Responses of Fine Root Morphological and Chemical Traits among Branch Orders to Forest Thinning in Pinus massoniana Plantations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Sample Collection
2.4. Fine Root Traits
2.5. Soil Properties
2.6. Statistics Analysis
3. Results
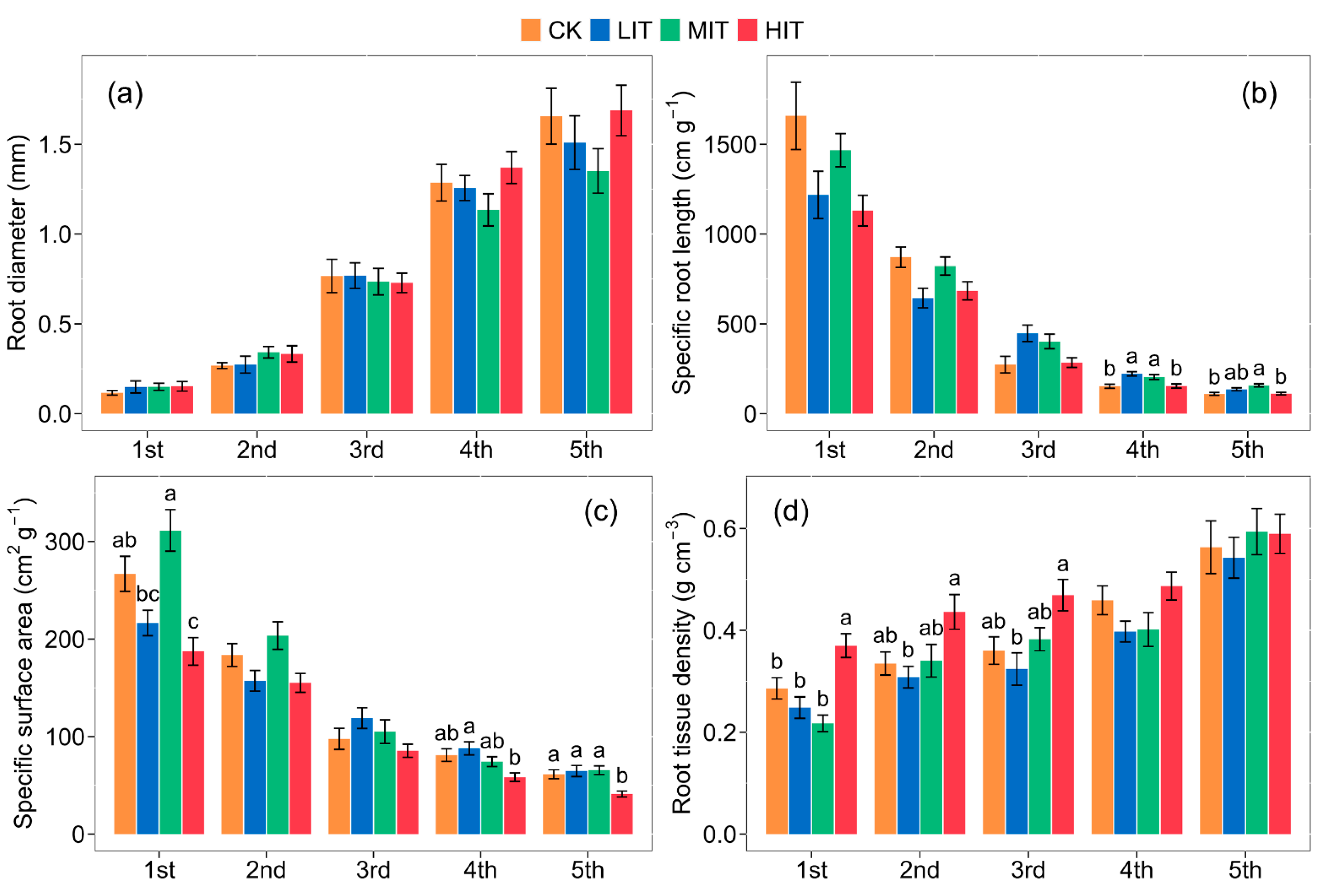
3.1. Fine Root Morphological Traits after Thinning
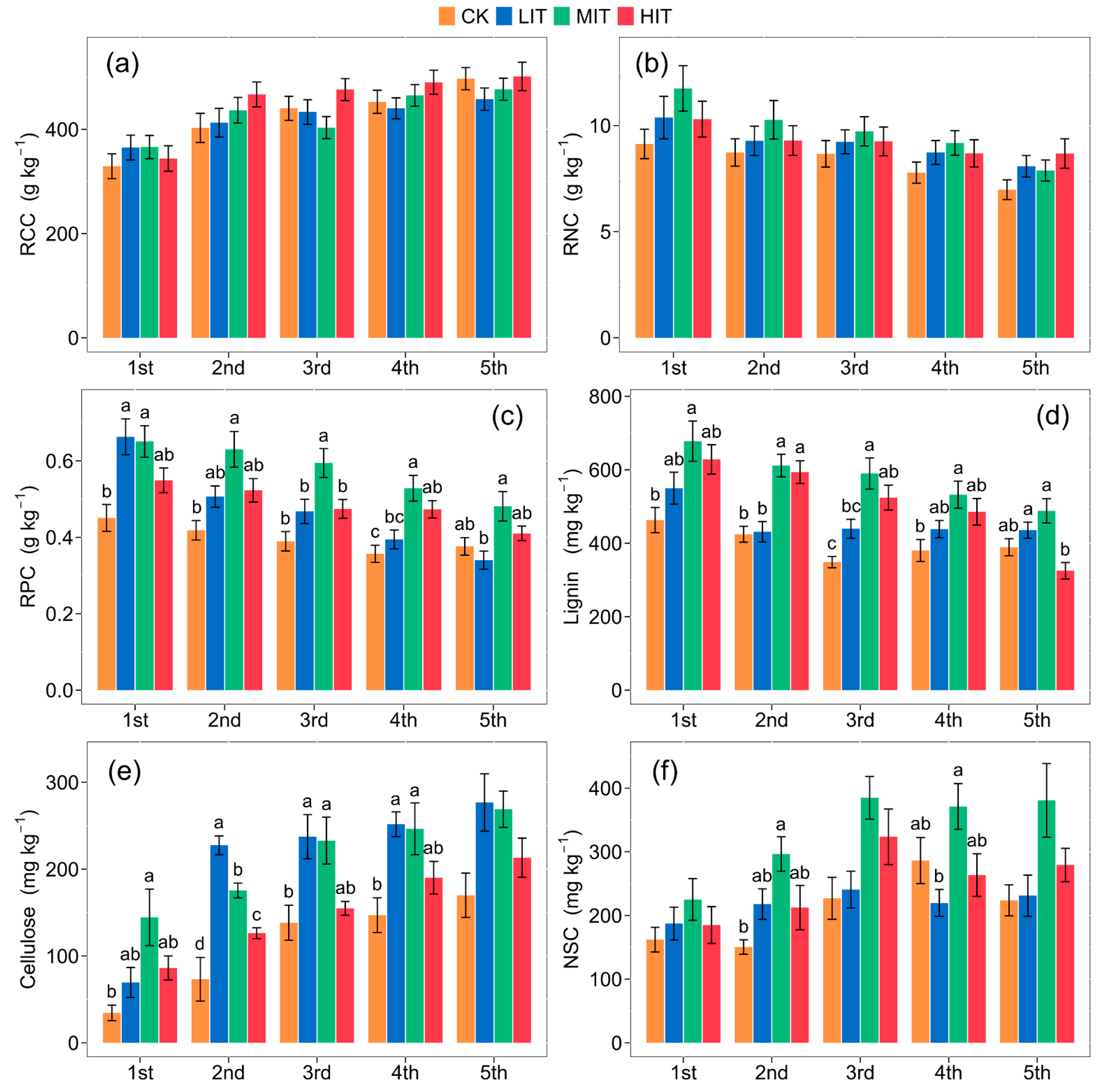
3.2. Fine Root Chemical Traits after Thinning
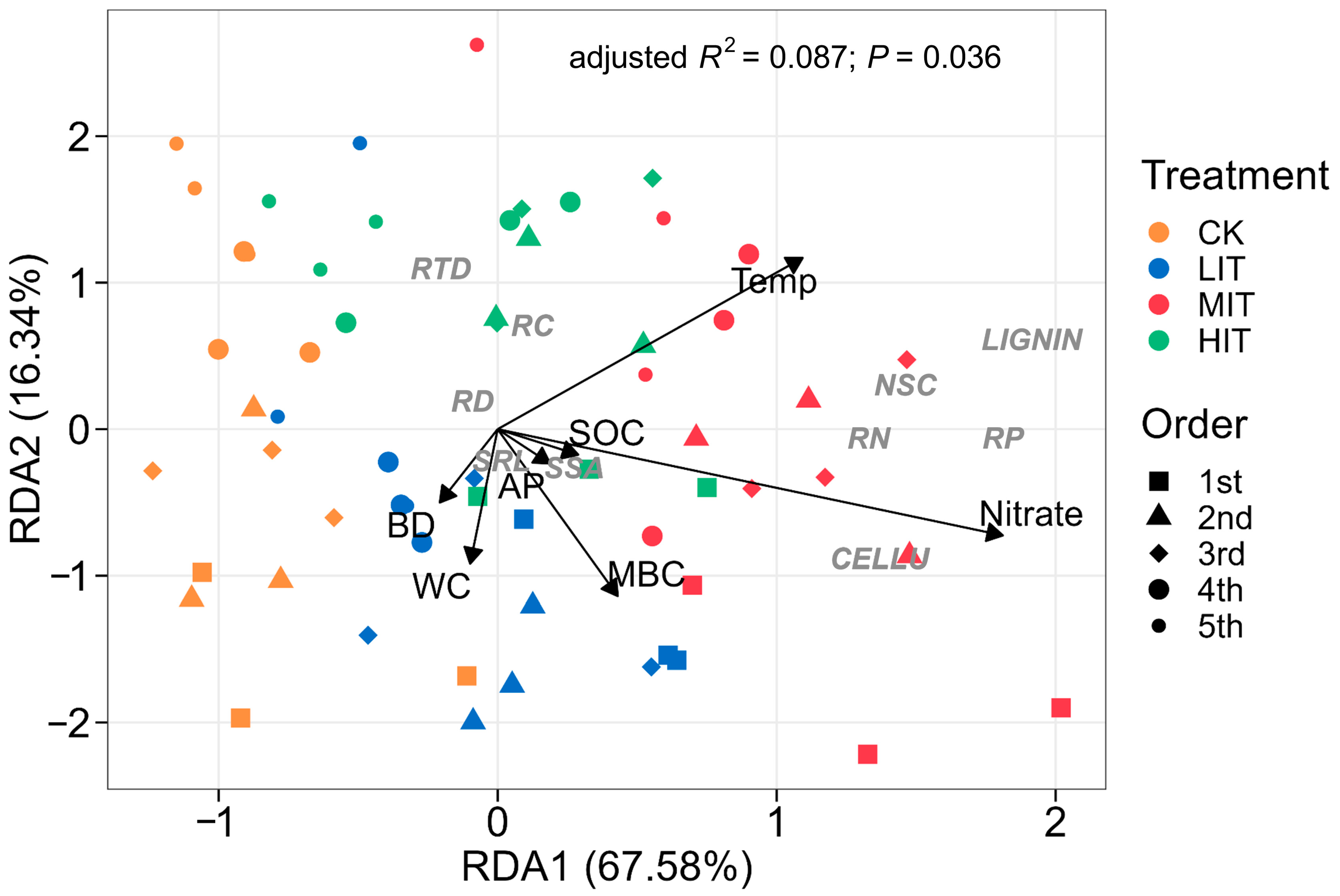
3.3. Soil Factors Affecting Fine Root Traits
4. Discussion
4.1. Fine Root Morpholofical Traits Variations
4.2. Fine Root Chemical Trait Variations
4.3. Variations in Fine Root Traits with Root Order
4.4. Soil Factors Affecting Fine Root Morphology and Chemistry
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fortier, J.; Truax, B.; Gagnon, D.; Lambert, F. Abiotic and Biotic Factors Controlling Fine Root Biomass, Carbon and Nutrients in Closed-Canopy Hybrid Poplar Stands on Post-Agricultural Land. Sci. Rep. 2019, 9, 6296. [Google Scholar] [CrossRef]
- Freschet, G.T.; Valverde-Barrantes, O.J.; Tucker, C.M.; Craine, J.M.; McCormack, M.L.; Violle, C.; Fort, F.; Blackwood, C.B.; Urban-Mead, K.R.; Iversen, C.M.; et al. Climate, Soil and Plant Functional Types as Drivers of Global Fine-Root Trait Variation. J. Ecol. 2017, 105, 1182–1196. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Li, Z.; Morreale, S.J.; Schneider, R.L.; Xu, D.; Lin, X. The Contributions of Root Morphological Characteristics and Soil Property to Soil Infiltration in a Reseeded Desert Steppe. Catena 2023, 225, 107020. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, B.; Wei, X.; Yu, M.; Cheng, X. Aboveground Litter Properties Determined the POC Root Functional Traits Mediate Rhizosphere Soil Carbon Stability in a Subtropical Forest. Soil Biol. Biochem. 2021, 162, 108431. [Google Scholar] [CrossRef]
- De Oliveira, C.D.C.; Durigan, G.; Putz, F.E. Thinning Temporarily Stimulates Tree Regeneration in a Restored Tropical Forest. Ecol. Eng. 2021, 171, 106390. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, H.; Lei, X. Simulation of Climate Change and Thinning Effects on Productivity of Larix olgensis Plantations in Northeast China Using 3-PGmix Model. J. Environ. Manag. 2020, 261, 110249. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, C.; Zhou, Z. Thinning Promotes the Nitrogen and Phosphorous Cycling in Forest Soils. Agric. For. Meteorol. 2021, 311, 108665. [Google Scholar] [CrossRef]
- Wang, D.; Olatunji, O.A.; Xiao, J. Thinning Increased Fine Root Production, Biomass, Turnover Rate and Understory Vegetation Yield in a Chinese Fir Plantation. For. Ecol. Manag. 2019, 440, 92–100. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, D.; Li, W.; Sun, D.; Jin, C.; Yuan, F.; Wang, A.; Wu, J. The Effects of Forest Thinning on Soil Carbon Stocks and Dynamics: A Meta-Analysis. For. Ecol. Manag. 2018, 429, 36–43. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Mommer, L.; De Vries, F.T. Going underground: Root traits as drivers of ecosystem processes. Trends Ecol. Evol. 2014, 29, 692–699. [Google Scholar] [CrossRef]
- Saleem, M.; Pervaiz, Z.H.; Contreras, J.; Lindenberger, J.H.; Hupp, B.M.; Chen, D.; Zhang, Q.; Wang, C.; Iqbal, J.; Twigg, P. Cover Crop Diversity Improves Multiple Soil Properties via Altering Root Architectural Traits. Rhizosphere 2020, 16, 100248. [Google Scholar] [CrossRef]
- Taylor, L.L.; Leake, J.R.; Quirk, J.; Hardy, K.; Banwart, S.A.; Beerling, D.J. Biological Weathering and the Long-Term Carbon Cycle: Integrating Mycorrhizal Evolution and Function into the Current Paradigm. Geobiology 2009, 7, 171–191. [Google Scholar] [CrossRef]
- Abalos, D.; De Deyn, G.B.; Kuyper, T.W.; van Groenigen, J.W. Plant Species Identity Surpasses Species Richness as a Key Driver of N2O Emissions from Grassland. Glob. Chang. Biol. 2014, 20, 265–275. [Google Scholar] [CrossRef]
- Quan, X.; Wang, C.; Zhang, Q.; Wang, X.; Luo, Y.; Bond-Lamberty, B. Dynamics of Fine Roots in Five Chinese Temperate Forests. J. Plant Res. 2010, 123, 497–507. [Google Scholar] [CrossRef]
- Hallett, P.D.; Feeney, D.S.; Bengough, A.G.; Rillig, M.C.; Scrimgeour, C.M.; Young, I.M. Disentangling the Impact of AM Fungi versus Roots on Soil Structure and Water Transport. Plant Soil 2009, 314, 183–196. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, M.; Chen, F.; Li, H. Variation in Fine Root Traits with Thinning Intensity in a Chinese Fir Plantation Insights from Branching Order and Functional Groups. Sci. Rep. 2021, 11, 22710. [Google Scholar] [CrossRef]
- Noguchi, K.; Han, Q.; Araki, M.G.; Kawasaki, T.; Kaneko, S.; Takahashi, M.; Chiba, Y. Fine-Root Dynamics in a Young Hinoki Cypress (Chamaecyparis obtusa) Stand for 3 Years Following Thinning. J. For. Res. 2011, 16, 284–291. [Google Scholar] [CrossRef]
- Akburak, S.; Makineci, E. Thinning Effects on Biomass and Element Concentrations of Roots in Adjacent Hornbeam and Oak Stands in Istanbul, Turkey. For. Ecosyst. 2021, 8, 1–10. [Google Scholar] [CrossRef]
- Bo, H.; Wen, C.; Song, L.; Yue, Y.; Nie, L. Fine-Root Responses of Populus Tomentosa Forests to Stand Density. Forests 2018, 9, 562. [Google Scholar] [CrossRef]
- Barrette, J.; Pothier, D.; Ward, C. Temporal Changes in Stem Decay and Dead and Sound Wood Volumes in the Northeastern Canadian Boreal Forest. Can. J. For. Res. 2013, 43, 234–244. [Google Scholar] [CrossRef]
- Meinen, C.; Hertel, D.; Leuschner, C. Biomass and Morphology of Fine Roots in Temperate Broad-Leaved Forests Differing in Tree Species Diversity: Is There Evidence of below-Ground Overyielding? Oecologia 2009, 161, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Montagnoli, A.; Dumroese, R.K.; Terzaghi, M.; Onelli, E.; Scippa, G.S.; Chiatante, D. Seasonality of Fine Root Dynamics and Activity of Root and Shoot Vascular Cambium in a Quercus ilex L. Forest (Italy). For. Ecol. Manag. 2019, 431, 26–34. [Google Scholar] [CrossRef]
- Cai, H.; Li, F.; Jin, G. Fine Root Biomass, Production and Turnover Rates in Plantations versus Natural Forests: Effects of Stand Characteristics and Soil Properties. Plant Soil 2019, 436, 463–474. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining Fine Roots Improves Understanding of Below-Ground Contributions to Terrestrial Biosphere Processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Iversen, C.M.; McCormack, M.L.; Powell, A.S.; Blackwood, C.B.; Freschet, G.T.; Kattge, J.; Roumet, C.; Stover, D.B.; Soudzilovskaia, N.A.; Valverde-Barrantes, O.J.; et al. A Global Fine-Root Ecology Database to Address below-Ground Challenges in Plant Ecology. New Phytol. 2017, 215, 15–26. [Google Scholar] [CrossRef]
- Wada, R.; Tanikawa, T.; Doi, R.; Hirano, Y. Variation in the Morphology of Fine Roots in Cryptomeria japonica Determined by Branch Order-Based Classification. Plant Soil 2019, 444, 139–151. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Wang, S.; Yang, L.; Gu, J. Intraspecific Variations of Anatomical, Morphological and Chemical Traits in Leaves and Absorptive Roots along Climate and Soil Gradients: A Case Study with Ginkgo biloba and Eucommia ulmoides. Plant Soil 2021, 469, 73–88. [Google Scholar] [CrossRef]
- Endo, I.; Kobatake, M.; Tanikawa, N.; Nakaji, T.; Ohashi, M.; Makita, N. Anatomical Patterns of Condensed Tannin in Fine Roots of Tree Species from a Coolerate Forest. Ann. Bot. 2021, 128, 59–71. [Google Scholar] [CrossRef]
- Yu, W.; Wang, C.; Huang, Z.; Wang, D.; Liu, G. Variations in the Traits of Fine Roots of Different Orders and Their Associations with Leaf Traits in 12 Co-Occuring Plant Species in a Semiarid Inland Dune. Plant Soil 2022, 472, 193–206. [Google Scholar] [CrossRef]
- Kong, D.; Wang, J.; Zeng, H.; Liu, M.; Miao, Y.; Wu, H.; Kardol, P. The nutrient absorption–transportation hypothesis: Optimizing structural traits in absorptive roots. New Phytol. 2017, 213, 1569–1572. [Google Scholar] [CrossRef] [PubMed]
- Zadworny, M.; McCormack, M.L.; Żytkowiak, R.; Karolewski, P.; Mucha, J.; Oleksyn, J. Patterns of Structural and Defense Investments in Fine Roots of Scots Pine (Pinus sylvestris L.) across a Strong Temperature and Latitudinal Gradient in Europe. Glob. Chang. Biol. 2017, 23, 1218–1231. [Google Scholar] [CrossRef]
- Beidler, K.V.; Pritchard, S.G. Maintaining Connectivity: Understanding the Role of Root Order and Mycelial Networks in Fine Root Decomposition of Woody Plants. Plant Soil 2017, 420, 19–36. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, J.; Wang, G.; Guan, Q.; Kuzyakov, Y. Fine Root Extension in Urban Forest Soil Depends on Organic Mulching. Agrofor. Syst. 2023, 97, 235–247. [Google Scholar] [CrossRef]
- Wang, G.; Liu, F.; Xue, S. Nitrogen Addition Enhanced Water Uptake by Affecting Fine Root Morphology and Coarse Root Anatomy of Chinese Pine Seedlings. Plant Soil 2017, 418, 177–189. [Google Scholar] [CrossRef]
- Püttsepp, Ü.; Lõhmus, K.; Persson, H.Å.; Ahlström, K. Fine-Root Distribution and Morphology in an Acidic Norway Spruce (Picea abies (L.) Karst.) Stand in SW Sweden in Relation to Granulated Wood Ash Application. For. Ecol. Manag. 2006, 221, 291–298. [Google Scholar] [CrossRef]
- Fort, F.; Cruz, P.; Lecloux, E.; Bittencourt de Oliveira, L.; Stroia, C.; Theau, J.P.; Jouany, C. Grassland Root Functional Parameters Vary According to a Community-Level Resource Acquisition–Conservation Trade-Off. J. Veg. Sci. 2016, 27, 749–758. [Google Scholar] [CrossRef]
- Bastida, F.; López-Mondéjar, R.; Baldrian, P.; Andrés-Abellán, M.; Jehmlich, N.; Torres, I.F.; García, C.; López-Serrano, F.R. When Drought Meets Forest Management: Effects on the Soil Microbial Community of a Holm Oak Forest Ecosystem. Sci. Total Environ. 2019, 662, 276–286. [Google Scholar] [CrossRef]
- Wagle, B.H.; Weiskittel, A.R.; Kizha, A.R.; Berrill, J.P.; D’Amato, A.W.; Marshall, D. Long-Term Influence of Commercial Thinning on Stand Structure and Yield with/without Pre-Commercial Thinning of Spruce-Fir in Northern Maine, USA. For. Ecol. Manag. 2022, 522, 120453. [Google Scholar] [CrossRef]
- Xiao, W.; Fei, F.; Diao, J.; Chen, B.J.W.; Guan, Q. Thinning Intensity Affects Microbial Functional Diversity and Enzymatic Activities Associated with Litter Decomposition in a Chinese Fir Plantation. J. For. Res. 2018, 29, 1337–1350. [Google Scholar] [CrossRef]
- Xu, M.; Liu, H.; Zhang, Q.; Zhang, Z.; Ren, C.; Feng, Y.; Yang, G.; Han, X.; Zhang, W. Effect of Forest Thinning on Soil Organic Carbon Stocks from the Perspective of Carbon-Degrading Enzymes. Catena 2022, 218, 106560. [Google Scholar] [CrossRef]
- Ye, Y.; Sun, X.; Zhao, J.; Wang, M.; Guan, Q. Establishing a Soil Quality Index to Assess the Effect of Thinning on Soil Quality in a Chinese Fir Plantation. Eur. J. For. Res. 2022, 141, 999–1009. [Google Scholar] [CrossRef]
- Chen, F.; Yuan, Y.J.; Yu, S.L.; Zhang, T. wen Influence of Climate Warming and Resin Collection on the Growth of Masson Pine (Pinus massoniana) in a Subtropical Forest, Southern China. Trees-Struct. Funct. 2015, 29, 1423–1430. [Google Scholar] [CrossRef]
- Ali, A.; Dai, D.; Akhtar, K.; Teng, M.; Yan, Z.; Urbina-Cardona, N.; Mullerova, J.; Zhou, Z. Response of Understory Vegetation, Tree Regeneration, and Soil Quality to Manipulated Stand Density in a Pinus massoniana Plantation. Glob. Ecol. Conserv. 2019, 20, e00775. [Google Scholar] [CrossRef]
- Mo, R.; Wang, Y.; Dong, S.; Ma, J.; Mo, Y. Ecosystem Service Evaluation and Multi-Objective Management of Pinus massoniana Lamb. Plantations in Guangxi, China. Forests 2023, 14, 213. [Google Scholar] [CrossRef]
- Yuan, Z.; Jin, X.; Xiao, W.; Wang, L.; Sun, Y.; Guan, Q.; Meshack, A.O. Comparing Soil Organic Carbon Stock and Fractions under Natural Secondary Forest and Pinus massoniana Plantation in Subtropical China. Catena 2022, 212, 106092. [Google Scholar] [CrossRef]
- Chen, X.L.; Wang, D.; Chen, X.; Wang, J.; Diao, J.J.; Zhang, J.Y.; Guan, Q.W. Soil Microbial Functional Diversity and Biomass as Affected by Different Thinning Intensities in a Chinese Fir Plantation. Appl. Soil Ecol. 2015, 92, 35–44. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; DeForest, J.L.; Burton, A.J.; Allen, M.F.; Ruess, R.W.; Hendrick, R.L. Fine Root Architecture of Nine North American Trees. Ecol. Monogr. 2002, 72, 293–309. [Google Scholar] [CrossRef]
- Van Soest, P.J. Use of detergents in the analysis of fibrous feeds. A rapid method for the determination of fiber and lignin. J. Assoc. Off. Agric. Chem. 1963, 46, 829–835. [Google Scholar] [CrossRef]
- Wan, S.; Xia, J.; Liu, W.; Niu, S. Photosynthetic overcompensation under nocturnal warming enhances grassland carbon sequestration. Ecology 2009, 90, 2700–2710. [Google Scholar] [CrossRef] [PubMed]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austraria, 2019. [Google Scholar]
- Assefa, D.; Godbold, D.L.; Belay, B.; Abiyu, A.; Rewald, B. Fine Root Morphology, Biochemistry and Litter Quality Indices of Fast- and Slow-Growing Woody Species in Ethiopian Highland Forest. Ecosystems 2018, 21, 482–494. [Google Scholar] [CrossRef]
- Luke Mccormack, M.; Adams, T.S.; Smithwick, E.A.H.; Eissenstat, D.M. Predicting Fine Root Lifespan from Plant Functional Traits in Temperate Trees. New Phytol. 2012, 195, 823–831. [Google Scholar] [CrossRef]
- Simpson, A.H.; Richardson, S.J.; Laughlin, D.C. Soil–Climate Interactions Explain Variation in Foliar, Stem, Root and Reproductive Traits across Temperate Forests. Glob. Ecol. Biogeogr. 2016, 25, 964–978. [Google Scholar] [CrossRef]
- Molina, A.J.; del Campo, A.D. The Effects of Experimental Thinning on Throughfall and Stemflow: A Contribution towards Hydrology-Oriented Silviculture in Aleppo Pine Plantations. For. Ecol. Manag. 2012, 269, 206–213. [Google Scholar] [CrossRef]
- Zhao, J.; Ye, Y.; Sun, X.; Shi, L.; Chen, X.; Guan, Q. Root Exudation Patterns of Chinese Fir after Thinning Relating to Root Characteristics and Soil Conditions. For. Ecol. Manag. 2023, 541, 121068. [Google Scholar] [CrossRef]
- Reich, P.B. The World-Wide “fast-Slow” Plant Economics Spectrum: A Traits Manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Kong, D.; Ma, C.; Zhang, Q.; Li, L.; Chen, X.; Zeng, H.; Guo, D. Leading Dimensions in Absorptive Root Trait Variation across 96 Subtropical Forest Species. New Phytol. 2014, 203, 863–872. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Tedersoo, L. Evolutionary History of Mycorrhizal Symbioses and Global Host Plant Diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef]
- Ostonen, I.; Truu, M.; Helmisaari, H.S.; Lukac, M.; Borken, W.; Vanguelova, E.; Godbold, D.L.; Lõhmus, K.; Zang, U.; Tedersoo, L.; et al. Adaptive Root Foraging Strategies along a Boreal–Temperate Forest Gradient. New Phytol. 2017, 215, 977–991. [Google Scholar] [CrossRef]
- Wahl, S.; Ryser, P. Root Tissue Structure Is Linked to Ecological Strategies of Grasses. New Phytol. 2000, 148, 459–471. [Google Scholar] [CrossRef]
- Yin, X.; Perry, J.A.; Dixon, R.K. Temporal changes in nutrient concentrations and contents of fine roots in a Quercus forest. For. Ecol. Manag. 1991, 44, 175–184. [Google Scholar] [CrossRef]
- Takahashi, Y.; Katoh, M. Root Response and Phosphorus Uptake with Enhancement in Available Phosphorus Level in Soil in the Presence of Water-Soluble Organic Matter Deriving from Organic Material. J. Environ. Manag. 2022, 322, 116038. [Google Scholar] [CrossRef]
- Li, W.; Jin, C.; Guan, D.; Wang, Q.; Wang, A.; Yuan, F.; Wu, J. The Effects of Simulated Nitrogen Deposition on Plant Root Traits: A Meta-Analysis. Soil Biol. Biochem. 2015, 82, 112–118. [Google Scholar] [CrossRef]
- Lõhmus, K.; Truu, M.; Truu, J.; Ostonen, I.; Kaar, E.; Vares, A.; Uri, V.; Alama, S.; Kanal, A. Functional Diversity of Culturable Bacterial Communities in the Rhizosphere in Relation to Fine-Root and Soil Parameters in Alder Stands on Forest, Abandoned Agricultural, and Oil-Shale Mining Areas. Plant Soil 2006, 283, 1–10. [Google Scholar] [CrossRef]
- Wang, P.; Guo, J.; Xu, X.; Yan, X.; Zhang, K.; Qiu, Y.; Zhao, Q.; Huang, K.; Luo, X.; Yang, F.; et al. Soil Acidification Alters Root Morphology, Increases Root Biomass but Reduces Root Decomposition in an Alpine Grassland. Environ. Pollut. 2020, 265, 115016. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, W.; Meng, M.; Fu, Z.; Xu, L.; Zha, Y.; Yue, J.; Zhang, S.; Zhang, J. Comparative Effects of Simulated Acid Rain of Different Ratios of SO42− to NO3− on Fine Root in Subtropical Plantation of China. Sci. Total Environ. 2018, 618, 336–346. [Google Scholar] [CrossRef]
- Nikolova, P.S.; Bauerle, T.L.; Häberle, K.H.; Blaschke, H.; Brunner, I.; Matyssek, R. Fine-Root Traits Reveal Contrasting Ecological Strategies in European Beech and Norway Spruce During Extreme Drought. Front. Plant Sci. 2020, 11, 1211. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, J.; Gong, L. The Morphological and Chemical Properties of Fine Roots Respond to Nitrogen Addition in a Temperate Schrenk’s Spruce (Picea schrenkiana) Forest. Sci. Rep. 2021, 11, 3839. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Makita, N.; Dannoura, M.; Wang, S.; Takahashi, K. The Composition of Non-Structural Carbohydrates Affects the Respiration and Morphology of Tree Fine Roots in Japan Alps. Rhizosphere 2023, 26, 100705. [Google Scholar] [CrossRef]
- Hishi, T.; Tateno, R.; Fukushima, K.; Fujimaki, R.; Itoh, M.; Tokuchi, N. Changes in the Anatomy, Morphology and Mycorrhizal Infection of Fine Root Systems of Cryptomeria japonica in Relation to Stand Ageing. Tree Physiol. 2017, 37, 61–70. [Google Scholar] [CrossRef]
- Doi, R.; Tanikawa, T.; Miyatani, K.; Hirano, Y. Intraspecific Variation in Morphological Traits of Root Branch Orders in Chamaecyparis obtusa. Plant Soil 2017, 416, 503–513. [Google Scholar] [CrossRef]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How Tree Roots Respond to Drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef]
- Li, Z.; Rubert-Nason, K.F.; Jamieson, M.A.; Raffa, K.F.; Lindroth, R.L. Root Secondary Metabolites in Populus tremuloides: Effects of Simulated Climate Warming, Defoliation, and Genotype. J. Chem. Ecol. 2021, 47, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Jia, L.; Wang, X.; Chen, W.; Xiong, D.; Chen, S.; Liu, X.; Yang, Z.; Yao, X.; Chen, T.; et al. Soil warming alters fine root lifespan, phenology, and architecture in a Cunninghamia lanceolata plantation. Agric. For. Meteorol. 2022, 327, 109201. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, J.; Melillo, J.M.; Butler, S.; Mohan, J.E. Root Standing Crop and Chemistry after Six Years of Soil Warming in a Temperate Forest. Tree Physiol. 2011, 31, 707–717. [Google Scholar] [CrossRef]
- Jiang, L.; Kou, L.; Li, S. Decomposition of leaf mixtures and absorptive-root mixtures synchronously changes with deposition of nitrogen and phosphorus. Soil Biol. Biochem. 2019, 138, 107602. [Google Scholar] [CrossRef]
- Sweeney, C.J.; de Vries, F.T.; van Dongen, B.E.; Bardgett, R.D. Root traits explain rhizosphere fungal community composition among temperate grassland plant species. New Phytol. 2021, 229, 1492–1507. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, J.; Weigelt, A.; Van Der Plas, F.; Laughlin, D.C.; Kuyper, T.W.; Guerrero-Ramirez, N.; Valverde-Barrantes, O.J.; Bruelheide, H.; Fresche, G.T.; Iversen, C.M.; et al. The Fungal Collaboration Gradient Dominates the Root Economics Space in Plants. Sci. Adv. 2020, 6, eaba3756. [Google Scholar] [CrossRef]
| Characteristics | CK | LIT | MIT | HIT |
|---|---|---|---|---|
| Stand density (tree ha−1) | 2591 ± 66 | 1944 ± 48 | 1453 ± 25 | 948 ± 20 |
| Tree DBH (cm) | 13.71 ± 1.54 | 17.63 ± 0.98 | 18.74 ± 3.60 | 19.52 ± 3.96 |
| Tree height (m) | 11.8 ± 2.12 | 13.7 ± 1.69 | 14.0 ± 2.77 | 14.4 ± 3.62 |
| Canopy density | 0.88 ± 0.01 | 0.80 ± 0.00 | 0.65 ± 0.01 | 0.50 ± 0.02 |
| Understory coverage (%) | 51.3 ± 1.23 | 62.1 ± 3.61 | 67.6 ± 2.17 | 80.5 ± 4.11 |
| Soil Properties | CK | LIT | MIT | HIT |
|---|---|---|---|---|
| Temperature (°C) | 25.91 ± 0.43 b | 26.12 ± 0.19 b | 26.98 ± 0.52 ab | 27.61 ± 0.69 a |
| Water content (%) | 24.98 ± 0.51 a | 24.11 ± 1.35 ab | 24.86 ± 0.82 a | 22.38 ± 1.01 b |
| pH | 4.56 ± 0.06 a | 4.53 ± 0.03 a | 4.47 ± 0.04 a | 4.48 ± 0.08 a |
| Bulk density | 1.11 ± 0.19 a | 1.08 ± 0.14 a | 1.04 ± 0.11 a | 1.05 ± 0.17 a |
| Organic C (g kg−1) | 28.73 ± 2.40 b | 34.33 ± 2.46 a | 30.6 ± 1.21 ab | 31.57 ± 2.10 ab |
| Total N (g kg−1) | 1.93 ± 0.15 b | 2.67 ± 0.15 a | 2.54 ± 0.12 a | 1.98 ± 0.06 b |
| Ammonium (mg kg−1) | 3.68 ± 0.39 b | 5.97 ± 0.36 a | 7.09 ± 0.70 a | 4.68 ± 0.89 b |
| Nitrate (mg kg−1) | 2.49 ± 0.05 b | 2.78 ± 0.35 b | 4.40 ± 0.17 a | 3.82 ± 0.58 a |
| Available P (mg kg−1) | 3.32 ± 1.21 a | 4.35 ± 1.32 a | 8.50 ± 3.08 a | 6.32 ± 2.31 a |
| Microbial biomass C (mg kg−1) | 623.59 ± 61.22 a | 759.79 ± 59.03 a | 689.81 ± 52.72 a | 637.38 ± 43.22 a |
| Microbial biomass N (mg kg−1) | 62.75 ± 17.60 b | 97.76 ± 16.76 a | 105.90 ± 14.03 a | 76.92 ± 7.36 ab |
| Treatment | Root Order | Treatment × Root Order | |
|---|---|---|---|
| Root diameter | 1.4246 | 208.9307 *** | 0.8760 |
| Specific root length | 4.2454 * | 259.0730 *** | 3.4064 ** |
| Specific surface area | 6.7396 * | 326.8082 *** | 7.1374 *** |
| Root tissue density | 9.9696 ** | 51.5888 *** | 0.9066 |
| Root C | 2.2400 | 18.6409 *** | 0.7702 |
| Root N | 2.5192 | 8.3423 * | 0.3854 |
| Root P | 24.1776 *** | 19.4464 *** | 1.8726 |
| Root lignin | 12.6885 ** | 21.3862 *** | 3.2998 ** |
| Root cellulose | 27.1723 *** | 30.6402 *** | 1.1862 |
| Root non-structural carbohydrate | 15.0373 *** | 8.3388 *** | 0.9170 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Sun, X.; Wang, D.; Wang, M.; Li, J.; Wang, J.; Guan, Q. Responses of Fine Root Morphological and Chemical Traits among Branch Orders to Forest Thinning in Pinus massoniana Plantations. Forests 2024, 15, 495. https://doi.org/10.3390/f15030495
Zhao J, Sun X, Wang D, Wang M, Li J, Wang J, Guan Q. Responses of Fine Root Morphological and Chemical Traits among Branch Orders to Forest Thinning in Pinus massoniana Plantations. Forests. 2024; 15(3):495. https://doi.org/10.3390/f15030495
Chicago/Turabian StyleZhao, Jiahao, Xiaodan Sun, Dong Wang, Meiquan Wang, Junjie Li, Jun Wang, and Qingwei Guan. 2024. "Responses of Fine Root Morphological and Chemical Traits among Branch Orders to Forest Thinning in Pinus massoniana Plantations" Forests 15, no. 3: 495. https://doi.org/10.3390/f15030495





