Distribution, Residue Dynamics, and Insecticidal Efficacy of Trunk-Injected Emamectin Benzoate in Pecan Trees
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Field Trial
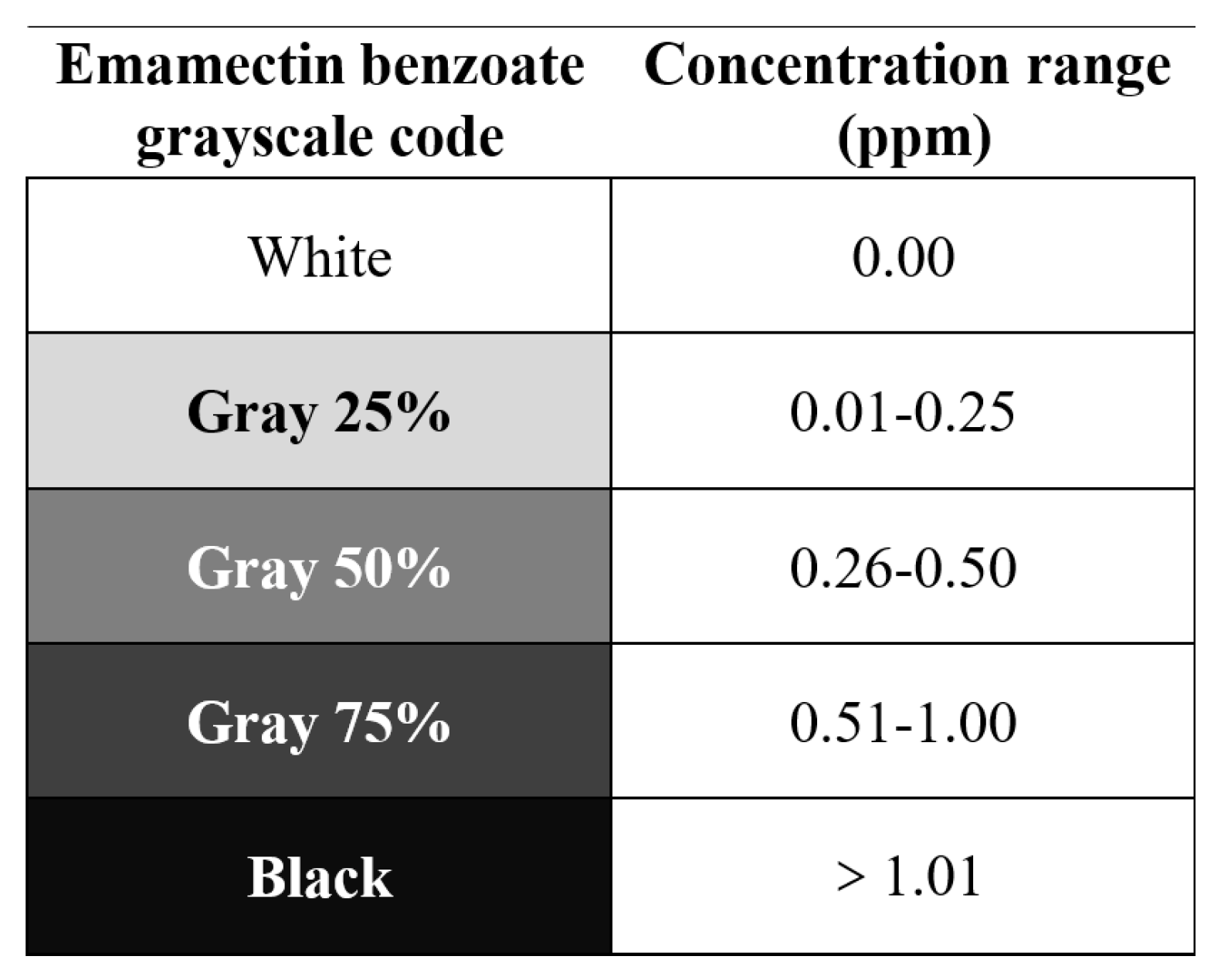
2.3. Sample Collection and Preparation for Determining Emamectin Benzoate Residue
2.4. Analytical Methods
2.4.1. Sample Extraction
2.4.2. UPLC-MS/MS Analysis
2.5. Assessment of Insecticidal Efficacy
2.6. Statistical Analysis
3. Results
3.1. Method Validation
3.1.1. Linear Equation and Matrix Effects
3.1.2. Recovery and LOQ
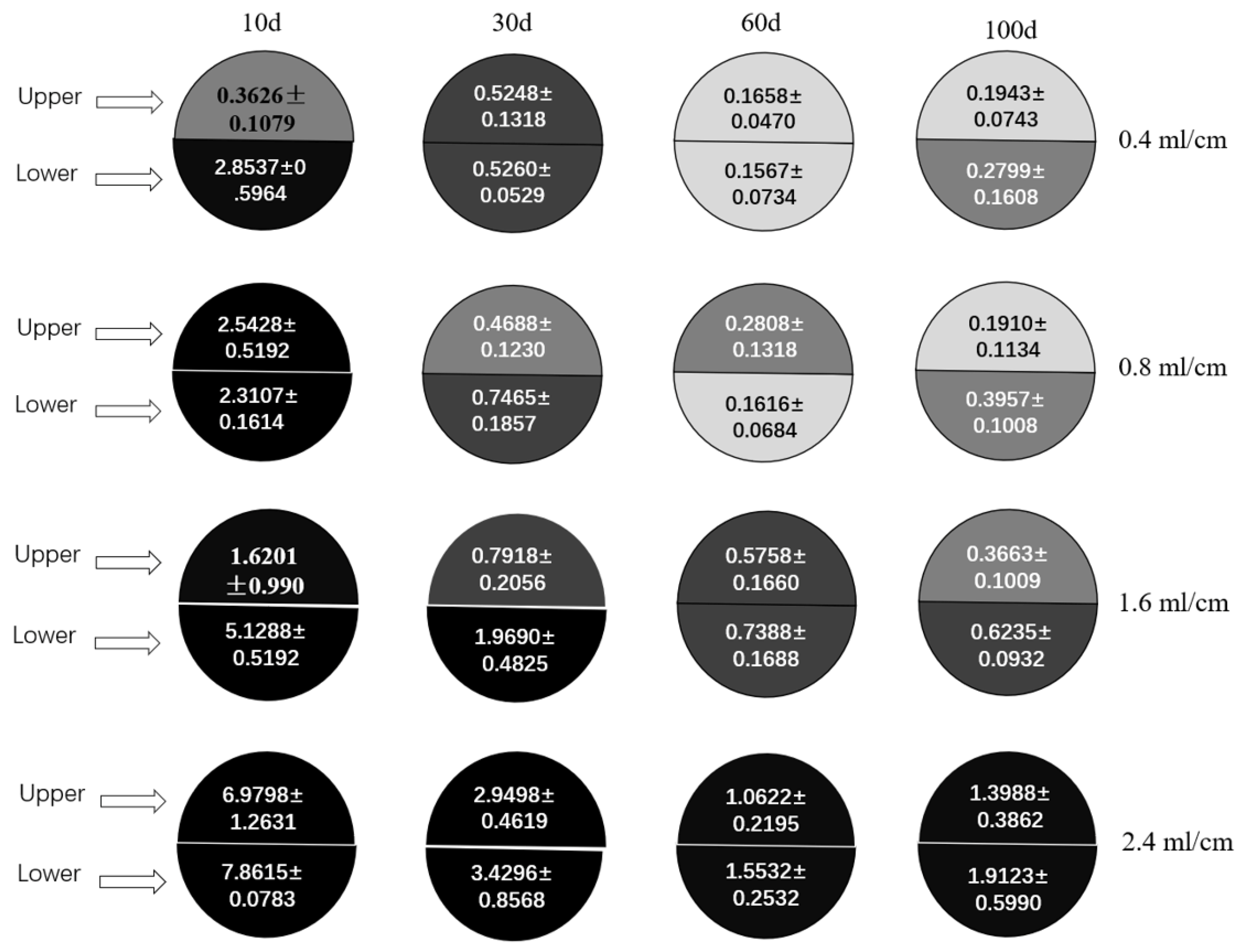
3.2. Distribution of Emamectin Benzoate in Pecan Crowns
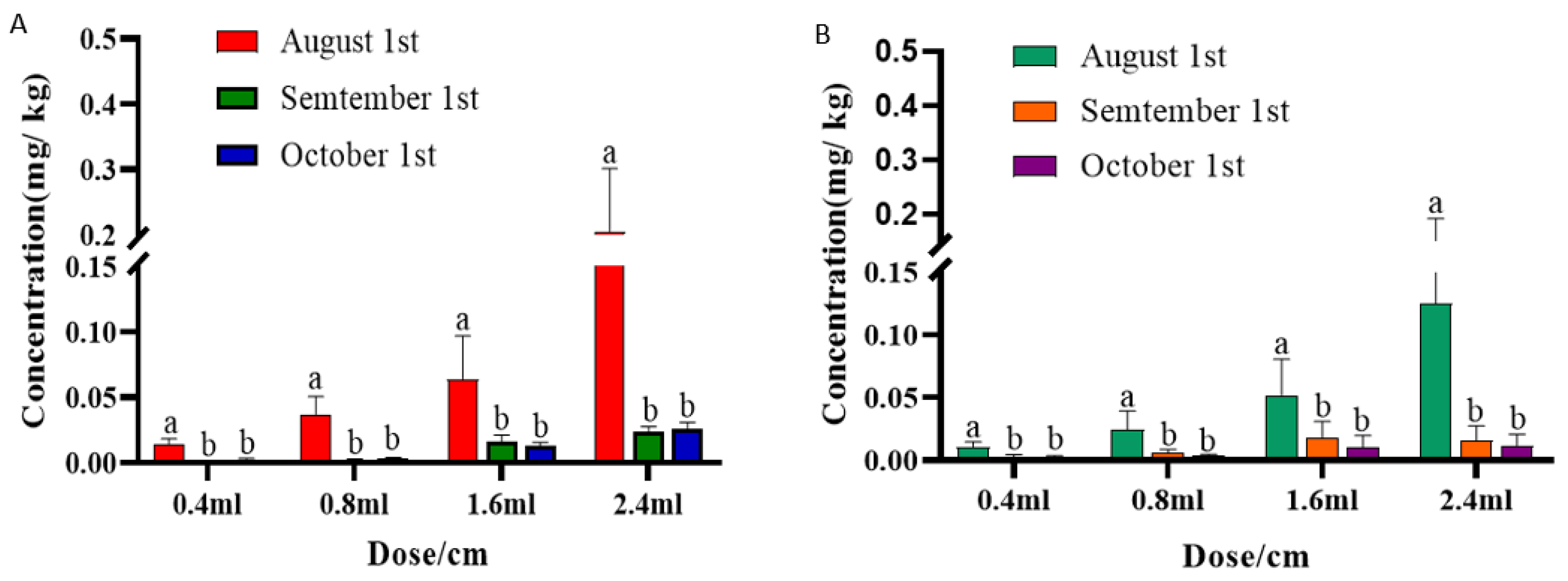
3.3. Final Residues of Emamectin Benzoate in Pecan Pericarps and Kernels
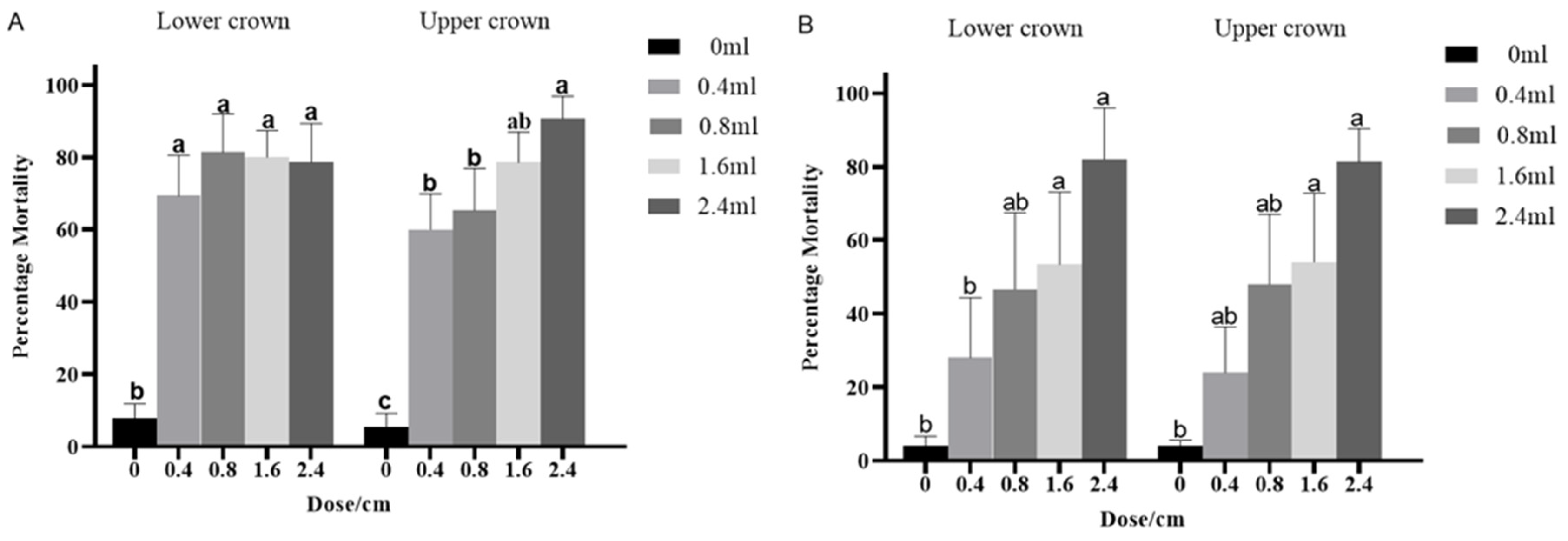
3.4. Insecticidal Efficacy of Emamectin Benzoate against H. cunea Larvae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, T.E.; Conner, P.J. Pecan. In Fruit Breeding; Springer: Berlin/Heidelberg, Germany, 2012; pp. 771–801. [Google Scholar]
- Hall, G.D. Pecan food potential in prehistoric North America. Econ. Bot. 2000, 54, 103–112. [Google Scholar] [CrossRef]
- Mo, Z.; Feng, G.; Su, W.; Liu, Z.; Peng, F. Transcriptomic analysis provides insights into grafting union development in pecan (Carya illinoinensis). Genes 2018, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Hänninen, H.; Lin, J.; Shen, S.; Zhang, R. Extending the cultivation area of Pecan (Carya illinoinensis) toward the south in southeastern subtropical China may cause increased cold damage. Front. Plant Sci. 2021, 12, 768963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Peng, F.; Li, Y. Pecan production in China. Sci. Hortic. 2015, 197, 719–727. [Google Scholar] [CrossRef]
- Zheng, X.-R.; Chen, J.-J. First report of leaf spot on pecan caused by Choanephora cucurbitarum in China. Plant Dis. 2023, 107, 2241. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X. Green prevention and control technology of main pests and diseases of Caray illinoensis. Anhui Agric. Sci. Bull. 2019, 25, 95–96. [Google Scholar]
- Slusher, E.K.; Cottrell, T.; Acebes-Doria, A.L. Effects of aphicides on pecan aphids and their parasitoids in pecan orchards. Insects 2021, 12, 241. [Google Scholar] [CrossRef]
- Pour, S.A.; Shahriari, M.; Zibaee, A.; Mojarab-Mahboubkar, M.; Sahebzadeh, N.; Hoda, H. Toxicity, antifeedant and physiological effects of trans-anethole against Hyphantria cunea Drury (Lep: Arctiidae). Pestic. Biochem. Physiol. 2022, 185, 105135. [Google Scholar] [CrossRef]
- Tan, M.; Wu, H.; Yan, S.; Jiang, D. Evaluating the toxic effects of tannic acid treatment on Hyphantria cunea larvae. Insects 2022, 13, 872. [Google Scholar] [CrossRef] [PubMed]
- Edosa, T.T.; Jo, Y.H.; Keshavarz, M.; Anh, Y.S.; Noh, M.Y.; Han, Y.S. Current status of the management of fall webworm, Hyphantria cunea: Towards the integrated pest management development. J. Appl. Entomol. 2019, 143, 1–10. [Google Scholar] [CrossRef]
- Berger, C.; Laurent, F. Trunk injection of plant protection products to protect trees from pests and diseases. Crop Prot. 2019, 124, 104831. [Google Scholar] [CrossRef]
- Byrne, F.J.; Almanzor, J.; Tellez, I.; Eskalen, A.; Grosman, D.M.; Morse, J.G. Evaluation of trunk-injected emamectin benzoate as a potential management strategy for Kuroshio shot hole borer in avocado trees. Crop Prot. 2020, 132, 105136. [Google Scholar] [CrossRef]
- Coslor, C.C.; Vandervoort, C.; Wise, J.C. Insecticide dose and seasonal timing of trunk injection in apples influence efficacy and residues in nectar and plant parts. Pest Manag. Sci. 2019, 75, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.; Suzuki, T.; Kawazu, K. Distribution and persistence of emamectin benzoate at efficacious concentrations in pine tissues after injection of a liquid formulation. Pest Manag. Sci. 2004, 60, 42–48. [Google Scholar] [CrossRef]
- Aćimović, S.G.; VanWoerkom, A.H.; Reeb, P.D.; Vandervoort, C.; Garavaglia, T.; Cregg, B.M.; Wise, J.C. Spatial and temporal distribution of trunk-injected imidacloprid in apple tree canopies. Pest Manag. Sci. 2014, 70, 1751–1760. [Google Scholar] [CrossRef]
- Wheeler, C.E.; Vandervoort, C.; Wise, J.C. Organic control of pear psylla in pear with trunk injection. Insects 2020, 11, 650. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, J.; Li, Y.; Li, J.; Shi, X.-H. Evaluation of the effectiveness of insecticide trunk injections for control of Latoia lepida (Cramer) in the sweet olive tree Osmanthus fragrans. Peerj 2016, 4, e2480. [Google Scholar] [CrossRef]
- Turcotte, R.M.; Lagalante, A.; Jones, J.; Cook, F.; Elliott, T.; Billings, A.A.; Park, Y.-L. Spatial and temporal distribution of imidacloprid within the crown of eastern hemlock. J. Insect Sci. 2017, 17, 22. [Google Scholar] [CrossRef]
- Kou, H.; Sun, Y.; Dong, Z.; Zhang, Z. Comparison between sustained effects of spray and injection thiamethoxam on apple aphids and non-target insects in apple orchard. Ecotoxicol. Environ. Saf. 2021, 207, 111307. [Google Scholar] [CrossRef]
- Roditakis, E.; Skarmoutsou, C.; Staurakaki, M. Toxicity of insecticides to populations of tomato borer Tuta absoluta (Meyrick) from Greece. Pest Manag. Sci. 2013, 69, 834–840. [Google Scholar] [CrossRef]
- Stavrakaki, M.; Ilias, A.; Ioannidis, P.; Vontas, J.; Roditakis, E. Investigating mechanisms associated with emamectin benzoate resistance in the tomato borer Tuta absoluta. J. Pest Sci. 2022, 95, 1163–1177. [Google Scholar] [CrossRef]
- Mashal, M.M.; Obeidat, B.F. The efficacy assessment of emamectin benzoate using micro injection system to control red palm weevil. Heliyon 2019, 5, e01833. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Fan, Q.; Chen, A.; Huang, J. Effects of trunk injection with emamectin benzoate on arthropod diversity. Pest Manag. Sci. 2023, 79, 935–946. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Mang, D.-Z.; Liao, H.; Ye, J.; Qian, J.-L.; Dong, S.-L.; Zhang, Y.-N.; He, P.; Zhang, Q.-H.; Purba, E.R. Functional disparity of three pheromone-binding proteins to different sex pheromone components in Hyphantria cunea (Drury). J. Agric. Food Chem. 2020, 69, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Luo, F.; Zhang, X.; Jiang, Y.; Lou, Z.; Chen, Z. Dissipation, transfer and safety evaluation of emamectin benzoate in tea. Food Chem. 2016, 202, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, P.; Zhang, F.; Li, Y.; Du, F.; Pan, C. Dissipation and residue behavior of emamectin benzoate on apple and cabbage field application. Ecotoxicol. Environ. Saf. 2012, 78, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Chen, L.; Guan, S.; Liu, J.; Liang, J.; Li, X.; Li, Z. Dissipation of emamectin benzoate residues in rice and rice-growing environments. Molecules 2020, 25, 483. [Google Scholar] [CrossRef]
- Yusoff, N.; Abd Ghani, I.; Othman, N.W.; Aizat, W.M.; Hassan, M. Toxicity and sublethal effect of farnesyl acetate on diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae). Insects 2021, 12, 109. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2022/1343; Official Journal of the European Union; European Union: Brussels, Belgium, 2022.
- EFSA; Anastassiadou, M.; Brancato, A.; Carrasco Cabrera, L.; Ferreira, L.; Greco, L.; Jarrah, S.; Kazocina, A.; Leuschner, R.; Magrans, J.O. Review of the existing maximum residue levels for emamectin according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2019, 17, e05803. [Google Scholar]
- EFSA; Bellisai, G.; Bernasconi, G.; Brancato, A.; Carrasco Cabrera, L.; Ferreira, L.; Giner, G.; Greco, L.; Jarrah, S.; Kazocina, A. Modification of the existing maximum residue levels for emamectin in various crops. EFSA J. 2021, 19, e06824. [Google Scholar]
- Yang, T.; Doherty, J.; Zhao, B.; Kinchla, A.J.; Clark, J.M.; He, L. Effectiveness of commercial and homemade washing agents in removing pesticide residues on and in apples. J. Agric. Food Chem. 2017, 65, 9744–9752. [Google Scholar] [CrossRef] [PubMed]
- Juraske, R.; Fantke, P.; Ramírez, A.C.R.; González, A. Pesticide residue dynamics in passion fruits: Comparing field trial and modelling results. Chemosphere 2012, 89, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Calvaruso, E.; Cammilleri, G.; Pulvirenti, A.; Lo Dico, G.M.; Lo Cascio, G.; Giaccone, V.; Vitale Badaco, V.; Ciprì, V.; Alessandra, M.M.; Vella, A. Residues of 165 pesticides in citrus fruits using LC-MS/MS: A study of the pesticides distribution from the peel to the pulp. Nat. Prod. Res. 2020, 34, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Archer, L.; Kunwar, S.; Alferez, F.; Batuman, O.; Albrecht, U. Trunk injection of oxytetracycline for huanglongbing management in mature grapefruit and sweet orange trees. Phytopathology 2023, 113, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.C.; Salamanca-Jimenez, A.; Doane, T.A.; Horwath, W.R. Carbon dioxide level and form of soil nitrogen regulate assimilation of atmospheric ammonia in young trees. Sci. Rep. 2015, 5, 13141. [Google Scholar] [CrossRef]
- Ding, W.; Li, S.; He, P.; Huang, S. Contribution and fate of maize residue-15N and urea-15N as affected by N fertilization regime. PLoS ONE 2019, 14, e0210176. [Google Scholar] [CrossRef]
- de la Puente, L.; Pedro Ferrio, J.; Palacio, S. Disentangling water sources in a gypsum plant community. Gypsum crystallization water is a key source of water for shallow-rooted plants. Ann. Bot. 2022, 129, 87–100. [Google Scholar] [CrossRef]
- Wise, J.C.; VanWoerkom, A.H.; Acimovic, S.G.; Sundin, G.W.; Cregg, B.M.; Vandervoort, C. Trunk Injection: A Discriminating Delivering System for Horticulture Crop IPM. Entomol. Ornithol. Herpetol. 2014, 3, 1. [Google Scholar]

| Samples | Linear Equation | Determination Coefficients (R2) | Slope Ratio (Matrix/Methanol) |
|---|---|---|---|
| Acetonitrile | y = 1657.63x + 6765.39 | 0.9999 | - |
| Leaves | y = 744.74x + 1656.97 | 0.9991 | 0.4493 |
| Pericarps | y = 1311.91x + 130.39 | 0.9999 | 0.7914 |
| Kernels | y = 629.91x + 739.42 | 0.9996 | 0.3800 |
| Samples | Spiked Level (mg/kg) | Recovery (%) | Average Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Leaves | 0.005 | 84.9, 91.6, 88.9, 89.7, 93.7 | 89.8 | 3.65 |
| 0.05 | 95.2, 92.6, 95.4, 97.9, 83.5 | 92.9 | 5.98 | |
| 0.1 | 80.2, 102.5, 95.8, 110.6, 102.6 | 98.3 | 11.58 | |
| Pericarps | 0.001 | 82.6, 79.4, 93.7, 95.7, 108.3 | 91.94 | 12.51 |
| 0.01 | 88.3, 95.9, 109.1, 91.6, 94.2 | 95.82 | 8.31 | |
| 0.1 | 100.5, 100.1, 85.7, 78.2, 89.5 | 90.8 | 10.55 | |
| Kernels | 0.001 | 75.3, 99.7, 93.1, 77.6, 77.4 | 84.62 | 13.05 |
| 0.01 | 85.2, 77.7, 77.2, 73.4, 79.7 | 78.64 | 5.49 | |
| 0.1 | 80.6, 81.0, 84.2, 79.9, 72.4 | 79.62 | 5.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.; Zhou, X.; Li, Y.; Zhou, M.; Yang, X.; Zhang, S.; Wickham, J.D.; Zhang, Q.-H.; Zhang, L. Distribution, Residue Dynamics, and Insecticidal Efficacy of Trunk-Injected Emamectin Benzoate in Pecan Trees. Forests 2024, 15, 535. https://doi.org/10.3390/f15030535
Liang Z, Zhou X, Li Y, Zhou M, Yang X, Zhang S, Wickham JD, Zhang Q-H, Zhang L. Distribution, Residue Dynamics, and Insecticidal Efficacy of Trunk-Injected Emamectin Benzoate in Pecan Trees. Forests. 2024; 15(3):535. https://doi.org/10.3390/f15030535
Chicago/Turabian StyleLiang, Zhi, Xi Zhou, Yinlong Li, Min Zhou, Xutao Yang, Shengnan Zhang, Jacob D. Wickham, Qing-He Zhang, and Longwa Zhang. 2024. "Distribution, Residue Dynamics, and Insecticidal Efficacy of Trunk-Injected Emamectin Benzoate in Pecan Trees" Forests 15, no. 3: 535. https://doi.org/10.3390/f15030535
APA StyleLiang, Z., Zhou, X., Li, Y., Zhou, M., Yang, X., Zhang, S., Wickham, J. D., Zhang, Q.-H., & Zhang, L. (2024). Distribution, Residue Dynamics, and Insecticidal Efficacy of Trunk-Injected Emamectin Benzoate in Pecan Trees. Forests, 15(3), 535. https://doi.org/10.3390/f15030535







