Variation and Correlation among Fine Root Traits of Desert Plants in Arid Areas of Northwest China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Measurement of Root Traits
2.4. Construction of Phylogeny
2.5. Measurement of Soil Water Content and Chemical Properties
2.6. Data Analysis
3. Results
3.1. Total Variation of Fine Root Traits
3.2. Intraspecific and Interspecific Variations among Fine Root Traits within Life Forms
3.3. Relationship between Fine Root Trait Variation and Root Economic Spectrum
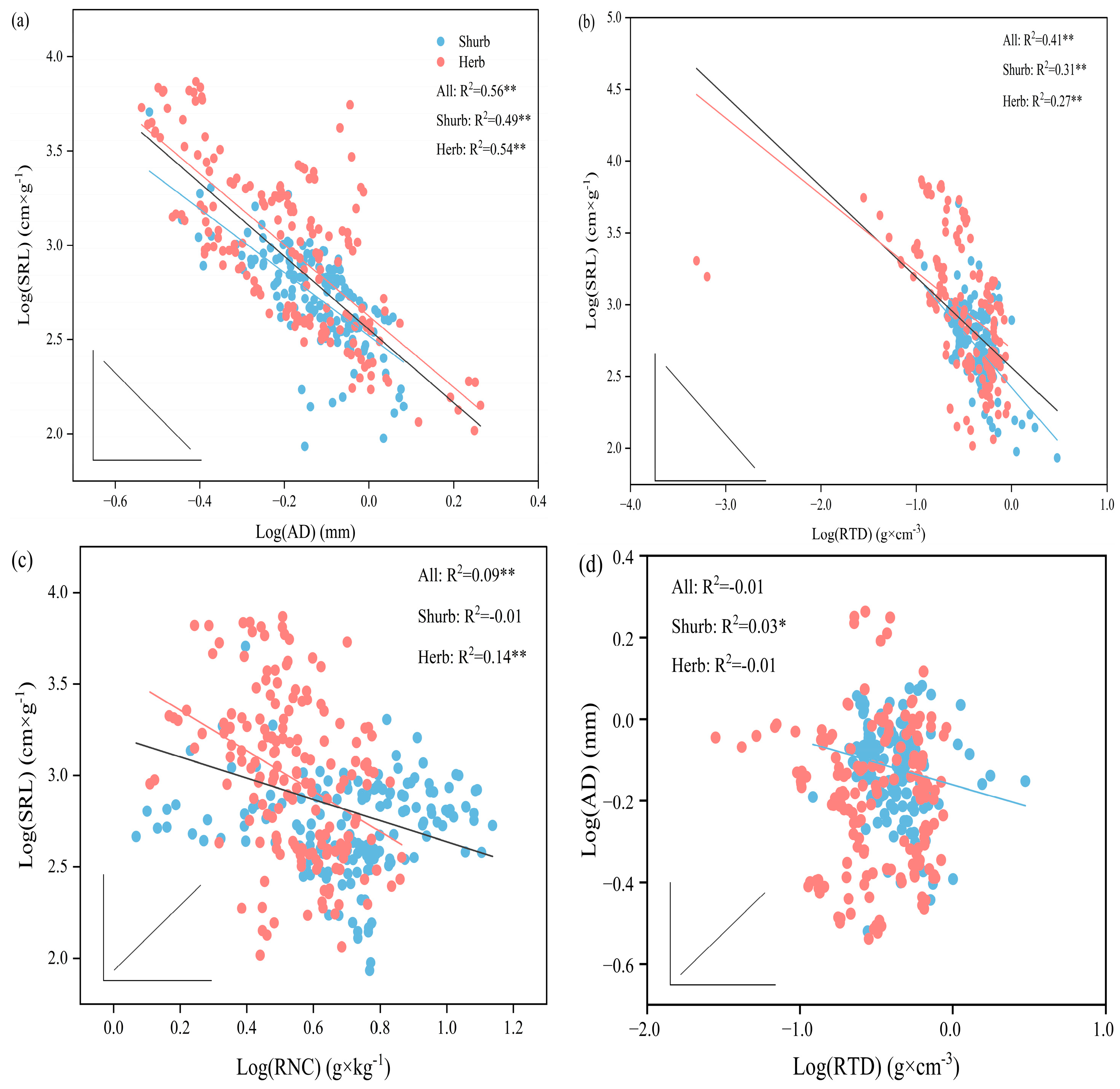
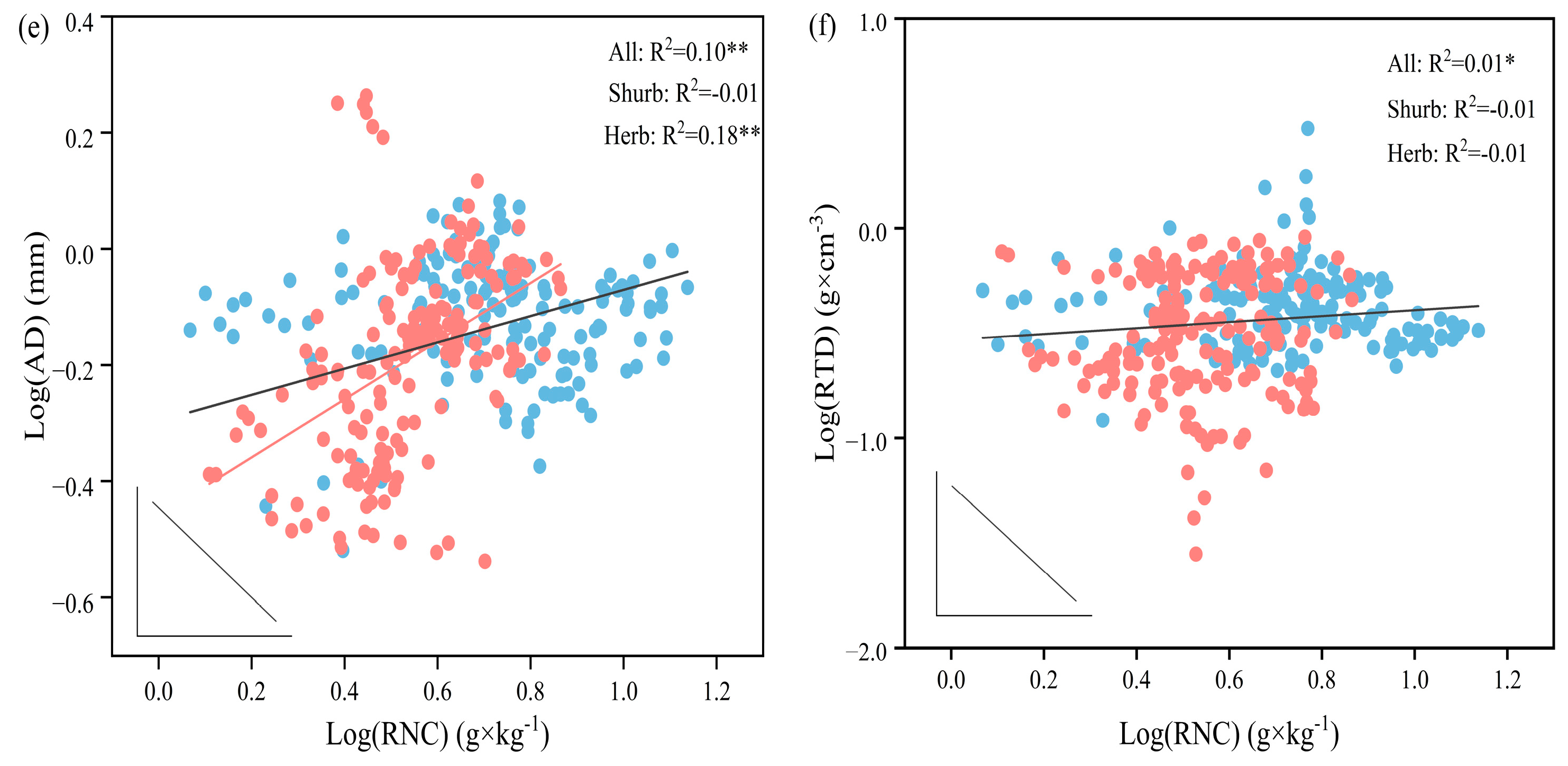
3.4. Correlation among Fine Root Traits
3.5. Correlation between Fine Root Traits and Environmental Factors
4. Discussion
4.1. Variation among Fine Root Traits Explains the Adaptation of Desert Plants to Heterogeneous Environments and Interspecific Competition
4.2. The Multidimensional Variation among Fine Root Traits Mitigates Potential Desert Environmental Stress
4.3. Influence of Soil and Climatic Conditions on Fine Root Traits in Desert Plants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zemunik, G.; Turner, B.L.; Lambers, H.; Laliberté, E. Diversity of plant nutrient-acquisition strategies increases during long-term ecosystem development. Nat. Plants 2015, 1, 15050. [Google Scholar] [CrossRef]
- Laliberte, E. Below-ground frontiers in trait-based plant ecology. New Phytol. 2017, 213, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- Reich, A.; Ewel, J.J.; Nadkarni, N.M.; Dawson, T.; Evans, R.D. Nitrogen isotope ratios shift with plant size in tropical bromeliads. Oecologia 2003, 137, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Bruelheide, H.; Dengler, J.; Purschke, O.; Lenoir, J.; Jimenez-Alfaro, B.; Hennekens, S.M.; Botta-Dukat, Z.; Chytry, M.; Field, R.; Jansen, F.; et al. Global trait-environment relationships of plant communities. Nat. Ecol. Evol. 2018, 2, 1906–1917. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Y.; Chen, H.Y.; Reich, P.B. Global-scale latitudinal patterns of plant fine-root nitrogen and phosphorus. Nat. Commun. 2011, 2, 344. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; DeForest, J.L.; Burton, A.J.; Allen, M.F.; Ruess, R.W.; Hendrick, R.L. Fine root architecture of nine north american trees. Ecol. Monogr. 2002, 72, 293–309. [Google Scholar] [CrossRef]
- Spasojevic, M.J.; Suding, K.N. Inferring community assembly mechanisms from functional diversity patterns: The importance of multiple assembly processes. J. Ecol. 2012, 100, 652–661. [Google Scholar] [CrossRef]
- Adler, P.B.; Fajardo, A.; Kleinhesselink, A.R.; Kraft, N.J. Trait-based tests of coexistence mechanisms. Ecol. Lett. 2013, 16, 1294–1306. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, H.Y.H.; Lian, J.; John, R.; Ronghua, L.; Liu, H.; Ye, W.; Berninger, F.; Ye, Q.; Timothy Paine, C.E. Using functional trait diversity patterns to disentangle the scale-dependent ecological processes in a subtropical forest. Funct. Ecol. 2018, 32, 1379–1389. [Google Scholar] [CrossRef]
- Tobner, C.M.; Paquette, A.; Messier, C. Interspecific coordination and intraspecific plasticity of fine root traits in North American temperate tree species. Front. Plant Sci. 2013, 4, 242. [Google Scholar] [CrossRef]
- Siefert, A.; Violle, C.; Chalmandrier, L.; Albert, C.H.; Taudiere, A.; Fajardo, A.; Aarssen, L.W.; Baraloto, C.; Carlucci, M.B.; Cianciaruso, M.V.; et al. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol. Lett. 2015, 18, 1406–1419. [Google Scholar] [CrossRef]
- Bu, W.; Schmid, B.; Liu, X.; Li, Y.; Härdtle, W.; von Oheimb, G.; Liang, Y.; Sun, Z.; Huang, Y.; Bruelheide, H.; et al. Interspecific and intraspecific variation in specific root length drives aboveground biodiversity effects in young experimental forest stands. J. Plant Ecol. 2017, 10, 158–169. [Google Scholar] [CrossRef]
- Doi, R.; Tanikawa, T.; Miyatani, K.; Hirano, Y. Intraspecific variation in morphological traits of root branch orders in Chamaecyparis obtusa. Plant Soil 2017, 416, 503–513. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Kramer-Walter, K.R.; Bellingham, P.J.; Millar, T.R.; Smissen, R.D.; Richardson, S.J.; Laughlin, D.C. Root traits are multidimensional: Specific root length is independent from root tissue density and the plant economic spectrum. J. Ecol. 2016, 104, 1299–1310. [Google Scholar] [CrossRef]
- Valverde-Barrantes, O.J.; Freschet, G.T.; Roumet, C.; Blackwood, C.B. A worldview of root traits: The influence of ancestry, growth form, climate and mycorrhizal association on the functional trait variation of fine-root tissues in seed plants. New Phytol. 2017, 215, 1562–1573. [Google Scholar] [CrossRef]
- Pinno, B.D.; Wilson, S.D. Fine root response to soil resource heterogeneity differs between grassland and forest. Plant Ecol. 2013, 214, 821–829. [Google Scholar] [CrossRef]
- Stearns, S.C. Trade-Offs in Life-History Evolution. Funct. Ecol. 1989, 3, 259–268. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, H.; Lu, Q.; Ma, J.; Shen, Y.; Wang, G. Different responses of leaf and root economics spectrum to grazing time at the community level in desert steppe, China. Sci. Total Environ. 2024, 909, 168547. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Wang, C.; Huang, Z.; Wang, D.; Liu, G. Variations in the traits of fine roots of different orders and their associations with leaf traits in 12 co-occuring plant species in a semiarid inland dune. Plant Soil 2022, 472, 193–206. [Google Scholar] [CrossRef]
- Zhang, G.; Mao, Z.; Maillard, P.; Brancheriau, L.; Gérard, B.; Engel, J.; Fortunel, C.; Heuret, P.; Maeght, J.L.; Martínez-Vilalta, J. Not all sweetness and light: Non-structural carbohydrate storage capacity in tree stems is decoupled from leaf but not from root economics. Funct. Ecol. 2023. Online Version of Record. [Google Scholar] [CrossRef]
- Kong, D.; Wang, J.; Wu, H.; Valverde-Barrantes, O.J.; Wang, R.; Zeng, H.; Kardol, P.; Zhang, H.; Feng, Y. Nonlinearity of root trait relationships and the root economics spectrum. Nat. Commun. 2019, 10, 2203. [Google Scholar] [CrossRef]
- Gillison, A.N. Plant functional types and traits at the community, ecosystem and world level. Veg. Ecol. 2013, 00, 347–386. [Google Scholar] [CrossRef]
- Warren, J.M.; Hanson, P.J.; Iversen, C.M.; Kumar, J.; Walker, A.P.; Wullschleger, S.D. Root structural and functional dynamics in terrestrial biosphere models—Evaluation and recommendations. New Phytol. 2015, 205, 59–78. [Google Scholar] [CrossRef] [PubMed]
- Fort, F.; Freschet, G.T.; Kroon, H. Plant ecological indicator values as predictors of fine-root trait variations. J. Ecol. 2020, 108, 1565–1577. [Google Scholar] [CrossRef]
- Weemstra, M.; Mommer, L.; Visser, E.J.; van Ruijven, J.; Kuyper, T.W.; Mohren, G.M.; Sterck, F.J. Towards a multidimensional root trait framework: A tree root review. New Phytol. 2016, 211, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Bai, W.; Zhang, Y.; Zhang, W.H. Multi-dimensional patterns of variation in root traits among coexisting herbaceous species in temperate steppes. J. Ecol. 2018, 106, 2320–2331. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Xu, G.; Zou, T. Ecophysiological response and morphological adjustment of two Central Asian desert shrubs towards variation in summer precipitation. Plant Cell Environ. 2007, 30, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.A.; Turner, N.C. Plant Productivity in the Arid and Semiarid Zones. Annu. Rev. Plant Physiol. 1978, 29, 277–317. [Google Scholar] [CrossRef]
- Montagnoli, A.; Terzaghi, M.; Di Iorio, A.; Scippa, G.; Chiatante, D. Fine-root seasonal pattern, production and turnover rate of European beech (Fagus sylvatica L.) stands in Italy Prealps: Possible implications of coppice conversion to high forest. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2012, 146, 1012–1022. [Google Scholar]
- Freschet, G.T.; Valverde-Barrantes, O.J.; Tucker, C.M.; Craine, J.M.; McCormack, M.L.; Violle, C.; Fort, F.; Blackwood, C.B.; Urban-Mead, K.R.; Iversen, C.M. Climate, soil and plant functional types as drivers of global fine-root trait variation. J. Ecol. 2017, 105, 1182–1196. [Google Scholar] [CrossRef]
- Hou, H.-Y. Vegetation of China With Reference to Its Geographical Distribution. Ann. Mo. Bot. Gard. 1983, 70, 509–549. [Google Scholar] [CrossRef]
- Cheng, J.H.; Chu, P.F.; Chen, D.M.; Bai, Y.F. Functional correlations between specific leaf area and specific root length along a regional environmental gradient in Inner Mongolia grasslands. Funct. Ecol. 2016, 30, 985–997. [Google Scholar] [CrossRef]
- Jackson, R.B.; Canadell, J.; Ehleringer, J.R.; Mooney, H.; Sala, O.; Schulze, E.-D. A global analysis of root distributions for terrestrial biomes. Oecologia 1996, 108, 389–411. [Google Scholar] [CrossRef] [PubMed]
- Bao, S. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Jin, Y.; Qian, H.V. PhyloMaker2: An updated and enlarged R package that can generate very large phylogenies for vascular plants. Plant Divers. 2022, 44, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Zanne, A.E.; Tank, D.C.; Cornwell, W.K.; Eastman, J.M.; Smith, S.A.; FitzJohn, R.G.; McGlinn, D.J.; O’Meara, B.C.; Moles, A.T.; Reich, P.B.; et al. Three keys to the radiation of angiosperms into freezing environments. Nature 2014, 506, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.O.; Donoghue, M.J. Phylomatic: Tree assembly for applied phylogenetics. Mol. Ecol. Notes 2005, 5, 181–183. [Google Scholar] [CrossRef]
- Zhang, J. Plantlist: Looking Up the Status of Plant Scientific Names Based on the Plant List Database. R Package Version 0.5.5. 2018. Available online: https://github.com/helixcn/plantlist/ (accessed on 20 January 2024).
- Liu, Y.; Wang, X.; Fan, D.; Lai, J.; Zavafer, A. The use of R in photosynthesis research. Funct. Plant Biol. 2021, 49, 565–572. [Google Scholar] [CrossRef]
- Gardner, W.H. Water content. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; Soil Science Society of America, Inc.: Madison, WI, USA, 1986; Volume 5, pp. 493–544. [Google Scholar]
- Lai, J.; Zou, Y.; Zhang, S.; Zhang, X.; Mao, L. glmm. hp: An R package for computing individual effect of predictors in generalized linear mixed models. J. Plant Ecol. 2022, 15, 1302–1307. [Google Scholar] [CrossRef]
- Lai, J.; Zhu, W.; Cui, D.; Mao, L. Extension of the glmm. hp package to zero-inflated generalized linear mixed models and multiple regression. J. Plant Ecol. 2023, 16, rtad038. [Google Scholar] [CrossRef]
- Zuo, E.; Sun, L.; Yan, J.; Chen, C.; Chen, C.; Lv, X. Rapidly detecting fennel origin of the near-infrared spectroscopy based on extreme learning machine. Sci. Rep. 2022, 12, 13593. [Google Scholar] [CrossRef] [PubMed]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO 5; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Violle, C.; Enquist, B.J.; McGill, B.J.; Jiang, L.; Albert, C.H.; Hulshof, C.; Jung, V.; Messier, J. The return of the variance: Intraspecific variability in community ecology. Trends Ecol. Evol. 2012, 27, 244–252. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Kichenin, E.; Wardle, D.A.; Peltzer, D.A.; Morse, C.W.; Freschet, G.T.; Kitajima, K. Contrasting effects of plant inter- and intraspecific variation on community-level trait measures along an environmental gradient. Funct. Ecol. 2013, 27, 1254–1261. [Google Scholar] [CrossRef]
- Tang, Q.; Huang, Y.; Ding, Y.; Zang, R. Interspecific and intraspecific variation in functional traits of subtropical evergreen and deciduous broad-leaved mixed forests. Biodivers. Sci. 2016, 24, 262–270. [Google Scholar] [CrossRef]
- Jung, V.; Violle, C.; Mondy, C.; Hoffmann, L.; Muller, S. Intraspecific variability and trait-based community assembly. J. Ecol. 2010, 98, 1134–1140. [Google Scholar] [CrossRef]
- Xu, Y. Envirotyping for deciphering environmental impacts on crop plants. Theor. Appl. Genet. 2016, 129, 653–673. [Google Scholar] [CrossRef]
- Lambers, H.; Mougel, C.; Jaillard, B.; Hinsinger, P. Plant-microbe-soil interactions in the rhizosphere: An evolutionary perspective. Plant Soil 2009, 321, 83–115. [Google Scholar] [CrossRef]
- Albert, C.H.; Thuiller, W.; Yoccoz, N.G.; Douzet, R.; Aubert, S.; Lavorel, S. A multi-trait approach reveals the structure and the relative importance of intra- vs. interspecific variability in plant traits. Funct. Ecol. 2010, 24, 1192–1201. [Google Scholar] [CrossRef]
- Jung, V.; Albert, C.H.; Violle, C.; Kunstler, G.; Loucougaray, G.; Spiegelberger, T.; Cornwell, W. Intraspecific trait variability mediates the response of subalpine grassland communities to extreme drought events. J. Ecol. 2014, 102, 45–53. [Google Scholar] [CrossRef]
- van Rooijen, N.M.; de Keersmaecker, W.; Ozinga, W.A.; Coppin, P.; Hennekens, S.M.; Schaminée, J.H.J.; Somers, B.; Honnay, O. Plant Species Diversity Mediates Ecosystem Stability of Natural Dune Grasslands in Response to Drought. Ecosystems 2015, 18, 1383–1394. [Google Scholar] [CrossRef]
- Albert, C.H.; Grassein, F.; Schurr, F.M.; Vieilledent, G.; Violle, C. When and how should intraspecific variability be considered in trait-based plant ecology? Perspect. Plant Ecol. Evol. Syst. 2011, 13, 217–225. [Google Scholar] [CrossRef]
- Niu, K.; Zhang, S.; Lechowicz, M.J.; Perez Carmona, C. Harsh environmental regimes increase the functional significance of intraspecific variation in plant communities. Funct. Ecol. 2020, 34, 1666–1677. [Google Scholar] [CrossRef]
- Cavin, L.; Mountford, E.P.; Peterken, G.F.; Jump, A.S.; Whitehead, D. Extreme drought alters competitive dominance within and between tree species in a mixed forest stand. Funct. Ecol. 2013, 27, 1424–1435. [Google Scholar] [CrossRef]
- O’Dell, R.E.; Rajakaruna, N. Intraspecific Variation, Adaptation, and Evolution. In Serpentine The Evolution and Ecology of a Model System; University of California Press: Berkeley, CA, USA, 2011; pp. 96–137. [Google Scholar]
- Albert, C.H. Intraspecific trait variability matters. J. Veg. Sci. 2015, 26, 7–8. [Google Scholar] [CrossRef]
- Auger, S.; Shipley, B.; de Bello, F. Inter-specific and intra-specific trait variation along short environmental gradients in an old-growth temperate forest. J. Veg. Sci. 2013, 24, 419–428. [Google Scholar] [CrossRef]
- Fajardo, A.; Siefert, A. Phenological variation of leaf functional traits within species. Oecologia 2016, 180, 951–959. [Google Scholar] [CrossRef]
- Jiang, F.; Xun, Y.; Cai, H.; Jin, G. Functional traits can improve our understanding of niche- and dispersal-based processes. Oecologia 2018, 186, 783–792. [Google Scholar] [CrossRef]
- Jiang, F.; Cadotte, M.W.; Jin, G. Individual-level leaf trait variation and correlation across biological and spatial scales. Ecol. Evol. 2021, 11, 5344–5354. [Google Scholar] [CrossRef]
- Ding, J.; Kong, D.; Zhang, Z.; Cai, Q.; Xiao, J.; Liu, Q.; Yin, H.; Wardle, D. Climate and soil nutrients differentially drive multidimensional fine root traits in ectomycorrhizal-dominated alpine coniferous forests. J. Ecol. 2020, 108, 2544–2556. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Q.; Zhao, N.; Xu, Z.; Zhu, X.; Jiao, C.; Yu, G.; He, N.; Niu, S. Different phylogenetic and environmental controls of first-order root morphological and nutrient traits: Evidence of multidimensional root traits. Funct. Ecol. 2017, 32, 29–39. [Google Scholar] [CrossRef]
- Sun, L.; Ataka, M.; Han, M.; Han, Y.; Gan, D.; Xu, T.; Guo, Y.; Zhu, B. Root exudation as a major competitive fine-root functional trait of 18 coexisting species in a subtropical forest. New Phytol. 2020, 229, 259–271. [Google Scholar] [CrossRef]
- Reich, P.B.; Cornelissen, H. The world-wide ‘fast-slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Prieto, I.; Roumet, C.; Cardinael, R.; Dupraz, C.; Jourdan, C.; Kim, J.H.; Maeght, J.L.; Mao, Z.; Pierret, A.; Portillo, N. Root functional parameters along a land-use gradient: Evidence of a community-level economics spectrum. J. Ecol. 2015, 103, 361–373. [Google Scholar] [CrossRef]
- Carmona, C.P.; Bueno, C.G.; Toussaint, A.; Träger, S.; Díaz, S.; Moora, M.; Munson, A.D.; Pärtel, M.; Zobel, M.; Tamme, R. Fine-root traits in the global spectrum of plant form and function. Nature 2021, 597, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Schenk, H.J.; Jackson, R.B. Rooting depths, lateral root spreads and below-ground/ above-ground allometries of plants in water-limited ecosystems. J. Ecol. 2002, 90, 480–494. [Google Scholar] [CrossRef]
- Song, C.J.; Ma, K.M.; Qu, L.Y.; Liu, Y.; Xu, X.L.; Fu, B.J.; Zhong, J.F. Interactive effects of water, nitrogen and phosphorus on the growth, biomass partitioning and water-use efficiency of Bauhinia faberi seedlings. J. Arid. Environ. 2010, 74, 1003–1012. [Google Scholar] [CrossRef]
- James, J.J.; Tiller, R.L.; Richards, J.H. Multiple resources limit plant growth and function in a saline-alkaline desert community. J. Ecol. 2005, 93, 113–126. [Google Scholar] [CrossRef]
- Holdaway, R.J.; Richardson, S.J.; Dickie, I.A.; Peltzer, D.A.; Coomes, D.A. Species-and community-level patterns in fine root traits along a 120 000-year soil chronosequence in temperate rain forest. J. Ecol. 2011, 99, 954–963. [Google Scholar] [CrossRef]
- Withington, J.M.; Reich, P.B.; Oleksyn, J.; Eissenstat, D.M. Comparisons of structure and life span in roots and leaves among temperate trees. Ecol. Monogr. 2006, 76, 381–397. [Google Scholar] [CrossRef]
- Comas, L.; Eissenstat, D. Patterns in root trait variation among 25 co-existing North American forest species. New Phytol. 2009, 182, 919–928. [Google Scholar] [CrossRef]
- Bergmann, J.; Weigelt, A.; van Der Plas, F.; Laughlin, D.C.; Kuyper, T.W.; Guerrero-Ramirez, N.; Valverde-Barrantes, O.J.; Bruelheide, H.; Freschet, G.T.; Iversen, C.M. The fungal collaboration gradient dominates the root economics space in plants. Sci. Adv. 2020, 6, eaba3756. [Google Scholar] [CrossRef]
- Stahl, U.; Kattge, J.; Reu, B.; Voigt, W.; Ogle, K.; Dickie, J.; Wirth, C. Whole-plant trait spectra of North American woody plant species reflect fundamental ecological strategies. Ecosphere 2013, 4, 2013. [Google Scholar] [CrossRef]
- Tjoelker, M.; Craine, J.M.; Wedin, D.; Reich, P.B.; Tilman, D. Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytol. 2005, 167, 493–508. [Google Scholar] [CrossRef]
- Eviner, V.T.; Chapin III, F.S. Functional Matrix: A Conceptual Framework for Predicting Multiple Plant Effects on Ecosystem Processes. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 455–485. [Google Scholar] [CrossRef]
- Krishna, M.; Winternitz, J.; Garkoti, S.C.; Penuelas, J. Functional leaf traits indicate phylogenetic signals in forests across an elevational gradient in the central Himalaya. J. Plant Res. 2021, 134, 753–764. [Google Scholar] [CrossRef]
- Ding, Y.; Zang, R.; Letcher, S.G.; Liu, S.; He, F. Disturbance regime changes the trait distribution, phylogenetic structure and community assembly of tropical rain forests. Oikos 2012, 121, 1263–1270. [Google Scholar] [CrossRef]
- Swenson, N.G. The assembly of tropical tree communities—The advances and shortcomings of phylogenetic and functional trait analyses. Ecography 2013, 36, 264–276. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, J.; Bai, W.; Zhang, Y.; Zhang, W.H. The response of root traits to precipitation change of herbaceous species in temperate steppes. Funct. Ecol. 2019, 33, 2030–2041. [Google Scholar] [CrossRef]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant root growth, architecture and function. Plant Soil 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Markesteijn, L.; Poorter, L. Seedling root morphology and biomass allocation of 62 tropical tree species in relation to drought-and shade-tolerance. J. Ecol. 2009, 97, 311–325. [Google Scholar] [CrossRef]
- Rojas-Botero, S.; Teixeira, L.H.; Prucker, P.; Kloska, V.; Kollmann, J.; Le Stradic, S. Root traits of grasslands rapidly respond to climate change, while community biomass mainly depends on functional composition. Funct. Ecol. 2023, 37, 1841–1855. [Google Scholar] [CrossRef]




| Trait | Acronym | Functions | Unit |
|---|---|---|---|
| Root tissue density | RTD | Transport, conservation, and defense | g/cm3 |
| Specific root length | SRL | Resource acquisition | cm/g |
| Specific root area | SRA | Resource acquisition | cm2/g |
| Average root diameter | AD | Transport, conservation, and defense | mm |
| Root carbon content | RCC | Transport, conservation, and defense | g/kg |
| Root nitrogen content | RNC | Resource acquisition | g/kg |
| Root phosphorus content | RPC | Resource acquisition | g/kg |
| Root carbon and nitrogen ratio | RCN | / | / |
| Root carbon and phosphorus ratio | RCP | / | / |
| Root nitrogen and phosphorus ratio | RNP | / | / |
| Traits | Unit | Mean | Standard Deviation | Minimum | Maximum | Coefficient of Variation (%) |
|---|---|---|---|---|---|---|
| AD | mm | 0.74 | 0.25 | 0.29 | 1.83 | 33.41 |
| SRL | cm/g | 1116.12 | 1328.38 | 85.73 | 7393.57 | 119.02 |
| SRA | cm2/g | 203.18 | 180.40 | 18.99 | 1579.65 | 88.79 |
| RTD | g/cm3 | 0.42 | 0.26 | 0.03 | 2.99 | 62.46 |
| RCC | g/kg | 399.60 | 92.99 | 152.69 | 849.72 | 23.27 |
| RNC | g/kg | 4.68 | 2.31 | 1.17 | 13.72 | 49.35 |
| RPC | g/kg | 1.01 | 0.58 | 0.14 | 4.44 | 57.77 |
| RCN | / | 104.81 | 56.69 | 27.90 | 345.12 | 54.09 |
| RNP | / | 6.26 | 5.06 | 0.55 | 33.30 | 80.75 |
| RCP | / | 538.16 | 373.04 | 42.62 | 3181.87 | 69.32 |
| Traits | Unit | Shrub | Herb | Community |
|---|---|---|---|---|
| AD | mm | 0.78 ± 0.18 a | 0.70 ± 0.29 b | 0.74 ± 0.25 |
| (13.51%/22.47%) | (12.36%/41.94%) | (12.86%/33.41%) | ||
| SRL | cm/g | 625.93 ± 480.89 a | 1572.52 ± 1663.51 b | 1116.12 ± 1326.86 |
| (40.35%/76.83%) | (35.58%/105.79%) | (37.68%/119.02%) | ||
| SRA | cm2/g | 137.91 ± 60.31 a | 263.94 ± 227.88 b | 203.18 ± 180.30 |
| (28.81%/43.73%) | (30.47%/86.34%) | (29.74%/88.79%) | ||
| RTD | g/cm3 | 0.46 ± 0.30 a | 0.38 ± 0.22 b | 0.42 ± 0.26 |
| (24.14%/64.54%) | (23.34%/57.44%) | (23.69%/62.46%) | ||
| RCC | g/kg | 423.08 ± 98.06 a | 377.74 ± 82.45 b | 399.60 ± 94.29 |
| (17.11%/23.18%) | (15.18%/21.83%) | (16.03%/23.27%) | ||
| RNC | g/kg | 5.79 ± 2.66 a | 3.65 ± 1.22 b | 4.68 ± 2.31 |
| (28.65%/46.00%) | (19.39%/33.54%) | (23.46%/49.35%) | ||
| RPC | g/kg | 0.96 ± 0.43 a | 1.05 ± 0.69 a | 1.01 ± 0.58 |
| (40.92%/44.53%) | (40.90%/66.05%) | (40.91%/57.77%) | ||
| RCN | / | 93.17 ± 60.83 a | 115.64 ± 50.35 b | 104.81 ± 56.66 |
| (40.13%/65.28%) | (27.59%/43.54%) | (33.11%/54.09%) | ||
| RNP | / | 7.79 ± 5.96 a | 4.84 ± 3.50 b | 6.26 ± 5.05 |
| (65.46%/76.47%) | (49.65%/72.37%) | (56.60%/80.75%) | ||
| RCP | / | 579.21 ± 438.98 a | 499.94 ± 295.12 b | 538.16 ± 372.53 |
| (59.33%/75.79%) | (45.24%/59.03%) | (51.44%/69.32%) |
| AD | SRL | SRA | RTD | RCC | RNC | RPC | RCN | RNP | RCP | |
|---|---|---|---|---|---|---|---|---|---|---|
| AD | −0.55 * | −0.17 | −0.24 | 0.09 | 0.47 | 0.32 | −0.58 * | 0.32 | −0.01 | |
| SRL | −0.54 ** | 0.84 ** | −0.38 | −0.17 | −0.46 | 0.10 | 0.46 | −0.39 | −0.09 | |
| SRA | −0.34 ** | 0.88 ** | −0.64 ** | −0.17 | −0.34 | 0.36 | 0.24 | −0.38 | −0.19 | |
| RTD | −0.05 | −0.40 ** | −0.54 ** | −0.23 | 0.04 | −0.19 | −0.07 | 0.01 | −0.14 | |
| RCC | −0.08 | −0.03 | 0.01 | −0.09 | 0.46 | −0.47 | −0.04 | 0.61 * | 0.73 ** | |
| RNC | 0.18 ** | −0.28 ** | −0.23 ** | 0.03 | 0.31 ** | −0.04 | −0.78 ** | 0.77 ** | 0.22 | |
| RPC | 0.39 ** | −0.08 | 0.03 | 0.02 | −0.29 ** | −0.11 * | −0.25 | −0.52 * | −0.74 ** | |
| RCN | −0.30 ** | 0.28 ** | 0.22 ** | −0.11 * | 0.23 ** | −0.70 ** | −0.07 | −0.53 * | 0.04 | |
| RNP | 0.08 | −0.15 ** | −0.15 ** | −0.04 | 0.27 ** | 0.61 ** | −0.56 ** | −0.41 ** | 0.74 ** | |
| RCP | −0.06 | 0.04 | −0.01 | −0.09 | 0.43 ** | 0.13 * | −0.66 ** | 0.06 | 0.79 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Wang, T.; Wang, H.; Yang, J.; Xie, T.; Zhang, Z.; He, C.; Shan, L. Variation and Correlation among Fine Root Traits of Desert Plants in Arid Areas of Northwest China. Forests 2024, 15, 476. https://doi.org/10.3390/f15030476
Ma J, Wang T, Wang H, Yang J, Xie T, Zhang Z, He C, Shan L. Variation and Correlation among Fine Root Traits of Desert Plants in Arid Areas of Northwest China. Forests. 2024; 15(3):476. https://doi.org/10.3390/f15030476
Chicago/Turabian StyleMa, Jing, Taotao Wang, Hongyong Wang, Jie Yang, Tingting Xie, Zhengzhong Zhang, Cai He, and Lishan Shan. 2024. "Variation and Correlation among Fine Root Traits of Desert Plants in Arid Areas of Northwest China" Forests 15, no. 3: 476. https://doi.org/10.3390/f15030476





