Density/n·hm−2 | Mean DBH/cm | Mean Height/m |
|---|---|---|---|---|---|---|---|---|
| P01 | 122°41′28.88″ | 53°22′3.16″ | 522.6 | 3 | southwest | 590 | 18.5 | 24.7 |
| P02 | 121°45′35.84″ | 53°19′20.97″ | 670.3 | 18 | southwest | 1060 | 15.5 | 22.9 |
| P03 | 121°24′2.67″ | 53°1′22.07″ | 436.3 | 3 | northeast | 470 | 18.1 | 21.6 |
| P04 | 122°33′16.06″ | 52°54′0.90″ | 498.5 | 8 | northeast | 790 | 19.4 | 26.6 |
| P05 | 123°20′1.81″ | 52°51′25.90″ | 567.4 | 2 | north | 540 | 16.3 | 26.2 |
| P06 | 125°0′58.02″ | 52°30′19.40″ | 368.3 | 7 | southeast | 720 | 18.7 | 18.6 |
| P07 | 125°51′19.44″ | 52°27′27.90″ | 370.6 | 2 | southeast | 490 | 13.9 | 14.5 |
| P08 | 122°23′32.41″ | 52°18′43.58″ | 741.5 | 22 | south | 880 | 16.4 | 22.3 |
| P09 | 121°27′1.31″ | 52°15′57.03″ | 690.7 | 13 | west | 950 | 12.8 | 21.3 |
| P10 | 124°3′3.28″ | 52°9′8.07″ | 506.8 | 2 | northeast | 640 | 9.1 | 10.1 |
| P11 | 124°56′20.70″ | 52°2′15.05″ | 481.3 | 2 | north | 710 | 13.5 | 13.6 |
| P12 | 121°30′36.51″ | 50°56′22.80″ | 850.2 | 12 | south | 490 | 22.2 | 29 |
| P13 | 126°30′22.12″ | 51°47′4.43″ | 283.3 | 3 | southeast | 690 | 16.3 | 15.9 |
| P14 | 120°50′24.53″ | 51°58′0.16″ | 483.2 | 6 | northeast | 500 | 23.1 | 13.9 |
| P15 | 123°52′56.99″ | 51°36′38.95″ | 721 | 3 | northeast | 490 | 15.6 | 12.2 |
| P16 | 125°44′4.35″ | 51°21′7.70″ | 405.1 | 17 | southwest | 500 | 17 | 26.8 |
| P17 | 120°26′38.38″ | 51°27′38.35″ | 589.7 | 10 | southwest | 530 | 25.4 | 17.8 |
| P18 | 124°41′11.50″ | 51°32′28.00″ | 560.5 | 3 | northeast | 530 | 9.9 | 17.4 |
| P19 | 121°53′29.55″ | 50°43′14.47″ | 822.5 | 9 | southeast | 640 | 21.2 | 20.8 |
| P20 | 122°10′8.93″ | 51°21′31.38″ | 855.5 | 23 | east | 810 | 13 | 24.5 |
| P21 | 122°31′0.17″ | 50°7′11.62″ | 572.6 | 1 | south | 300 | 25.3 | 29.3 |
| P22 | 122°26′54.67″ | 49°31′15.08″ | 590.8 | 2 | southeast | 260 | 30.5 | 33.9 |
| P23 | 121°20′10.55″ | 48°30′52.53″ | 997 | 10 | west | 340 | 31.2 | 14.8 |
| P24 | 121°14′22.65″ | 47°57′11.19″ | 755.6 | 11 | north | 540 | 22.4 | 17.9 |
| P25 | 121°22′43.57″ | 47°31′53.92″ | 610.8 | 18 | northeast | 400 | 25.4 | 15.6 |
| P26 | 120°38′33.79″ | 47°31′34.67″ | 1087.7 | 12 | northeast | 670 | 20.5 | 17.6 |
| Plots | Sample Depth | Common Interval | Mean Correlation Coefficient between Trees | Expressed Population Signal | Signal Noise Ratio | Mean Stand Age at Breast Height |
|---|---|---|---|---|---|---|
| P01 | 59 | 1954–2012 | 0.53 | 0.97 | 32.1 | 45 |
| P02 | 106 | 1978–2015 | 0.44 | 0.98 | 43.4 | 44 |
| P03 | 47 | 1988–2016 | 0.31 | 0.95 | 18.93 | 83 |
| P04 | 79 | 1991–2016 | 0.24 | 0.93 | 13.06 | 49 |
| P05 | 54 | 1995–2016 | 0.6 | 0.99 | 81.5 | 40 |
| P06 | 72 | 1985–2016 | 0.31 | 0.88 | 7.08 | 24 |
| P07 | 49 | 1969–2012 | 0.61 | 0.99 | 70.96 | 35 |
| P08 | 88 | 1997–2016 | 0.11 | 0.74 | 2.88 | 49 |
| P09 | 95 | 1992–2016 | 0.3 | 0.97 | 27.93 | 50 |
| P10 | 64 | 1980–2016 | 0.15 | 0.84 | 5.08 | 21 |
| P11 | 71 | 1982–2013 | 0.42 | 0.96 | 26.81 | 37 |
| P12 | 50 | 1991–2013 | 0.2 | 0.82 | 4.6 | 31 |
| P13 | 69 | 1958–2016 | 0.61 | 0.98 | 55.04 | 39 |
| P14 | 49 | 1968–2016 | 0.36 | 0.93 | 14.07 | 129 |
| P15 | 53 | 1984–2016 | 0.6 | 0.98 | 45.9 | 31 |
| P16 | 53 | 1975–2016 | 0.37 | 0.97 | 27.75 | 37 |
| P17 | 81 | 1991–2016 | 0.76 | 0.97 | 37.49 | 48 |
| P18 | 50 | 1937–2016 | 0.65 | 0.95 | 18.8 | 47 |
| P19 | 49 | 2000–2016 | 0.48 | 0.97 | 29.78 | 61 |
| P20 | 64 | 1975–2014 | 0.68 | 0.98 | 47.06 | 49 |
| P21 | 30 | 1977–2016 | 0.46 | 0.97 | 31.71 | 29 |
| P22 | 26 | 1984–2015 | 0.71 | 0.98 | 60.62 | 37 |
| P23 | 34 | 1904–2016 | 0.29 | 0.92 | 11.6 | 38 |
| P24 | 54 | 1983–2014 | 0.19 | 0.84 | 5.1 | 45 |
| P25 | 40 | 1976–2016 | 0.64 | 0.96 | 22.86 | 45 |
| P26 | 67 | 1975–2016 | 0.72 | 0.98 | 55.14 | 48 |
| Drought Level | Drought Intensity | SPEI |
|---|---|---|
| 1 | Non-drought | −0.5 < SPEI |
| 2 | mild | −1.0 < SPEI ≤ −0.5 |
| 3 | moderate | −1.5 < SPEI ≤ −1.0 |
| 4 | severe | −2.0 < SPEI ≤ −1.5 |
| 5 | extreme | SPEI ≤ −2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zhu, Y.; Pang, R.; Gao, L. Can Growth Increase of Small Trees after Drought Compensate for Large Trees’ Growth Loss? Forests 2024, 15, 448. https://doi.org/10.3390/f15030448
Liu M, Zhu Y, Pang R, Gao L. Can Growth Increase of Small Trees after Drought Compensate for Large Trees’ Growth Loss? Forests. 2024; 15(3):448. https://doi.org/10.3390/f15030448
Chicago/Turabian StyleLiu, Mingqian, Yihong Zhu, Rongrong Pang, and Lushuang Gao. 2024. "Can Growth Increase of Small Trees after Drought Compensate for Large Trees’ Growth Loss?" Forests 15, no. 3: 448. https://doi.org/10.3390/f15030448





