Changing Relationship between Specific Leaf Area and Leaf Matter Dry Content of Moso Bamboo Phyllostachys pubescens syn. edulis (Poales: Poaceae) under the Stress of Pantana phyllostachysae (Lepidoptera: Lymantriidae)
Abstract
:1. Introduction
2. Materials and Methods
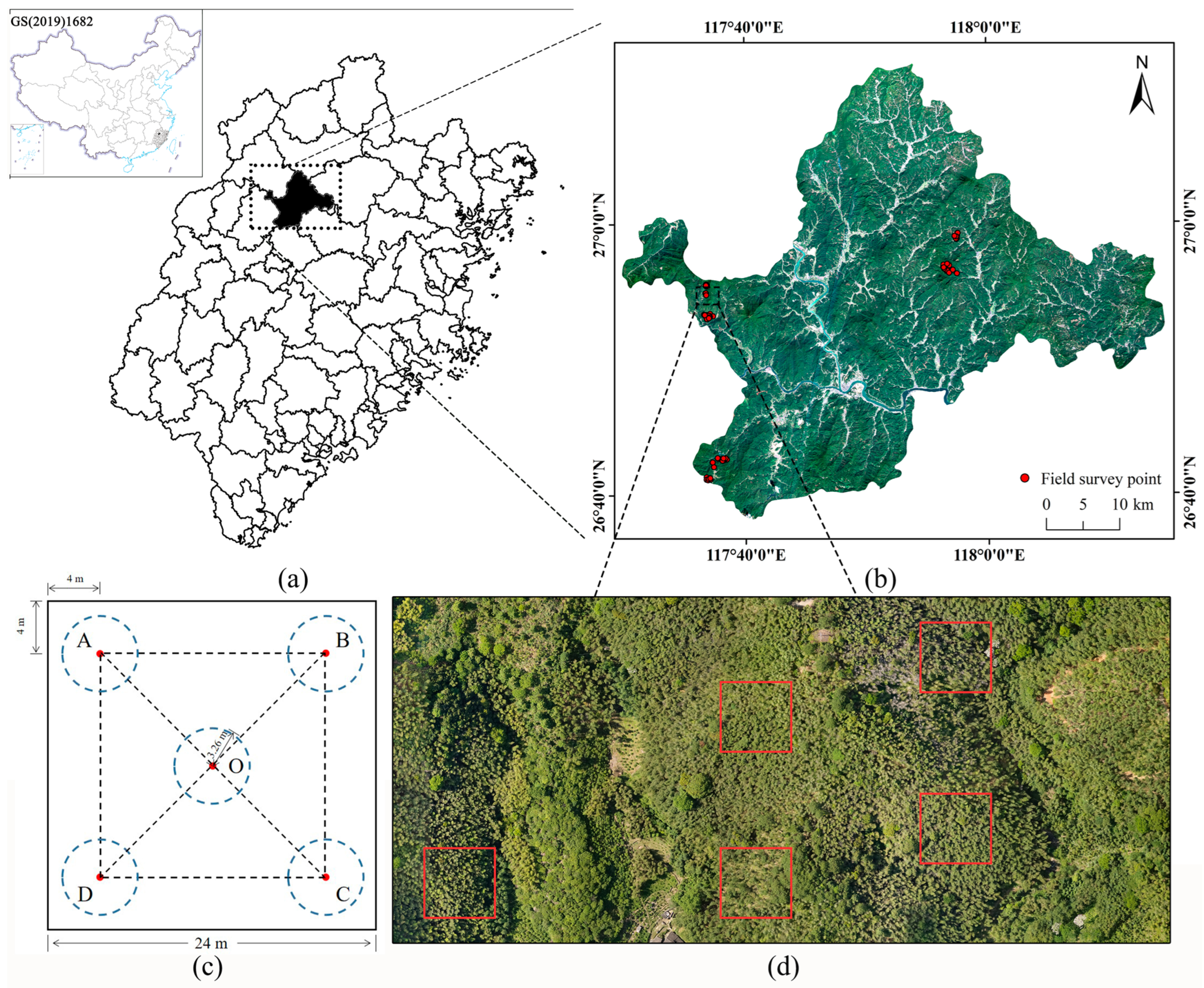
2.1. Field Research
2.2. Species Research
2.3. Measurement of Leaf Functional Traits
- (1)
- A sample of 835 leaves was selected from the sample plot, and each leaf was numbered.
- (2)
- Leaf area (LA) was combined with the YMJ-C leaf area meter to obtain an image of each leaf, supplemented by image processing software to carry out measurements [47].
- (3)
- Leaf fresh weight (m0) was measured on the spot during field collection. The measured leaves were then placed in a self-sealing bag, sealed, and stored in a liquid nitrogen tank to maintain freshness.
- (4)
- For leaf-saturated fresh weight (m1), after bringing the liquid nitrogen tank indoors, we retrieved the leaf samples, and placed them in distilled water for storage for approximately 24 h. Afterward, we removed them, quickly absorbed water from the surface of the leaves with filter paper, and weighed them on an electronic balance accurate to one part per million to obtain the saturated fresh weight (m1).
- (5)
- After obtaining the saturated fresh weight of a leaf, we dried it in an oven (heating it at 105 °C for 30 min and then continuing to dry it at 80 °C until a constant weight was reached). We weighed the dried leaf to obtain the dried weight (m2).
- (6)
- Specific leaf area (SLA) is defined as the ratio of leaf area to leaf dry weight, and the results are usually expressed in cm2/g and calculated using Equation (1):
- (7)
- Leaf dry matter content (LDMC), defined as the ratio of leaf dry weight to leaf saturated fresh weight, was calculated using Equation (2):
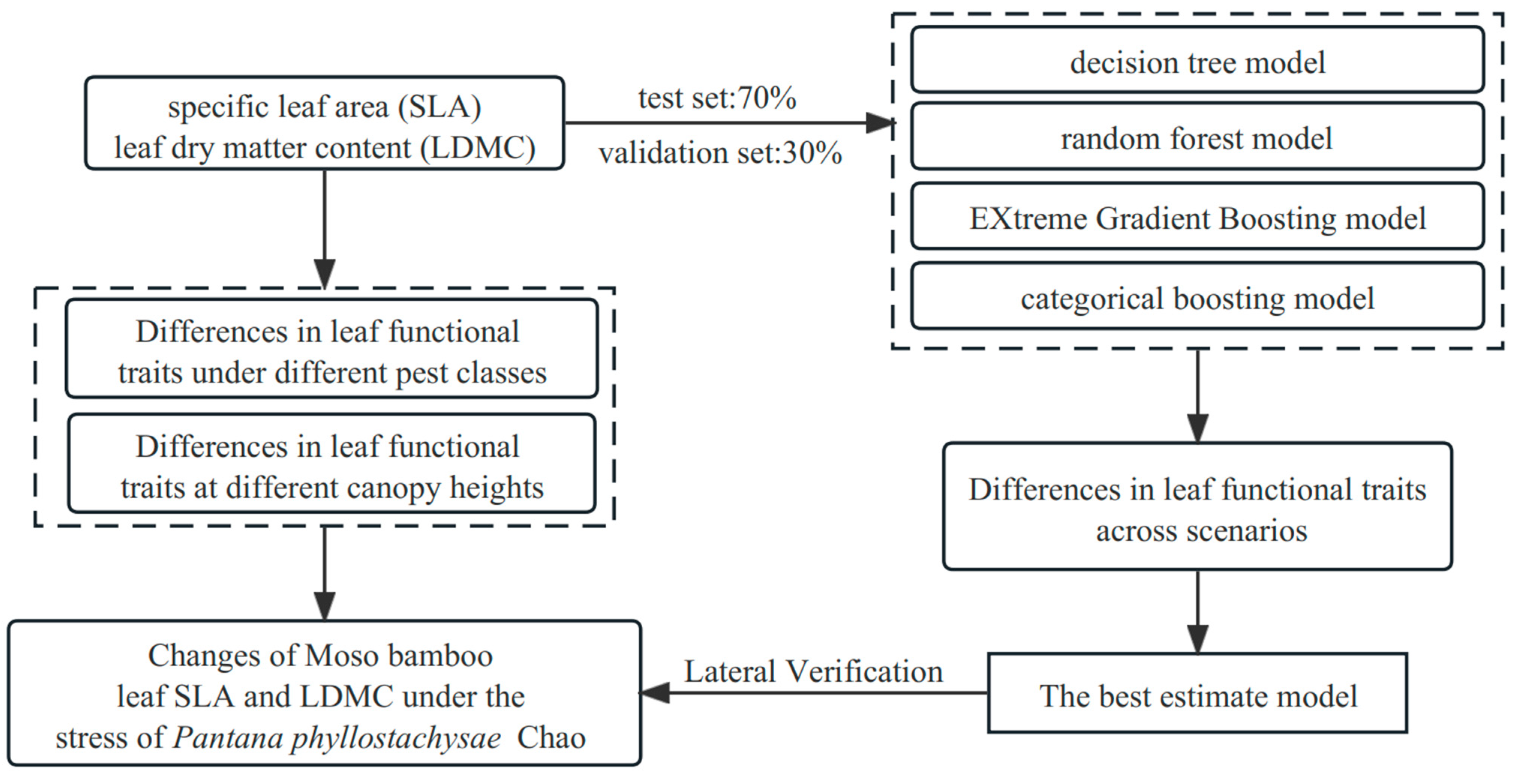
2.4. Relationship Modeling and Analysis
3. Results and Analysis
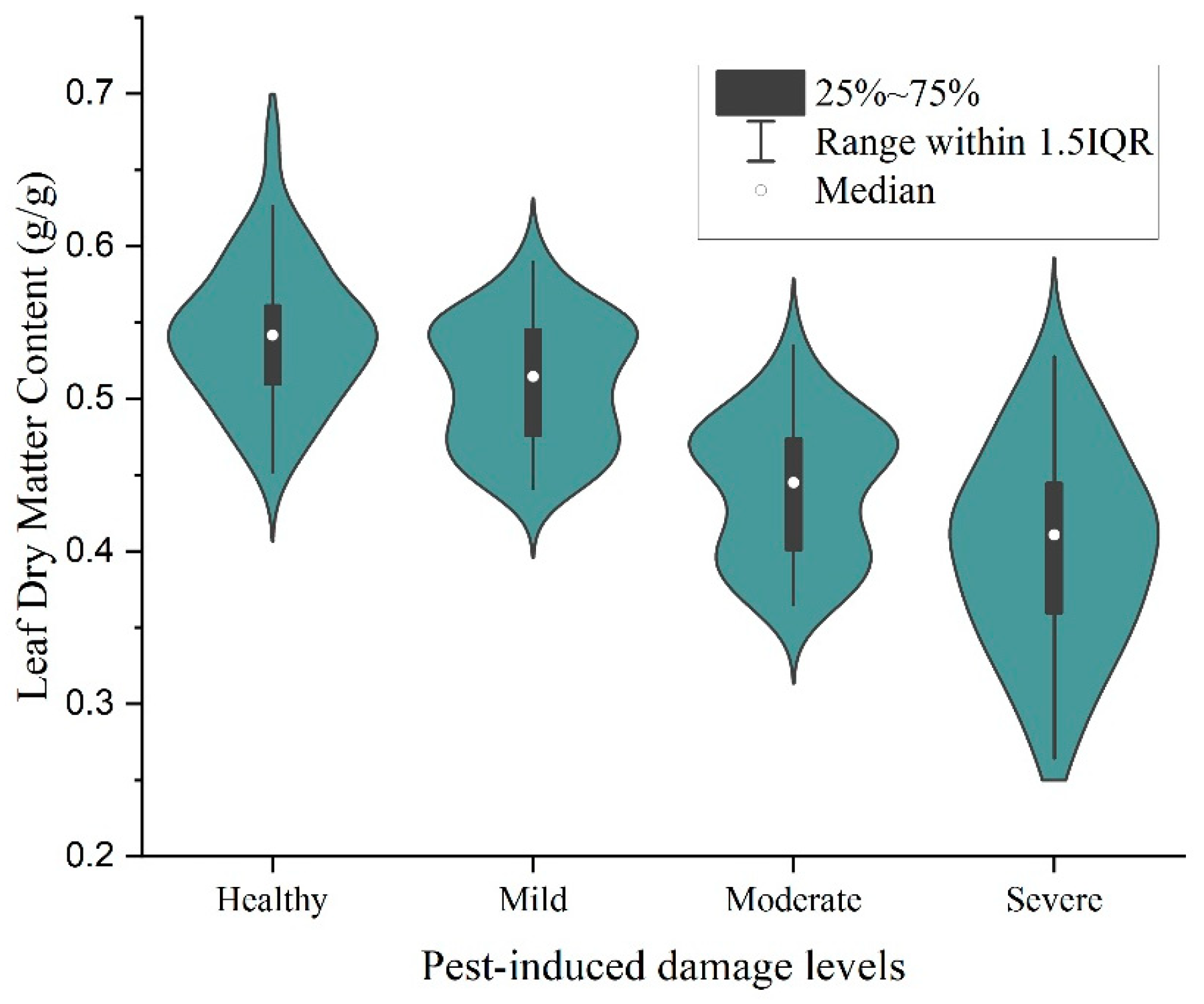
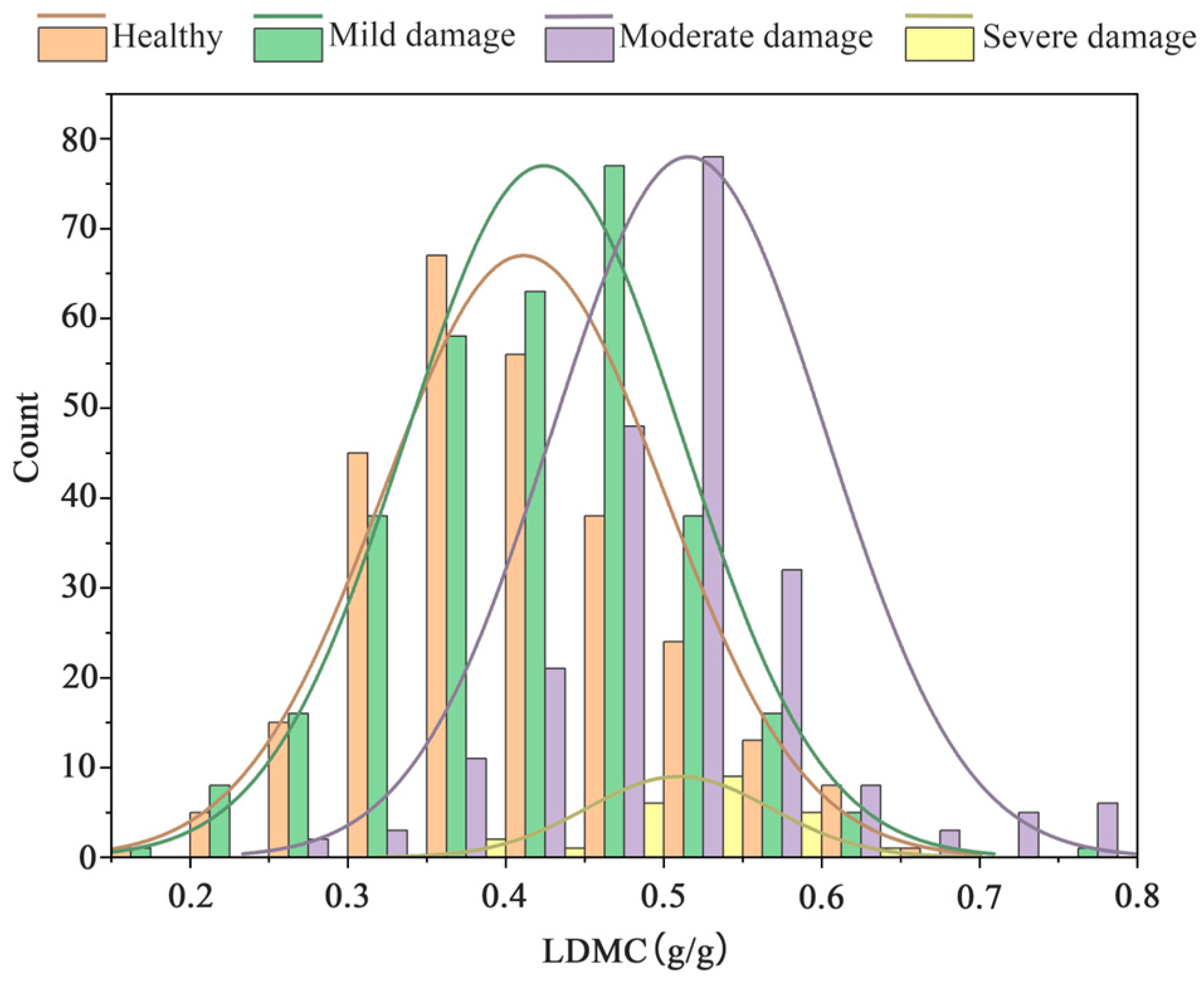
3.1. Descriptive Statistical Analysis of Data under Different Pest Levels of Stress
3.2. Analysis of the Relationship between SLA and LDMC of Ph. pubescens Leaves under Different Insect Pest Levels of Stress
3.3. Analysis of the Relationship between SLA and LDMC of Ph. pubescens Leaves at Different Canopy Heights
3.4. Modeling and Analysis of the Changing Patterns of SLA and LDMC in Ph. pubescens Leaves under Pest Stress
- (1)
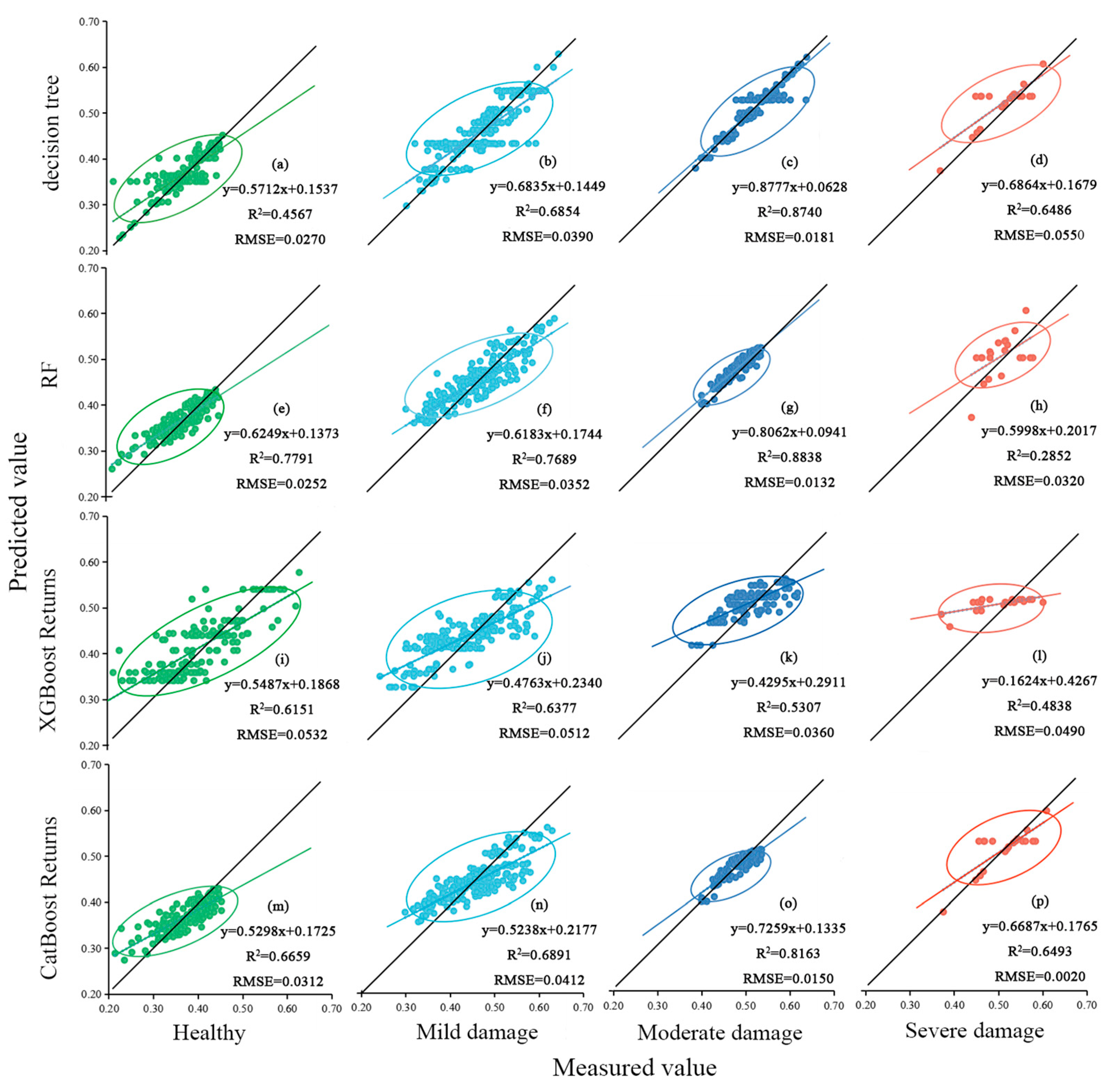
- Analyzing the models’ R2 values, the RF regression model is better than the other models, and the R2 values for the RF regression model vary across different hazard levels: 0.696 for the full sample, 0.755 for healthy leaves, 0.740 for leaves with mild infestation, 0.879 for leaves with moderate infestation, and 0.717 for leaves with severe infestation.
- (2)
- Upon scrutinizing the RMSE of the models, the RF regression model exhibited a commendable level of stability. The RMSE values for different hazard levels were consistently low, standing at 0.044 for the full sample, 0.025 for healthy leaves, 0.035 for leaves with mild infestation, 0.013 for leaves with moderate infestation, and 0.032 for severe infestation samples.
3.4.1. Changes in SLA and LDMC under Different Pest Level Stresses
3.4.2. Changes in SLA and LDMC under Different Canopy Heights
4. Discussion
4.1. Factors Influencing Differences in Leaf Functional Traits
4.2. Indications of Changes in Leaf Functional Traits on Resource Utilization in the Canopy Vertical Space
4.3. Analysis of the Relationship between Physiological Metabolic Processes and Leaf Functional Traits
5. Conclusions
- (1)
- Pure forests of Ph. pubescens have a high leaf density, and the canopy as a whole has a high sense of vertical hierarchy and a high degree of coverage. The understory of mixed forests of Ph. pubescens is rich in vegetation, and usually consists of horsetail pine, Pinus massoniana Lamb. (Pinales: Pinaceae), long-stalked mizuna, Brassica rapa subsp. nipposinica (L. H. Bailey) Hanelt (Capparales: Brassicaceae), and deciduous trees mixed with Ph. pubescens to form various types of mixed forests, in which Ph. pubescens generally resides in the second layer, and taller species such as pines reside in the first layer. Under the stress of the P. phyllostachysae, the SLA exhibited a notable downward trend across various pest levels as canopy height increased. This trend was primarily driven by light conditions. Conversely, the LDMC in Ph. pubescens displayed an initial increase followed by a decrease with canopy height, showcasing the plant’s adaptive responses to the light environment and its strategic use of water resources. Leaves situated at the upper canopy faced the imminent risk of substantial water loss in a high-radiant-energy environment. In response, plant leaves employed mechanisms such as increasing cuticle thickness and closing stomata to mitigate further water loss.
- (2)
- The analysis of Ph. pubescens leaf SLA and LDMC revealed a significant negative correlation under different levels of P. phyllostachysae stress, but the correlation was not significant under heavy damage. The leaf area in a healthy state was larger than that when infested by a pest; the higher the infestation level, the more likely the leaf area was defoliated, while leaf density was higher and leaf saturation fresh weight was higher. There was a general trend of decreasing LDMC with increasing leaf SLA at different canopy heights, but the range of values was not consistent. Analyzing the accuracy of four models in the paper for investigating the leaves of Ph. pubescens forests under different levels of the P. phyllostachysae stress and different canopy heights, the results showed that the CatBoost model was suitable for heavy damage monitoring from the point of view of different infestation levels, and RF was used in the other cases, whereas the decision tree model was the best one to be used for predicting different canopy heights.
- (3)
- The SLA and LDMC play pivotal roles in reflecting the growth rate of plants and their resource utilization efficiency. In the case of Ph. pubescens leaves, SLA exhibited a decreasing trend with an escalating infestation level from the poisonous moth, establishing a negative correlation with the degree of infestation. Conversely, LDMC displayed an increasing trend, highlighting that leaves in a healthy state possessed a higher SLA and lower LDMC. The potential existence of chance values in LDMC is attributed to the inconsistently decreasing pattern of leaf area under varying pest levels, suggesting that the leaf area in the healthy state might be relatively small. The impact of insect pest stress on leaf water content in Ph. pubescens forests becomes more pronounced with a higher degree of infestation. Spatial and temporal variability in insect damage to Ph. pubescens leaves across different canopy levels, seasons, or geographic locations may contribute to a more comprehensive understanding of the intrinsic mechanisms operating in Ph. pubescens forests under the influence of P. phyllostachysae. Plants “talk” to each other by means of chemical or physical signals, and when they are infested by pests, the amount of biochemical components and the relationship between them will change, which is on the one hand a physiological change to resist the attack, and on the other hand a warning signal to the plants in the same community, especially to the plants of the same species, of the existence of a potential danger. In this study, this subtle “dialogue” was detected by remote sensing, which is of great significance to protect the health of bamboo forests. This dimension adds depth to this research perspective and opens avenues for future investigations.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, H.Q.; Mao, F.J.; Li, X.J.; Zhou, G.M.; Xu, X.J.; Han, N.; Sun, S.B.; Gao, G.L.; Cui, L.; Li, Y.G.; et al. Mapping global bamboo forest distribution using multisource remote sensing data. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2018, 11, 1458–1471. [Google Scholar] [CrossRef]
- Cai, C.J.; Yang, Z.H.; Liu, L.; Lai, Y.S.; Lei, J.J.; Fan, S.H.; Tang, X.L. Consistent Effects of Canopy vs. Understory Nitrogen Addition on Soil Respiration and Net Ecosystem Production in Moso Bamboo Forests. Forests 2021, 12, 1427. [Google Scholar] [CrossRef]
- Yen, T.M.; Lee, J.S. Comparing aboveground carbon sequestration between Moso bamboo (Phyllostachys heterocycla) and China fir (Cunninghamia lanceolata) forests based on the allometric model. For. Ecol. Manag. 2011, 261, 995–1002. [Google Scholar] [CrossRef]
- Ouyang, M.; Yang, C.; Tian, D.; Pan, J.; Chen, G.P.; Su, H.J.; Yan, Z.B.; Ji, C.J.; Tang, Z.Y.; Fang, J.Y. A field-based estimation of moso bamboo forest biomass in China. For. Ecol. Manag. 2022, 505, 119885. [Google Scholar] [CrossRef]
- Seidl, R.; Klonner, G.; Rammer, W.; Essl, F.; Moreno, A.; Neumann, M.; Dullinger, S. Invasive alien pests threaten the carbon stored in Europe’s forests. Nat. Commun. 2018, 9, 1626. [Google Scholar] [CrossRef]
- Baxter, I.R.; Vitek, O.; Lahner, B.; Muthukumar, B.; Borghi, M.; Morrissey, J.; Guerinot, M.L.; Salt, D.E. The leaf ionome as a multivariable system to detect a plant’s physiological status. Proc. Natl. Acad. Sci. USA 2008, 105, 12081–12086. [Google Scholar] [CrossRef] [PubMed]
- Roa-Fuentes, L.L.; Templer, P.H.; Campo, J. Effects of precipitation regime and soil nitrogen on leaf traits in seasonally dry tropical forests of the Yucatan Peninsula, Mexico. Oecologia 2015, 179, 585–597. [Google Scholar] [CrossRef]
- Münchinger, I.K.; Hajek, P.; Akdogan, B.; Caicoya, A.T.; Kunert, N. Leaf thermal tolerance and sensitivity of temperate tree species are correlated with leaf physiological and functional drought resistance traits. J. For. Res. 2023, 34, 63–76. [Google Scholar] [CrossRef]
- Ochoa-Beltrán, A.; Martínez-Villa, J.A.; Kennedy, P.G.; Salgado-Negret, B.; Duque, A. Plant Trait Assembly in Species-Rich Forests at Varying Elevations in the Northwest Andes of Colombia. Land 2021, 10, 1057. [Google Scholar] [CrossRef]
- Long, Q.Z.; Du, H.; Su, L.; Zeng, F.D.; Lian, Z.W.; Peng, W.X.; Liu, K.P.; Tan, W.N. Variation of plant functional traits and adaptive strategies in Karst evergreen deciduous broad-leaved forest. Acta Ecol. Sin. 2023, 43, 8875–8883. [Google Scholar]
- Ajilogba, C.F.; Walker, S. Using crop modeling to find solutions for wheat diseases: A review. Front. Environ. Sci. 2023, 10, 987765. [Google Scholar] [CrossRef]
- Haris, M.; Huaasin, T.; Mohamed, H.I.; Khan, A.; Ansari, M.S.; Tauseef, A.; Khan, A.A.; Akhtar, N. Nanotechnology-A new frontier of nano-farming in agricultural and food production and its development. Sci. Total Environ. 2023, 857, 159639. [Google Scholar] [CrossRef] [PubMed]
- Chianucci, F.; Ferrara, C.; Pollastrini, M.; Corona, P. Development of digital photographic approaches to assess leaf traits in broadleaf tree species. Ecol. Indic. 2019, 106, 105547. [Google Scholar] [CrossRef]
- Zhu, X.X.; Wen, Z.M.; Zheng, C.; Gao, Y.; Zhang, G.Y.; Wang, W.W.; Diao, X.C.; Wan, J.F. Functional traits of plant life forms and their responses to environmental factors in the Yanhe river basin. Res. Soil Water Conserv. 2023, 30, 328–336. [Google Scholar]
- Yang, J.Y.; Wang, Z.J. Exploring urban remnant forest soil bacterial diversity responses to woody plant leaf functional traits. Plant Soil 2023, 494, 463–475. [Google Scholar] [CrossRef]
- Begum, G.; Gao, J.; Xue, W.; Yu, F.H. Plant-soil feedbacks in Hydrocotyle vulgaris: Genotypic differences and relations to functional traits. Ecol. Indic. 2023, 146, 109766. [Google Scholar] [CrossRef]
- Orzech, K.; Wanic, M.; Copeland, L. The Effects of Soil Compaction and Different Tillage Systems on the Bulk Density and Moisture Content of Soil and the Yields of Winter Oilseed Rape and Cereals. Agriculture 2021, 11, 666. [Google Scholar] [CrossRef]
- Tasset, E.; Boulanger, T.; Diquelou, S.; Laine, P.; Lemauviel-Lavenant, S. Plant trait to fodder quality relationships at both species and community levels in wet grasslands. Ecol. Indic. 2019, 97, 389–397. [Google Scholar] [CrossRef]
- Garrish, V.; Cernusak, L.A.; Winter, K.; Turner, B.L. Nitrogen to phosphorus ratio of plant biomass versus soil solution in a tropical pioneer tree, Ficus insipida. J. Exp. Bot. 2010, 61, 3735. [Google Scholar] [CrossRef]
- Siegwolf, R.; Lehmann, M.; Goldsmith, G.; Sidorova, O.C.; Mirande-Ney, C.; Timoveeva, G.; Weight, R.B.; Saurer, M. Updating the dual C and O isotope-Gas-exchange model: A concept to understand plant responses to the environment and its implications for tree rings. Plant Cell Environ. 2023, 46, 2606–2627. [Google Scholar] [CrossRef] [PubMed]
- Kumordzi, B.B.; Bello, F.D.; Freschet, G.T.; Bagousse-Pinguet, Y.L.; Lep, J.; Wardle, D.A. Linkage of plant trait space to successional age and species richness in boreal forest understorey vegetation. J. Ecol. 2015, 103, 1610–1620. [Google Scholar] [CrossRef]
- Canham, C.A.; Woods, C.; Setterfield, S.A.; Veneklaas, E.J.; Freestone, F.L.; Beesley, L.S.; Douglas, M.M. Functional traits of riparian trees in the lower Fitzroy River, Western Australia. Ecohydrology 2023, 16, e2488. [Google Scholar] [CrossRef]
- Carvalho, C.E.; Sfair, J.C.; Eller, C.B.; Menezes, B.S.; Menezes, M.O.T.; Araujo, F.S. Tree height, leaf thickness and seed size drive Caatinga plants’ sensitivity to climate change. J. Biogeogr. 2023, 50, 2057–2068. [Google Scholar] [CrossRef]
- Alvi, A.F.; Iqbal, N.; Albaqami, M.; Khan, N.A. The emerging key role of reactive sulfur species in abiotic stress tolerance in plants. Physiol. Plant. 2023, 175, e13945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, W.; Huang, S.; Xu, P.; Cao, Z.; Chen, M.; Lin, X. The potential role of plasma membrane proteins in response to Zn stress in rice roots based on iTRAQ and PRM under low Cd condition. J. Hazard. Mater. 2022, 429, 128324. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.R.; Sarvestani, R.; Mohammadi, B.; Baghery, A. Drought stress-induced changes at physiological and biochemical levels in some common vetch (Vicia sativa L.) genotypes. J. Agric. Sci. Technol. 2014, 16, 505–516. [Google Scholar]
- Monarca, S.; Feretti, D.; Zerbini, I.; Alberti, A.; Zani, C.; Resola, S.; Gelatti, U.; Nardi, G. Soil Contamination Detected Using Bacterial and Plant Mutagenicity Tests and Chemical Analyses. Environ. Res. 2002, 88, 64–69. [Google Scholar] [CrossRef]
- Liz, F.A.; Adam, K.; Jan, A.; Peter, N.M.; Alan, C.; Fritz, S.; Jii, D. Comparative anatomy of leaf petioles in temperate trees and shrubs: The role of plant size, environment and phylogeny. Ann. Bot. 2022, 129, 567–582. [Google Scholar]
- Koch, G.W.; Sillett, S.C.; Jennings, G.M.; Davis, S.D. The limits to tree height. Nature 2004, 428, 851–854. [Google Scholar] [CrossRef]
- Wilson, P.J.; Thompson, K.; Hodgson, J.G. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol. 2010, 143, 155–162. [Google Scholar] [CrossRef]
- Wangchuk, K.; Darabant, A.; Wangdi, J.; Nirola, H.; Wangdi, J.; Gratzer, G. Climate warming decreases plant diversity but increases community biomass in high-altitude grasslands. Rangel. Ecol. Manag. 2021, 75, 51–57. [Google Scholar] [CrossRef]
- Hu, Y.T.; Sun, Z.H.; Zeng, Y.L.; Ouyang, S.; Chen, L.; Lei, P.F.; Deng, X.W.; Zhao, Z.H.; Fang, X.; Xiang, W.H. Tree-level stomatal regulation is more closely related to xylem hydraulic traits than to leaf photosynthetic traits across diverse tree species. Agric. For. Meteorol. 2023, 329, 109291. [Google Scholar] [CrossRef]
- Cui, E.Q.; Weng, E.S.; Yan, E.R.; Xia, J.Y. Robust leaf trait relationships across species under global environmental changes. Nat. Commun. 2020, 11, 2999. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, X.F.; Yang, X.J. Changes of aquatic plants in Donghu Lake of Wuhan based 1990–2020 Landsat images. Chin. J. Plant Ecol. 2022, 46, 1551–1561. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Cai, X.B.; Song, X.X.; Suo, J.R.; Wang, Z.; Li, E.H.; Wang, X.L. Remote sensing information extraction of aquatic plants in Honghu Lake based on decision tree. Wetl. Sci. 2018, 16, 213–222. [Google Scholar]
- Ghosh, A.; Joshi, P.K. A comparison of selected classification algorithms for mapping bamboo patches in lower Gangetic plains using very high resolution WorldView 2 imagery. Int. J. Appl. Earth Obs. Geoinf. 2014, 26, 298–311. [Google Scholar] [CrossRef]
- Wang, Q.; Chang, Q.R.; Li, K.; Chen, X.K.; Miu, H.L.; Shi, B.T.; Zeng, X.L.; Li, Z.F. Estimation of winter wheat canopy chlorophyll content based on principal component analysis and random forest regression. J. Triticeae Crops 2024, 43, 1–11. [Google Scholar]
- Yoon, H.I.; Lee, H.; Yang, J.S.; Choi, J.H.; Jung, D.H.; Park, Y.J.; Park, J.E.; Kim, S.M.; Park, S.H. Predicting models for plant metabolites based on PLSR, AdaBoost, XGBoost, and LightGBM algorithms using hyperspectral imaging of brassica juncea. Agriculture 2023, 13, 1477. [Google Scholar] [CrossRef]
- Wang, L.Q.; Wang, S.Q.; Sun, D.Y.; Li, J.S.; Zhu, Y.X.; Xu, Y.J.; Zhang, H.L. XGBoost-Based inversion of phytoplankton pigment concentrations from field measured fluorescence excitation spectra. Acta Opt. Sin. 2022, 42, 215–224. [Google Scholar]
- Zhou, G.L.; Ni, Z.Y.; Zhao, Y.B.; Luan, J.W. Identification of bamboo species based on extreme gradient boosting (XGBoost) using Zhuhai-1 orbita hyperspectral remote sensing imagery. Sensors 2022, 22, 5434. [Google Scholar] [CrossRef]
- Xu, Z.H.; Zhang, Q.; Xiang, S.Y.; Li, Y.F.; Huang, X.Y.; Zhang, Y.W.; Zhou, X.; Li, Z.L.; Yao, X.; Li, Q.S.; et al. Monitoring the severity of Pantana phyllostachysae Chao infestation in Moso bamboo forests based on UAV multi-spectral remote sensing feature selection. Forests 2022, 13, 418. [Google Scholar] [CrossRef]
- Sun, M.L.; Yu, K.Y.; Zhang, X.P.; Zhao, G.J.; Chen, Y.C.; Chen, X.Y.; Huang, X.; Liu, J. Research on estimation of single wood accumulation of fir based on airborne Lidar point cloud data and Catboost algorithm. J. Southwest For. Univ. (Nat. Sci.) 2024, 44, 1–9. [Google Scholar]
- Xu, Z.H.; Zhang, Y.W.; Li, Z.L.; Xiang, S.Y.; Zhang, Q.; Li, Y.F.; Zhou, X.; Yu, H.; Shen, W.L. Improved “slice-based class-oriented” remote sensing algorithm for bamboo forest information extraction and its application. Remote Sens. Technol. Appl. 2023, 38, 393–404. [Google Scholar]
- Xu, Z.H.; He, A.Q.; Zhang, Y.W.; Hao, Z.B.; Li, Y.F.; Xiang, S.Y.; Li, B.; Chen, L.Y.; Yu, H.; Shen, W.L.; et al. Retrieving chlorophyll content and equivalent water thickness of Moso bamboo (Phyllostachys pubescens) forests under Pantana phyllostachysae Chao-induced stress from Sentinel-2A/B images in a multiple LUTs-based PROSAIL framework. For. Ecosyst. 2023, 10, 100–108. [Google Scholar] [CrossRef]
- Huang, X.; Xu, Z.; Yang, X.; Shi, J.; Hu, X.; Ju, W. Monitoring the severity of Pantana phyllostachysae Chao on bamboo using leaf hyperspectral data. Remote Sens. 2021, 13, 4146. [Google Scholar] [CrossRef]
- Chen, L.Y.; He, A.Q.; Xu, Z.H.; Li, B.; Zhang, H.F.; Li, G.T.; Guo, X.Y.; Li, Z.L. Mapping aboveground biomass of Moso bamboo (Phyllostachys pubescens) forests under Pantana phyllostachysae Chao-induced stress using Sentinel-2 imagery. Ecol. Indic. 2024, 158, 111564. [Google Scholar] [CrossRef]
- Xu, Z.H.; Sun, L.; Li, B. Design of leaf area measurement system based on Android smartphone. Lab. Technol. Manag. 2023, 40, 89–94. [Google Scholar]
- Samuel, A.L. Some studies in machine learning using the game of checkers. IBM J. Res. Dev. 2000, 44, 206–226. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–23. [Google Scholar] [CrossRef]
- Tianqi, C.; Guestrin, C.E. XGBoost: A scalable tree boosting system. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Prokhorenkova, L.; Gusev, G.; Vorobev, A.; Dorogush, A.V.; Gulin, A. CatBoost: Unbiased boosting with categorical features. arXiv 2019, arXiv:1706.09516. [Google Scholar]
- Edna, E.; Robyn, H.L.; Alethea, A.; Timothy, C. Technology assessment and resource allocation for predictive genetic testing: A study of the perspectives of Canadian genetic health care providers. BMC Med. Ethics 2009, 10, 6. [Google Scholar]
- Griffin-Nolan, R.; Sandel, B. Global intraspecific trait–climate relationships for grasses are linked to a species’ typical form and function. Ecography 2023, 2023, e06586. [Google Scholar] [CrossRef]
- Gao, J.; Wang, J.F.; Wang, K.Q.; Li, Y.H.; Zhang, X. Patterns and drivers of community specific leaf area in China—ScienceDirect. Glob. Ecol. Conserv. 2023, 33, e02331. [Google Scholar]
- Day, M.E.; Greenwood, M.S.; White, A.S. Age-related changes in foliar morphology and physiology in red spruce and their influence on declining photosynthetic rates and productivity with tree age. Tree Physiol. 2001, 21, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Vendramini, F.; Díaz, S.; Gurvich, D.E.; Wilson, P.J.; Thompson, K.; Hodgson, J.G. Leaf traits as indicators of resource—Use strategy in floras with succulent species. New Phytol. 2002, 154, 147–157. [Google Scholar] [CrossRef]
- Ryan, M.G.; Yoder, B.J. Hydraulic limits to tree height and tree growth. Bioscience 1997, 47, 235–242. [Google Scholar] [CrossRef]
- Nykiel, M.; Gietler, M.; Fidler, J.; Graska, J.; RybarczykPłońska, A.; Prabucka, B.; Muszyńska, E.; Bocianowski, J.; Labudda, M. Differential water deficit in leaves is a principal factor modifying barley response to drought stress. Int. J. Mol. Sci. 2022, 23, 15240. [Google Scholar] [CrossRef]
- Miller, C.D.; Forthman, M.; Miller, C.W.; Kimball, R.T. Extracting ‘legacy loci’ from an invertebrate sequence capture data set. Zool. Scr. 2022, 51, 14–31. [Google Scholar] [CrossRef]
- Miller, C.W.; Kimball, R.T.; Michael, F. The evolution of multi-component weapons in the superfamily of leaf-footed bugs. Evolution 2024, 2024, qpae011. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Ali, A.M.; Darvishzadeh, R.; Skidmore, A.K.; van Duren, I.; Heiden, U.; Heurich, M. Estimating leaf functional traits by inversion of prospect: Assessing leaf dry matter content and specific leaf area in mixed mountainous forest. Int. J. Appl. Earth Obs. Geoinf. 2016, 45, 66–76. [Google Scholar] [CrossRef]
- Smart, S.M.; Glanville, H.C.; Blanes, M.D.C.; Mercado, L.M.; Emmett, B.A.; Jones, D.L.; Cosby, B.J.; Marrs, R.H.; Butler, A.; Marshall, M.R.; et al. Leaf dry matter content is better at predicting above-ground net primary production than specific leaf area. Funct. Ecol. 2017, 31, 1336–1344. [Google Scholar] [CrossRef]
- Baek, G.; Lim, H.; Noh, N.J.; Kim, C. No impact of nitrogen fertilization on carbon sequestration in a temperatePinus densifloraforest. Sci. Rep. 2023, 13, 1743. [Google Scholar] [CrossRef] [PubMed]




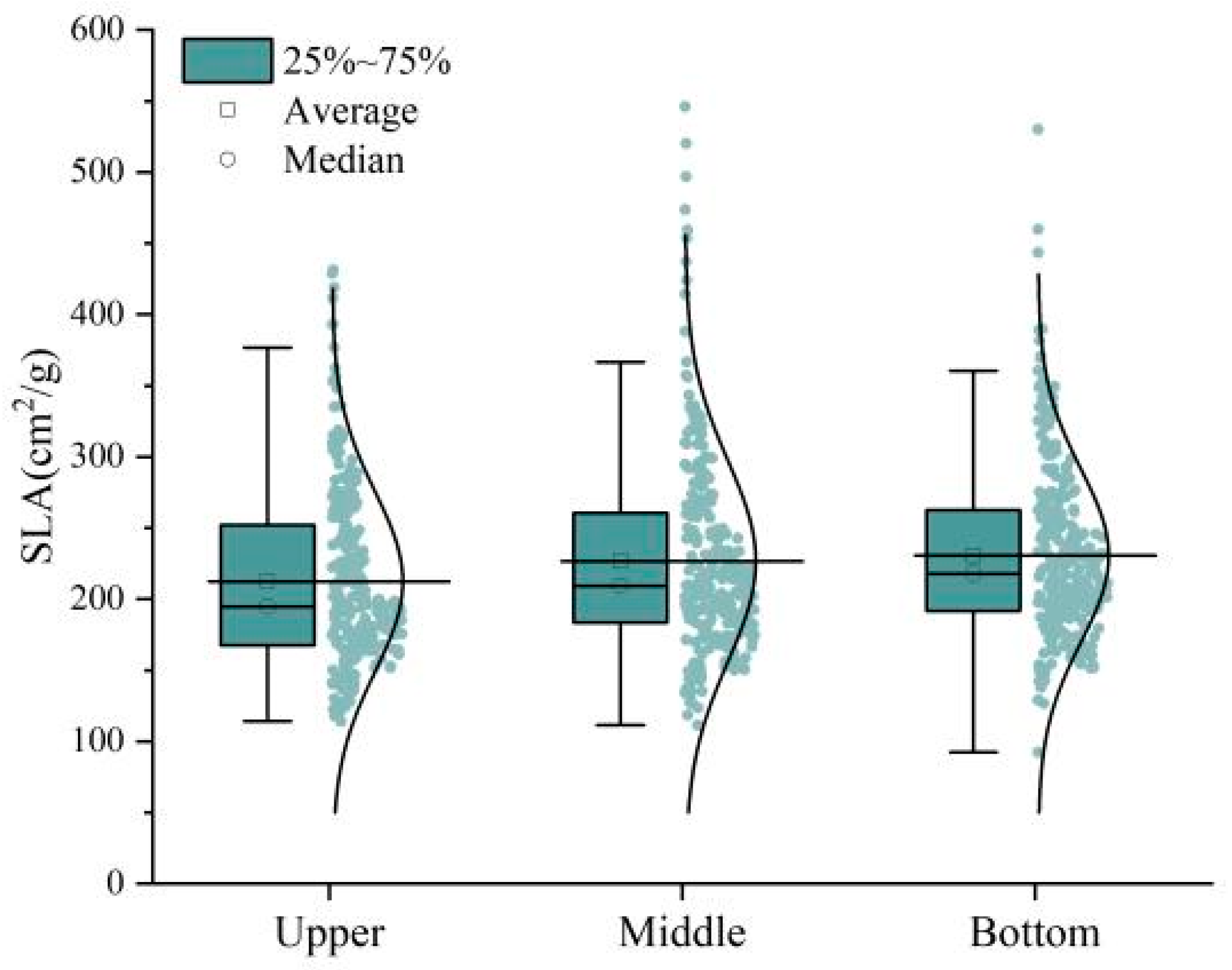



| Dry Matter Type | Pest Levels | Number of Leaves/Piece | Mean (cm2/g) | SD (cm2/g) | Min. (cm2/g) | Max. (cm2/g) | CV (%) | Skewness | Kurtosis |
|---|---|---|---|---|---|---|---|---|---|
| SLA | Full sample | 835 | 218.041 | 54.391 | 92.374 | 389.693 | 0.28 | 1.72 | 2.28 |
| Healthy | 267 | 230.644 | 52.197 | 126.721 | 389.693 | 0.23 | 0.53 | 0.23 | |
| Mild damage | 306 | 229.118 | 58.282 | 92.374 | 381.73 | 0.25 | 0.51 | 0.33 | |
| Middle damage | 188 | 190.646 | 34.261 | 126.553 | 300.68 | 0.18 | 0.76 | 0.62 | |
| Severe damage | 74 | 150.992 | 21.286 | 114.152 | 190.87 | 0.14 | 0.15 | 1.14 |
| Dry Matter Type | Pest Levels | Number of Leaves/Piece | Mean (g/g) | SD (g/g) | Min. (g/g) | Max. (g/g) | CV (%) | Skewness | Kurtosis |
|---|---|---|---|---|---|---|---|---|---|
| LDMC | Full sample | 835 | 0.451 | 0.089 | 0.198 | 0.643 | 0.19 | 0.14 | 0.56 |
| Healthy | 267 | 0.412 | 0.091 | 0.198 | 0.643 | 0.21 | 0.39 | 0.07 | |
| Mild damage | 306 | 0.434 | 0.084 | 0.243 | 0.629 | 0.18 | 0.05 | 0.56 | |
| Middle damage | 188 | 0.508 | 0.053 | 0.384 | 0.622 | 0.10 | 0.13 | 0.19 | |
| Severe damage | 74 | 0.512 | 0.066 | 0.373 | 0.612 | 0.12 | 0.61 | 0.02 |
| Evaluation Indicators | R | R2 | Adjusted R2 | Standard Deviation |
|---|---|---|---|---|
| Full sample | 0.728 a | 0.530 | 0.539 | 0.0670 |
| Upper | 0.718 a | 0.515 | 0.513 | 0.0707 |
| Middle | 0.750 a | 0.563 | 0.561 | 0.6497 |
| Lower | 0.719 a | 0.516 | 0.515 | 0.6517 |
| (R2, RMSE) | Decision Tree | RF | XGBoost | CatBoost |
|---|---|---|---|---|
| Full sample | (0.658, 0.047) | (0.704, 0.044) | (0.597, 0.053) | (0.588, 0.052) |
| Healthy | (0.457, 0.027) | (0.779, 0.025) | (0.615, 0.053) | (0.666, 0.031) |
| Mild damage | (0.685, 0.039) | (0.769, 0.035) | (0.638, 0.051) | (0.689, 0.041) |
| Moderate damage | (0.874, 0.018) | (0.884, 0.013) | (0.531, 0.036) | (0.816, 0.015) |
| Severe damage | (0.649, 0.055) | (0.285, 0.032) | (0.484, 0.049) | (0.649, 0.002) |
| (R2, RMSE) | Decision Tree | RF | XGBoost | CatBoost |
|---|---|---|---|---|
| Full sample | (0.658, 0.051) | (0.704, 0.048) | (0.597, 0.064) | (0.588, 0.058) |
| Upper | (0.913, 0.032) | (0.886, 0.038) | (0.687, 0.064) | (0.832, 0.046) |
| Middle | (0.917, 0.030) | (0.913, 0.032) | (0.623, 0.063) | (0.874, 0.038) |
| Bottom | (0.870, 0.038) | (0.885, 0.036) | (0.693, 0.046) | (0.823, 0.045) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, W.; Xu, Z.; Qin, N.; Chen, L.; Yang, Y.; Zhang, H.; Yu, X.; He, A.; Sun, L.; Li, X. Changing Relationship between Specific Leaf Area and Leaf Matter Dry Content of Moso Bamboo Phyllostachys pubescens syn. edulis (Poales: Poaceae) under the Stress of Pantana phyllostachysae (Lepidoptera: Lymantriidae). Forests 2024, 15, 540. https://doi.org/10.3390/f15030540
Shen W, Xu Z, Qin N, Chen L, Yang Y, Zhang H, Yu X, He A, Sun L, Li X. Changing Relationship between Specific Leaf Area and Leaf Matter Dry Content of Moso Bamboo Phyllostachys pubescens syn. edulis (Poales: Poaceae) under the Stress of Pantana phyllostachysae (Lepidoptera: Lymantriidae). Forests. 2024; 15(3):540. https://doi.org/10.3390/f15030540
Chicago/Turabian StyleShen, Wanling, Zhanghua Xu, Na Qin, Lingyan Chen, Yuanyao Yang, Huafeng Zhang, Xier Yu, Anqi He, Lei Sun, and Xia Li. 2024. "Changing Relationship between Specific Leaf Area and Leaf Matter Dry Content of Moso Bamboo Phyllostachys pubescens syn. edulis (Poales: Poaceae) under the Stress of Pantana phyllostachysae (Lepidoptera: Lymantriidae)" Forests 15, no. 3: 540. https://doi.org/10.3390/f15030540
APA StyleShen, W., Xu, Z., Qin, N., Chen, L., Yang, Y., Zhang, H., Yu, X., He, A., Sun, L., & Li, X. (2024). Changing Relationship between Specific Leaf Area and Leaf Matter Dry Content of Moso Bamboo Phyllostachys pubescens syn. edulis (Poales: Poaceae) under the Stress of Pantana phyllostachysae (Lepidoptera: Lymantriidae). Forests, 15(3), 540. https://doi.org/10.3390/f15030540







