Modelling Distribution of an Endangered Longhorn Beetle, Callipogon relictus (Coleoptera: Cerambycidae), in Northeast Asia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Distribution Point Collection
2.2. Climate Data Collection and Environmental Variables
2.3. Species Distribution Model
2.4. Change of Suitable Area under Different Climate Pathways
3. Results
3.1. Reliability Analysis of Models Established for C. relictus
3.2. Potential Suitable Areas for C. relictus under Future Climate Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Semenov, A.P. Callipogon (Eoxenus) relictus sp. n. new species of Cerambycidae in fauna of Russia. Russ. Entomol. J. 1899, 32, 562–580. [Google Scholar]
- Semenov, A.P. Another undescribed male Callipogon (Eoxenus) relictus Sem. (Coleoptera, Cerambycidae). Rus. Entomol. Obozr. 1903, 6, 372–373. [Google Scholar]
- Yi, D.A.; Kuprin, A.V.; Bae, Y.J. Distribution of the longhorned beetle Callipogon relictus (Coleoptera: Cerambycidae) in Northeast Asia. Zootaxa 2018, 4369, 101–108. [Google Scholar] [CrossRef]
- Kuprin, A.; Bezborodov, V. Areal of Callipogon relictus Semenov, 1899 (Coleoptera, Cerambycidae) in the Russian Far East. Biol. Bull. 2012, 39, 387–391. [Google Scholar] [CrossRef]
- Kuprin, A.V. The Longicorn Beetles (Insecta, Coleoptera: Cerambycoidae) of the Ussuri Nature Reserve and Adjacent Territories. Far East. Entomol. 2016, 309, 21–28. [Google Scholar]
- Yi, D.A.; Kuprin, A.; Bae, Y.J. Morphological anomalies of endangered Korean relict long-horned beetle Callipogon relictus (Cerambycidae, Coleoptera) during ontogenesis and possible causes of their occurrence. ZooKeys 2017, 714, 53–60. [Google Scholar] [CrossRef]
- Yi, D.A.; Kuprin, A.; Lee, Y.; Bae, Y.J. Newly developed fungal diet for artificial rearing of the endangered long-horned beetle Callipogon relictus (Coleoptera: Cerambycidae). Entomol. Res. 2017, 47, 373–379. [Google Scholar] [CrossRef]
- Yi, D.A.; Kuprin, A.; Bae, Y.J. Effects of temperature on instar number and larval development in the endangered longhorn beetle Callipogon relictus (Coleoptera: Cerambycidae) raised on an artificial diet. Can. Entomol. 2019, 151, 537–544. [Google Scholar] [CrossRef]
- Kang, J.H.; Yi, D.A.; Kuprin, A.V.; Han, C.; Bae, Y.J. Phylogeographic Investigation of an Endangered Longhorn Beetle, Callipogon relictus (Coleoptera: Cerambycidae), in Northeast Asia: Implications for Future Restoration in Korea. Insects 2021, 12, 555. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, C.; Kuprin, A.V.; Kang, J.H.; Lee, B.W.; Oh, S.H.; Lim, J. Survey research on the habitation and biological information of Callipogon relictus Semenov in Gwangneung forest, Korea and Ussurisky nature reserve, Russia (Coleoptera, Cerambycidae, Prioninae). ZooKeys 2018, 792, 45–68. [Google Scholar] [CrossRef]
- Yi, D.A.; Kuprin, A.; Bae, Y.J. First Record of Callipogon relictus Semenov, 1899 (Coleoptera: Cerambycidae: Prioninae) from Lazovsky Nature Reserve, Primorsky Region, Russia. Entomol. News 2017, 126, 421–423. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, C.; Choi, I.J.; Kuprin, A.V.; Lim, J. A review of host plants of Callipogon (Eoxenus) relictus Semenov (Coleoptera: Cerambycidae: Prioninae), a Korea natural monument, with a new host, Quercus aliena Blume. J. Asia Pac. Entomol. 2019, 22, 353–358. [Google Scholar] [CrossRef]
- Byun, B.K.; Kwon, T.S.; Weon, G.J.; Jo, D.G.; Lee, B.W.; Lee, Y.M.; Choi, H.J.; Kim, C.H.; Lee, S.H.; Bae, Y.S. Occurrence of Callipogon relictus Semenov (Coleoptera: Cerambycidae) in the Gwangneung Forest, Korea with suggestion for the conservation. Korean J. Appl. Entomol. 2007, 46, 19–25. [Google Scholar] [CrossRef]
- Kim, C.W.; Yoon, I.B.; Nam, S.H. On the habitats and habits of Callipogon relictus. (Coleoptera: Cerambycidae). Nat. Conserv. 1976, 11, 5–16. [Google Scholar]
- Li, J.; Drumont, A.; Xueping, G.; Wei, G. The checklist of Northeast China’s subfamily Prioninae and biological observations of Callipogon (Eoxenus) relictus Semenov-Tian-Shanskij 1899 (Coleoptera, Cerambycidae, Prioninae). Les Cah. Magellanes 2012, 9, 50–56. [Google Scholar]
- Phillips, S.; Dudík, M. Modeling of species distributions with MAXENT: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Thomaes, A.; Kervyn, T.; Maes, D. Applying species distribution modelling for the conservation of the threatened saproxylic Stag Beetle (Lucanus cervus). Biol. Conserv. 2008, 141, 1400–1410. [Google Scholar] [CrossRef]
- Campanaro, A.; Redolfi DeZan, L.; Hardersen, S.; Antonini, G.; Chiari, S.; Cini, A.; Mancini, E.; Mosconi, F.; Rossi de Gasperis, S.; Solano, E.; et al. Guidelines for the monitoring of Rosalia alpina. Nat. Conserv. 2017, 20, 165–203. [Google Scholar] [CrossRef]
- Bosso, L.; Rebelo, H.; Garonna, A.P.; Russo, D.; Bosso, L.; Rebelo, H.; Garonna, A.P.; Russo, D. Modelling geographic distribution and detecting conservation gaps in Italy for the threatened beetle Rosalia alpina. J. Nat. Conserv. 2013, 21, 72–80. [Google Scholar] [CrossRef]
- Maurizi, E.; Campanaro, A.; Maura, M.; Mosconi, F.; Simone, S.; Zauli, A.; Audisio, P.; Carpaneto, G.M. Guidelines for the monitoring of Osmoderma eremita and closely related species. Nat. Conserv. 2017, 20, 79–128. [Google Scholar] [CrossRef]
- Hardersen, S.; Bardiani, M.; Chiari, S.; Maura, M.; Maurizi, E.; Roversi, P.; Franco, M.; Bologna, M. Guidelines for the monitoring of Morimus asper funereus and Morimus asper asper. Nat. Conserv. 2017, 20, 205–236. [Google Scholar] [CrossRef]
- Storozhenko, S.Y.; Molodtsov, V.V.; Sergeev, M.G. The mysterious Amurian grig Paracyphoderris erebeus Storozhenko, 1980 (Orthoptera: Prophalangopsidae): New data on its distribution, ecology and biology. Insects 2023, 14, 789. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-W.; Jung, J.-M.; Nam, Y.; Jung, J.-K.; Jung, S.; Lee, W.-H. Spatial ensemble modeling for predicting the potential distribution of Lymantria dispar asiatica (Lepidoptera: Erebidae: Lymantriinae) in South Korea. Environ. Monit. Assess. 2022, 194, 889. [Google Scholar] [CrossRef]
- Lee, W.-H.; Song, J.-W.; Yoon, S.-H.; Jung, J.-M. Spatial evaluation of machine learning-based species distribution models for prediction of invasive ant species distribution. Appl. Sci. 2022, 12, 10260. [Google Scholar] [CrossRef]
- Sung, S.; Kwon, Y.-S.; Lee, D.K.; Cho, Y. Predicting the potential distribution of an invasive species, Solenopsis invicta Buren (Hymenoptera: Formicidae), under climate change using species distribution models. Entomol. Res. 2018, 48, 505–513. [Google Scholar] [CrossRef]
- Byeon, D.; Lee, J.-H.; Lee, H.-S.; Park, Y.; Jung, S.; Lee, W.-H. Prediction of spatiotemporal invasive risk by the red imported fire ant (Hymenoptera: Formicidae) in South Korea. Agronomy 2020, 10, 875. [Google Scholar] [CrossRef]
- Chen, S.; Ding, F.; Hao, M.; Jiang, D. Mapping the potential global distribution of red imported fire ant (Solenopsis invicta Buren) based on a machine learning method. Sustainability 2020, 12, 10182. [Google Scholar] [CrossRef]
- Zhang, Q.-C.; Wang, J.-G.; Lei, Y.-H. Predicting distribution of the Asian longhorned beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae) and its natural enemies in China. Insects 2022, 13, 687. [Google Scholar] [CrossRef] [PubMed]
- Kuprin, A.V.; Bezborodov, V.G.; Yi, D.A.; Kotlyar, A.K. Developmental biology and ecological peculiarities of the relict longhorn beetle Callipogon relictus Semenov, 1899 (Coleoptera, Cerambycidae). Entomol. Rev. 2014, 93, 1080–1085. [Google Scholar] [CrossRef]
- Bezborodov, V.G. Chorology and population structure of Callipogon relictus Semenov, 1899 (Coleoptera, Cerambycidae) in East Asia. Euroasian Entomol. J. 2016, 15, 393–398. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- WorldClim. Available online: https://worldclim.org/ (accessed on 21 December 2022).
- Séférian, R.; Nabat, P.; Michou, M.; Saint-Martin, D.; Voldoire, A.; Colin, J.; Decharme, B.; Delire, C.; Berthet, S.; Chevallier, M.; et al. Evaluation of CNRM Earth system model, CNRM-ESM2-1: Role of Earth system processes in present-day and future climate. J. Adv. Model. Earth Syst. 2019, 11, 4182–4227. [Google Scholar] [CrossRef]
- Fawcett, T. Introduction to ROC Analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Scheldeman, X.; Zonneveld, M. Training Manual on Spatial Analysis of Plant Diversity and Distribution; Biodiversity International: Rome, Italy, 2010; p. 179. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Morales, N.S.; Fernández, I.C.; Baca-González, V. MaxEnt’s parameter configuration and small samples: Are we paying attention to recommendations? A Systematic review. PeerJ 2017, 5, e3093. [Google Scholar] [CrossRef] [PubMed]
- Meinshausen, M.; Nicholls, Z.R.J.; Lewis, J.; Gidden, M.J.; Vogel, E.; Freund, M.; Beyerle, U.; Gessner, C.; Nauels, A.; Bauer, N.; et al. The shared socio-economic pathway (SSP) greenhouse gas concentrations and their extensions to 2500. Geosci. Model Dev. 2020, 13, 3571–3605. [Google Scholar] [CrossRef]
- The Intergovernmental Panel on Climate Change (IPCC). Available online: https://www.ipcc.ch/ (accessed on 2 December 2023).
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Ahmadzadeh, F.; Flecks, M.; Carretero, M.A.; Böhme, W.; Ilgaz, C.; Engler, J.O.; Harris, D.J.; Üzüm, N.; Rödder, D. Rapid lizard radiation lacking niche conservatism: Ecological diversification within a complex landscape. J. Biogeogr. 2013, 9, 1807–1818. [Google Scholar] [CrossRef]
- Byun, B.K. Research Report on a Natural Monument, Callipogon relictus Semenov in the Gwangneung Forest; Korea Nature Arboretum: Chuncheon, Republic of Korea, 2006; 46p. [Google Scholar]
- Bisi, F.; Wauters, L.A.; Preatoni, D.G.; Martinoli, A. Interspecific competition mediated by climate change: Which interaction between brown and mountain hare in the Alps? Mamm. Biol. 2015, 80, 424–430. [Google Scholar] [CrossRef]
- Van der Putten, W.H.; Macel, M.; Visser, M.E. Predicting species distribution and abundance responses to climate change: Why it is essential to include biotic interactions across trophic levels. Philos. Trans. R Soc. Lond B Biol. Sci. 2010, 365, 2025–2034. [Google Scholar] [CrossRef]
- Franić, I.; Allan, E.; Prospero, S.; Adamson, K.; Attorre, F.; Auger-Rozenberg, M.-A.; Augustin, S.; Avtzis, D.; Baert, W.; Barta, M.; et al. Climate, host and geography shape insect and fungal communities of trees. Sci. Rep. 2023, 13, 11570. [Google Scholar] [CrossRef] [PubMed]
- Keena, M.A.; Moore, P.M. Effects of temperature on Anoplophora glabripennis (Coleoptera: Cerambycidae) larvae and pupae. Environ. Entomol. 2010, 39, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Sheingauz, A.S. Developing Far East’s Forests and Using their Efficiency from Mid 19th to Mid 20th Century. Spat. Econ. 2008, 1, 118–146. [Google Scholar] [CrossRef]
- Manko, Y.I. Forestry chronicle in the Russian Far East during transition from capitalism to socialism (1917–1922). Vestn. FEB RAS 2014, 3, 95–110. [Google Scholar]

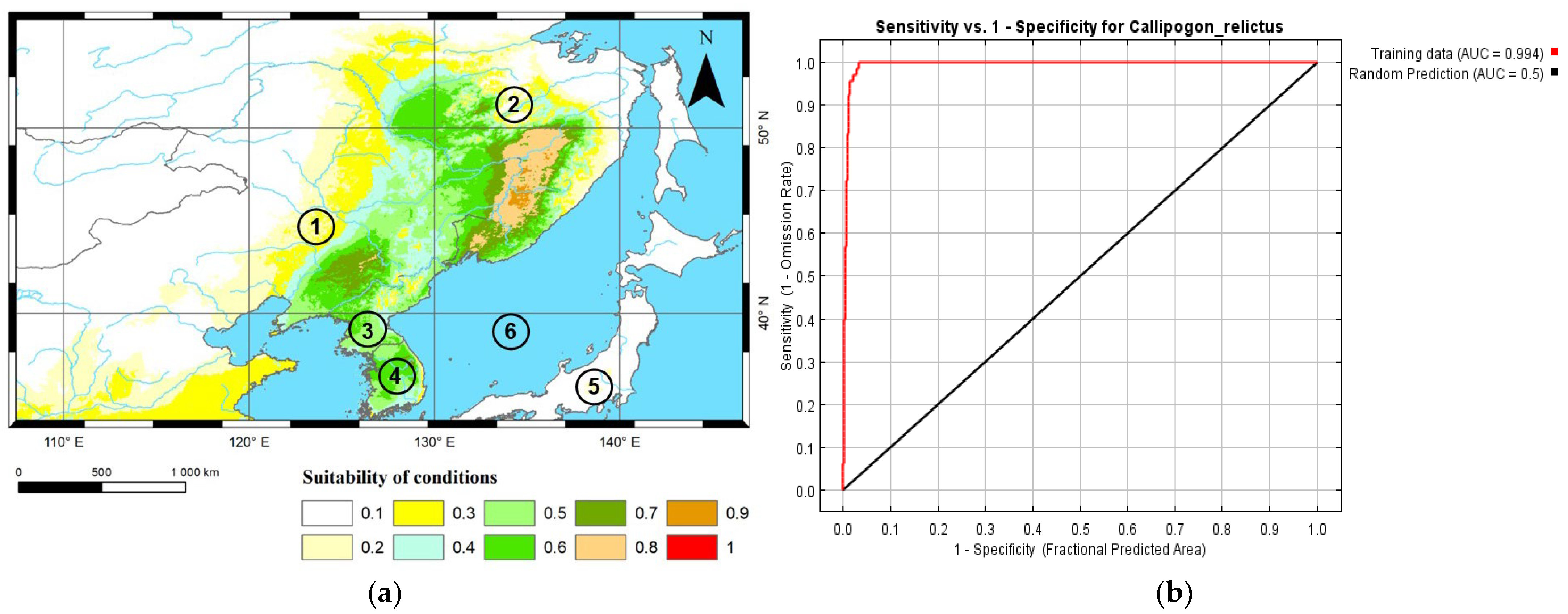

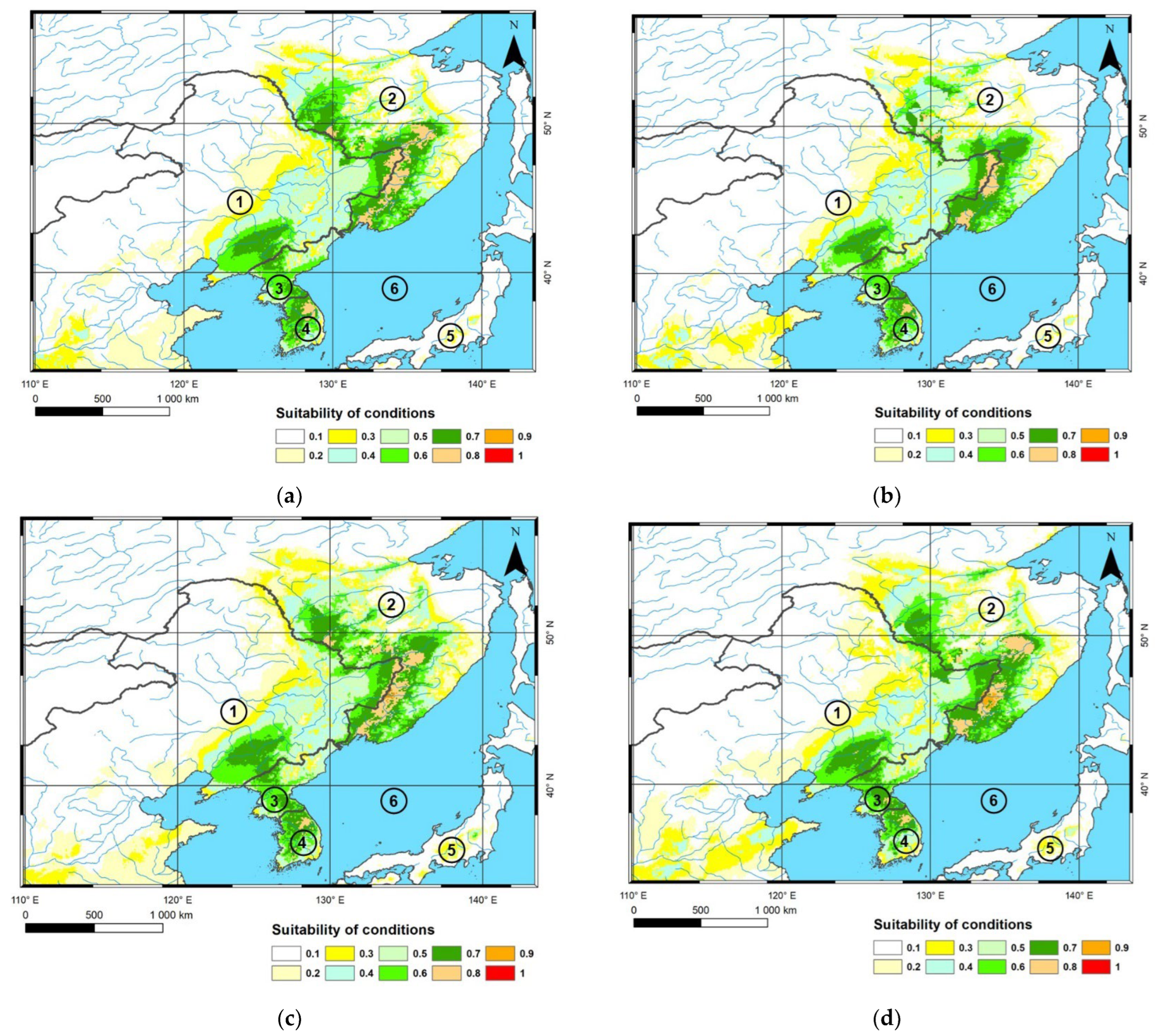
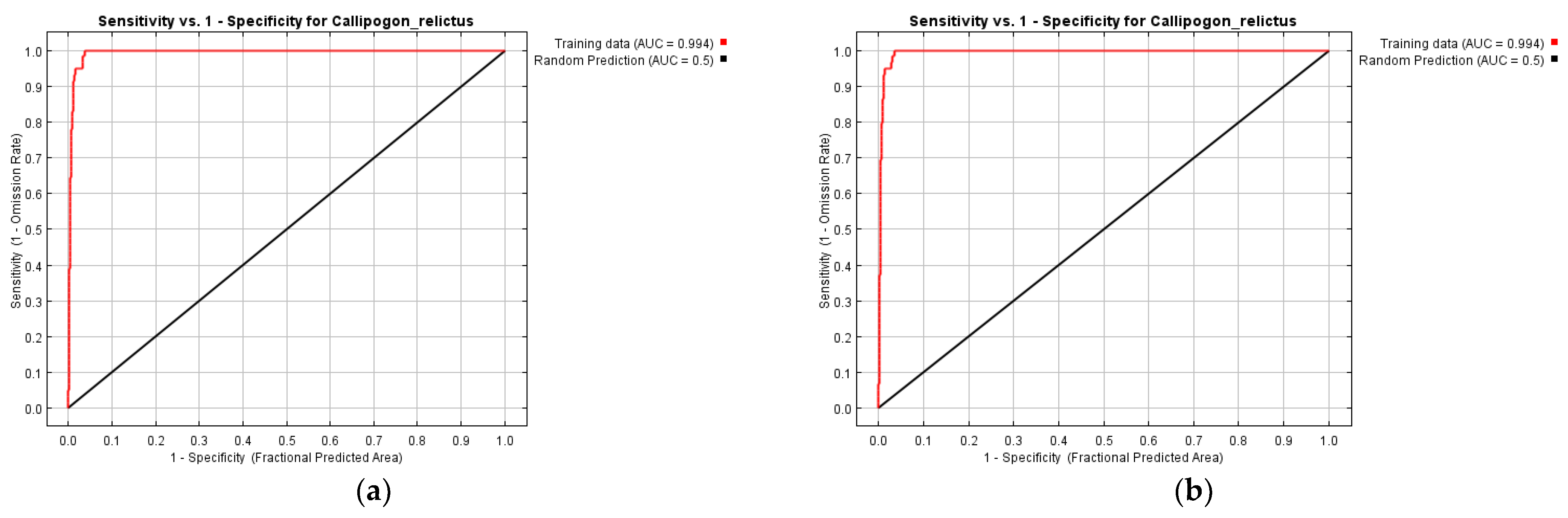
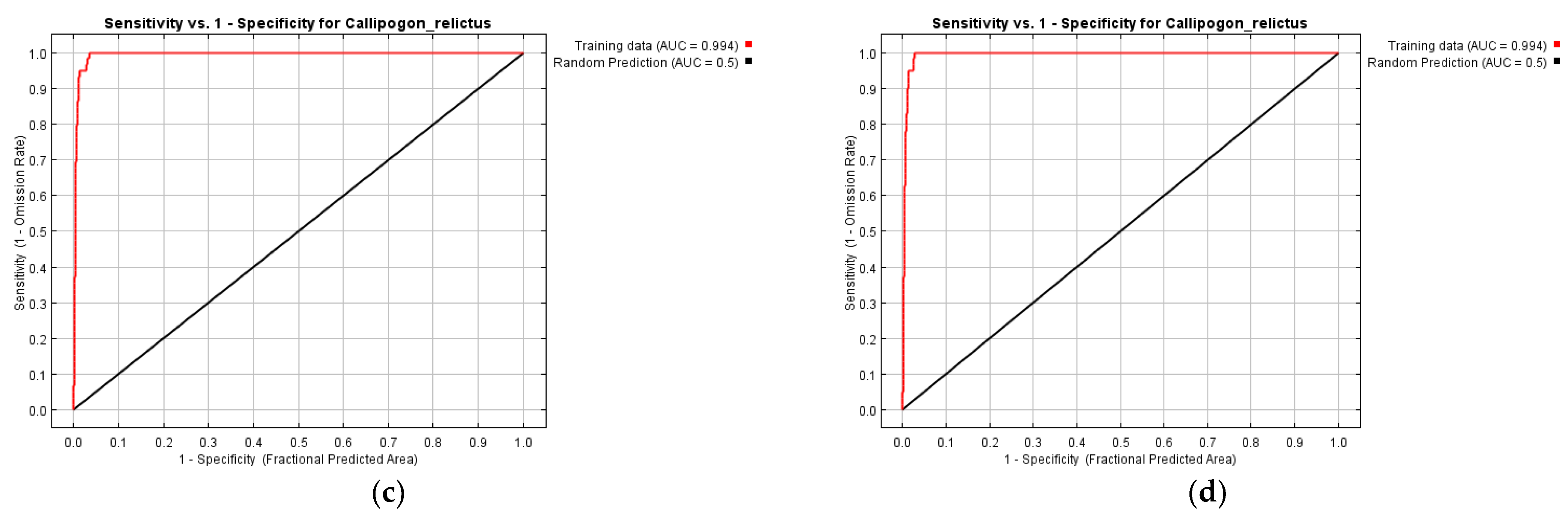
| Variable | Percent Contribution | Permutation Importance | Min | Max | Mean | SD | CV, % |
|---|---|---|---|---|---|---|---|
| BIO_1 | 3.8 | 73.7 | −4.9 | 13.9 | 4.92 | 3.73 | 75.8 |
| BIO_4 | 27.4 | 15.1 | - | - | - | - | - |
| BIO_12 | 7.7 | 1.6 | 420 | 1480 | 836 | 253 | 30.2 |
| BIO_13 | 2.2 | 0.9 | 108 | 439 | 187 | 83 | 44.4 |
| BIO_18 | 42.3 | 0.9 | 257 | 890 | 456 | 167 | 35.9 |
| BIO_15 | 16.6 | 7.8 | - | - | - | - | - |
| Suitable Area | Unsuitable Area | |||
|---|---|---|---|---|
| Area (×104 km2) | Proportion (%) | Area (×104 km2) | Proportion (%) | |
| Current | 6.35 | 5.89 | 101.57 | 94.11 |
| RPC 2.6 | 1.42 | 2.97 | 46.39 | 97.03 |
| RPC 4.5 | 0.87 | 2.04 | 41.78 | 97.96 |
| RPC 6.0 | 1.32 | 2.73 | 47.06 | 97.27 |
| RPC 8.5 | 1.22 | 2.43 | 49.25 | 97.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuprin, A.; Shevchenko, N.; Baklanova, V. Modelling Distribution of an Endangered Longhorn Beetle, Callipogon relictus (Coleoptera: Cerambycidae), in Northeast Asia. Forests 2024, 15, 598. https://doi.org/10.3390/f15040598
Kuprin A, Shevchenko N, Baklanova V. Modelling Distribution of an Endangered Longhorn Beetle, Callipogon relictus (Coleoptera: Cerambycidae), in Northeast Asia. Forests. 2024; 15(4):598. https://doi.org/10.3390/f15040598
Chicago/Turabian StyleKuprin, Alexander, Nicolaj Shevchenko, and Vladislava Baklanova. 2024. "Modelling Distribution of an Endangered Longhorn Beetle, Callipogon relictus (Coleoptera: Cerambycidae), in Northeast Asia" Forests 15, no. 4: 598. https://doi.org/10.3390/f15040598





