Seasonal Variation in Foliar Mite Diversity and Abundance in Leaf Domatia of Three Native South African Forest Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Study Species
2.3. Sampling Protocol
2.4. Data Analysis
3. Results
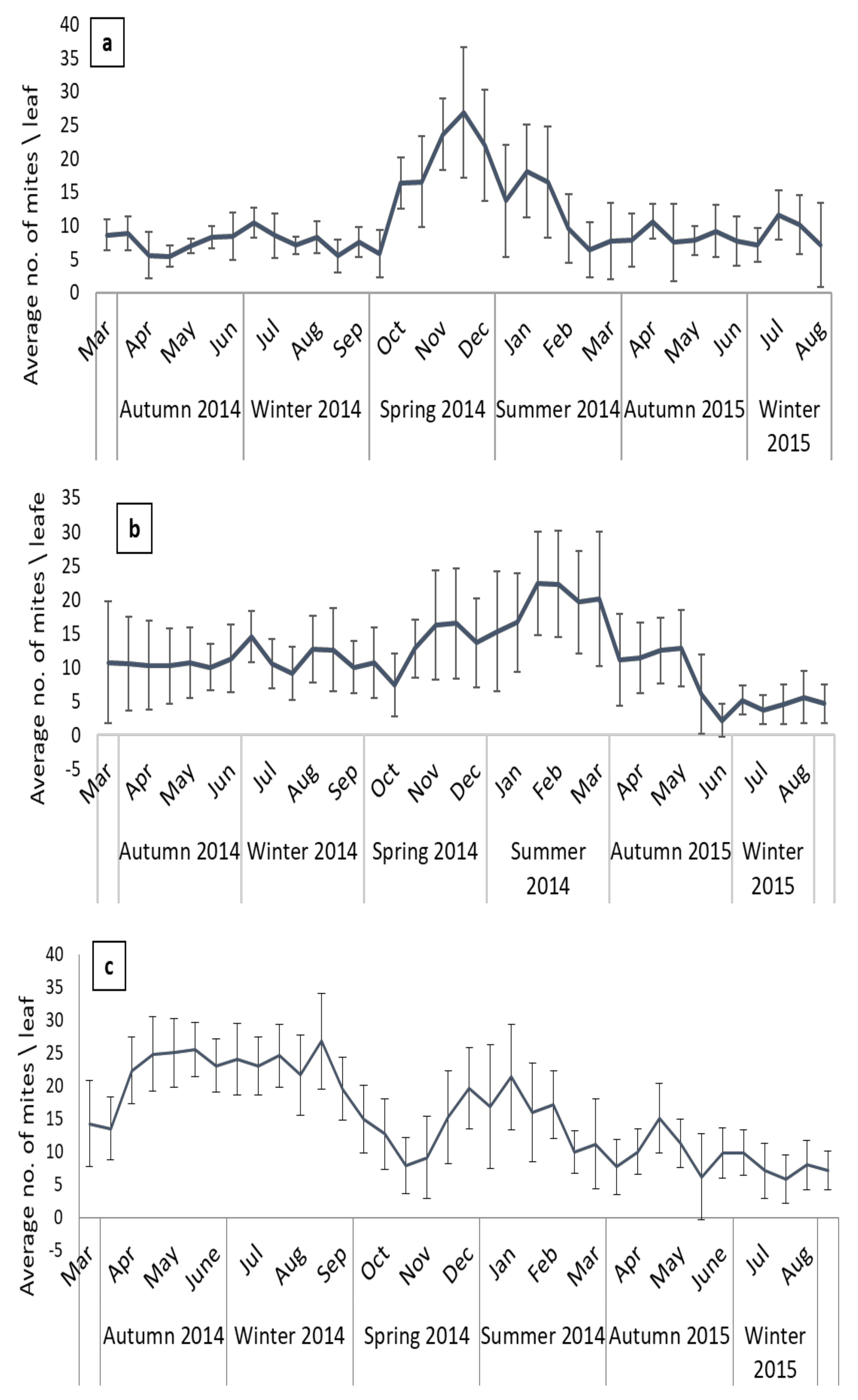
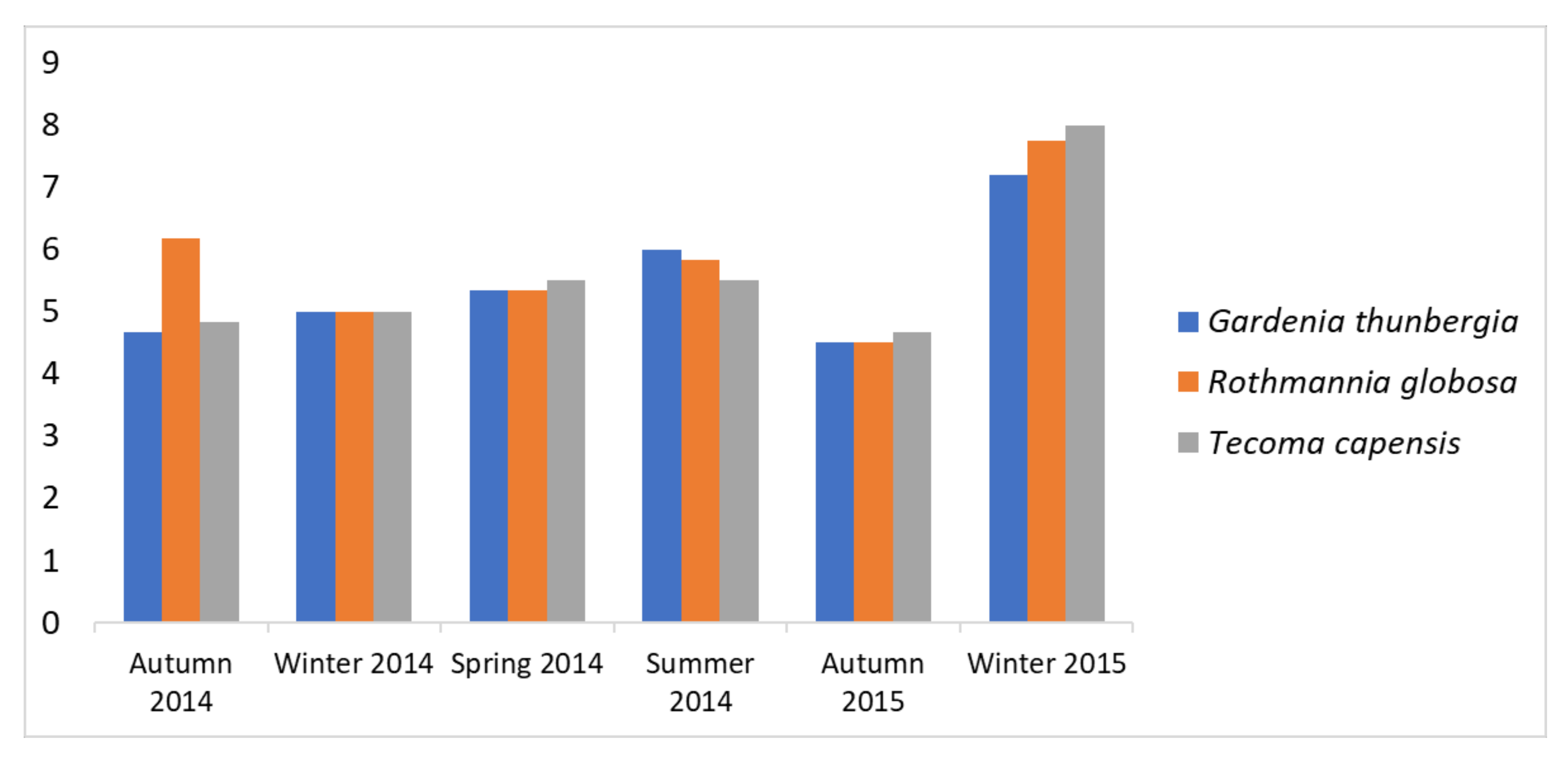
3.1. Seasonal Patterns in Mite Abundance and Species Richness
3.2. Seasonal Patterns in Mite Species Richness
3.3. Relationships between Environmental Variables and Mite Abundance and Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krantz, G.W. A Manual of Acarology; Texas Tech University: Lubbock, TX, USA, 1978. [Google Scholar]
- Nazar, M.Z.; Freed, S.; Hussain, S.; Sumra, M.W.; Shah, M.S.; Naeem, A. Characteristics of biochemical resistance mechanism of novel insecticides in Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Crop Prot. 2020, 138, 105320. [Google Scholar] [CrossRef]
- Salmane, I.; Brumelis, G. Species list and habitat preference of Mesostigmata mites (Acari, Parasitiformes) in Latvia. Acarologia 2010, 50, 373–394. [Google Scholar] [CrossRef]
- Huhta, V.; Hanninen, S.M. Effects of temperature and moisture fluctuations on an experimental soil microarthropods community. Pedobiologia 2001, 45, 279–286. [Google Scholar] [CrossRef]
- Devi, M.; Challa, N. Impact of weather parameters on seasonality of phytophagous mites. J. Entomol. Zool. 2019, 7, 1095–1100. [Google Scholar]
- Uvarov, A.V. Effects of diurnal temperature fluctuations on population response of forest floor mites. Pedobiologia 2003, 47, 331–339. [Google Scholar] [CrossRef]
- Zundel, C.; Nagel, P.; Hanna, R.; Korner, F.; Scheidegger, U. Environment and host-plant genotype effects on the seasonal dynamics of a predatory mite on cassava in sub-humid tropical Africa. Agric. For. Entomol. 2009, 11, 321–331. [Google Scholar] [CrossRef]
- Nishida, S. Seasonal and Regional Changes in Mite Fauna of Domatia in Viburnum odoratissimum var. awabuki (Viburnaceae). Acta Phytotaxon. Geobot. 2023, 74, 141–155. [Google Scholar]
- Toyoshima, S.; Amano, H. Diversity and abundance of phytoseiid mites on Magnolia hypoleuca Siebold et Zuccarini, a candidate source of natural enemies in natural vegetation. Appl. Entomol. Zool. 2006, 41, 349–355. [Google Scholar] [CrossRef]
- Kopačka, M.; Zemek, R. Species Composition and Seasonal Abundance of Predatory Mites (Acari: Phytoseiidae) Inhabiting Aesculus hippocastanum (Sapindaceae). Forests 2023, 14, 942. [Google Scholar] [CrossRef]
- Kolokytha, P.D.; Fantinou, A.A.; Papadoulis, G.T. Temperature and diet effects on immature development of predatory mite Typhlodromus athenas Swirski and Ragusa (Acari: Phyotseiidae). Environ. Entomol. 2011, 40, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Vermaak, M. The Diversity and Ecology of Mites (Acari) in Vineyards. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2019. [Google Scholar]
- Duso, C.; Fontana, P.; Malagnini, V. Diversity and abundance of phytoseiid mites (Acari: Phytoseiidae) in vineyards and the surrounding vegetation in northeastern Italy. Acarologia 2004, 44, 31–47. [Google Scholar]
- Mucina, L.; Rutherford, M.C. The Vegetation of South Africa, Lesotho and Swaziland; Strelitzia: South African National Biodiversity Institute: Pretoria, South Africa, 2006. [Google Scholar]
- Boon, R. Pooley’s Trees of Eastern South Africa: A Complete Guide; Flora and Fauna Publications Trust: Durban, South Africa, 2010. [Google Scholar]
- Dharani, N. Field Guide to Common Trees and Shrubs of East Africa; Struik Publishers: Cape Town, South Africa, 2002. [Google Scholar]
- Situngu, S.; Barker, N.P. Position, position, position: Mites occupying leaf domatia are not uniformly distributed in the tree canopy. S. Afr. J. Bot. 2017, 108, 23–28. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 5 January 2024).
- Kishimoto, H. Species composition and seasonal occurrence of spider mites (Acari: Tetranychidae) and their predators in Japanese pear orchards with different agrochemical spraying programs. Appl. Entomol. Zool. 2002, 37, 603–615. [Google Scholar] [CrossRef]
- Walter, D.E.; O’Dowd, D.J. Beneath biodiversity: Factors influencing the diversity and abundance of canopy mites. Selbyana 1995, 1, 12–20. [Google Scholar]
- Onzo, A.; Hanna, R.; Zannou, I.; Sabelis, M.W.; Yaninek, J.S. Dynamics of refuge use: Diurnal, vertical migration by predatory and herbivorous mites within cassava plants. Oikos 2003, 101, 59–69. [Google Scholar] [CrossRef]
- Prado, J.; Witte, A.R.; Frank, S.; Sadof, C.S. Do leaf domatia mediate intraguild predation and host resistance to Oligonychus acaris (Shimer) on red sunset maple (Acer rubrum)? Biological Control 2015, 90, 187–192. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Cunha, D.F.; Pallini, A.; Sabelis, M.W.; Janssen, A. Leaf domatia reduce intraguild predation among predatory mites. Ecol. Entomol. 2011, 36, 435–441. [Google Scholar] [CrossRef]
- Irmler, U. Climatic and litter fall effects on Collembolan and oribatid mites species and communities in a beech wood based on a 7-yearyear investigation. Eur. J. Soil Biol. 2006, 42, 51–62. [Google Scholar] [CrossRef]
- Schmidt, A.R.; Jancke, S.; Lindquist, E.E.; Ragazzi, E.; Roghi, G.; Nascimbene, P.C.; Schmidt, K.; Wappler, T.; Grimaldi, D.A. Arthropods in amber from the Triassic period. Proc. Natl. Acad. Sci. USA 2012, 109, 14796–14801. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Watanabe, M.; Takeda, M. UV tolerance in the two-spotted spider mite, Tetranychus urticae. J. Insect Physiol. 2009, 55, 649–654. [Google Scholar] [CrossRef]

| Mite Species | Feeding Guild | Gardenia thunbergia | Rothmannia globosa | Tecomaria capensis |
|---|---|---|---|---|
| Agistemus tranatalensis Meyer | Predacious | (6; 8) | (3; 9) | (6; 10) |
| Neoseiulus californicus (McGregor) | Predacious | (24; 128) | (19; 28) | |
| Brevipalpus sp. | Phytophagous | (12; 56) | (1; 2) | |
| Bunaxella zebedielensis | Predacious | (12; 17) | ||
| Eriophyiodea (unknown) | Phytophagous | (3; 23) | (16; 177) | (6; 49) |
| Euseius addoensis (McMurtry) | Predacious | (11; 20) | (26; 51) | (32; 62) |
| Oribatid sp. | Mycophagous | (12; 25) | (17; 23) | |
| Panonychus ulmi Koch | Phytophagous | (14; 21) | ||
| Saproglyphus sp. | Mycophagous | (24; 46) | (34.211) | (19; 71) |
| Siculobata sicula (BERLESE 1892) | mycophagous/Saprophytic | (1; 5) | ||
| Tetranychus sp. | Phytophagous | (18; 56) | (5; 26) | |
| Tetranychus sp. (new) | Phytophagous | (2; 3) | ||
| Tetratriophtydeus myacanthus (Ueckermann) | Predacious | (18; 97) | (2; 6) | (5; 11) |
| Tydeus munsteri Meyer and Ryke | Predacious | (25; 158) | (25; 174) | (34; 462) |
| Typhlodromus microbullatus Van der Merwe | Predacious | (6; 14) | (10; 19) | (1; 1) |
| Gardenia thunbergia | Rothmannia globosa | Tecomeria capensis | ||
|---|---|---|---|---|
| Min. Temp | Std. error | 0.33 | 0.37 | 0.46 |
| T value | 2.57 | 0.17 | −1.17 | |
| p value | 0.56 | 0.85 | 0.24 | |
| Max. Temp | Std. error | 0.20 | 0.22 | 0.27 |
| T value | 1.99 | 0.78 | 0.23 | |
| p value | 0.05 | 0.43 | 0.81 | |
| Rel. Humidity @ 08:00 | Std. error | 0.10 | 0.19 | 0.14 |
| T value | −0.16 | −1.74 | −0.53 | |
| p value | 0.87 | 0.092 | 0.59 | |
| Rel. Humidity @ 14:00 | Std. error | 0.05 | 0.05 | 0.06 |
| T value | 1.03 | 1.56 | 1.06 | |
| p value | 0.30 | 0.12 | 0.29 | |
| Rain the day before | Std. error | 0.27 | 0.30 | 0.38 |
| T value | −0.98 | −1.37 | −0.24 | |
| p value | 0.33 | 0.17 | 0.80 | |
| Accumulative rainfall | Std. error | 0.04 | 0.04 | 0.05 |
| T value | 0.17 | 1.86 | −1.50 | |
| p value | 0.86 | 0.07 | 0.14 |
| Gardenai thunbergia | Rothmannia globosa | Tecomeria capensis | ||
|---|---|---|---|---|
| Min. Temp | Std. error | 0.10 | 0.09 | 0.08 |
| T value | −0.37 | −1.59 | −1.11 | |
| p value | 0.70 | 0.12 | 0.27 | |
| Max. Temp | Std. error | 0.06 | 0.05 | 0.05 |
| T value | −0.409 | 0.52 | 0.36 | |
| p value | 0.68 | 0.60 | 0.71 | |
| Rel. Humidity @ 08:00 | Std. error | 0.03 | 0.03 | 0.02 |
| T value | −2.08 | 0.36 | −0.07 | |
| p value | 0.04 * | 0.71 | 0.93 | |
| Rel. Humidity @ 14:00 | Std. error | 0.01 | 0.01 | 0.01 |
| T value | −0.95 | 0.98 | 0.12 | |
| p value | 0.34 | 0.33 | 0.89 | |
| Rain the day before | Std. error | 0.08 | 0.08 | 0.07 |
| T value | 0.28 | −0.84 | −0.11 | |
| p value | 0.78 | 0.40 | 0.91 | |
| Accumulative rainfall | Std. error | 0.01 | 0.01 | 0.01 |
| T value | 0.67 | −0.08 | 0.29 | |
| p value | 0.57 | 0.93 | 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Situngu, S.; Barker, N.P. Seasonal Variation in Foliar Mite Diversity and Abundance in Leaf Domatia of Three Native South African Forest Species. Forests 2024, 15, 467. https://doi.org/10.3390/f15030467
Situngu S, Barker NP. Seasonal Variation in Foliar Mite Diversity and Abundance in Leaf Domatia of Three Native South African Forest Species. Forests. 2024; 15(3):467. https://doi.org/10.3390/f15030467
Chicago/Turabian StyleSitungu, Sivuyisiwe, and Nigel P. Barker. 2024. "Seasonal Variation in Foliar Mite Diversity and Abundance in Leaf Domatia of Three Native South African Forest Species" Forests 15, no. 3: 467. https://doi.org/10.3390/f15030467





