Fragmentation and Connectivity in dehesa Ecosystems Associated with Cerambyx spp. Dispersion and Control: A Graph-Theory Approach
Abstract
:1. Introduction
2. Materials and Methods
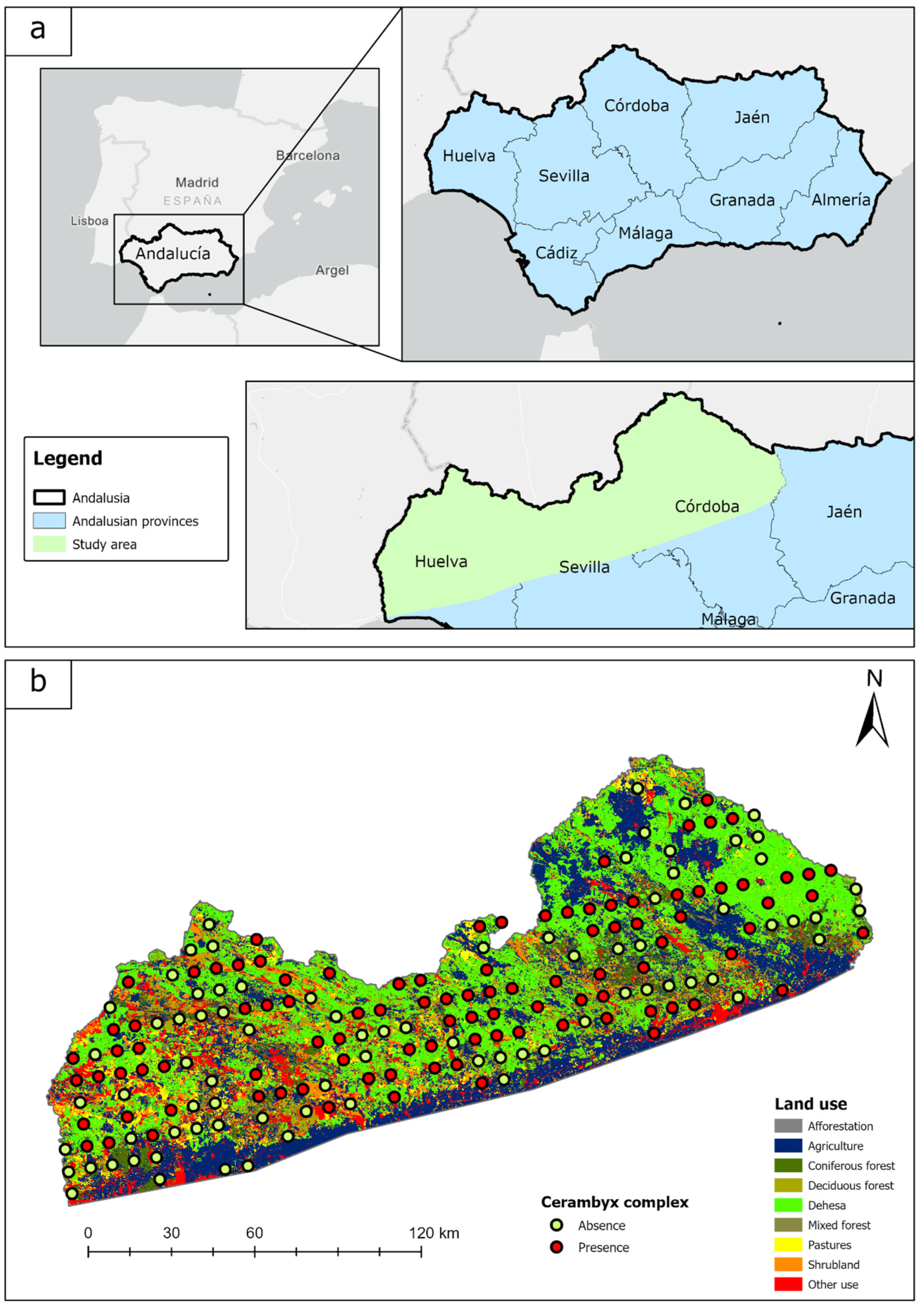
2.1. Study Area
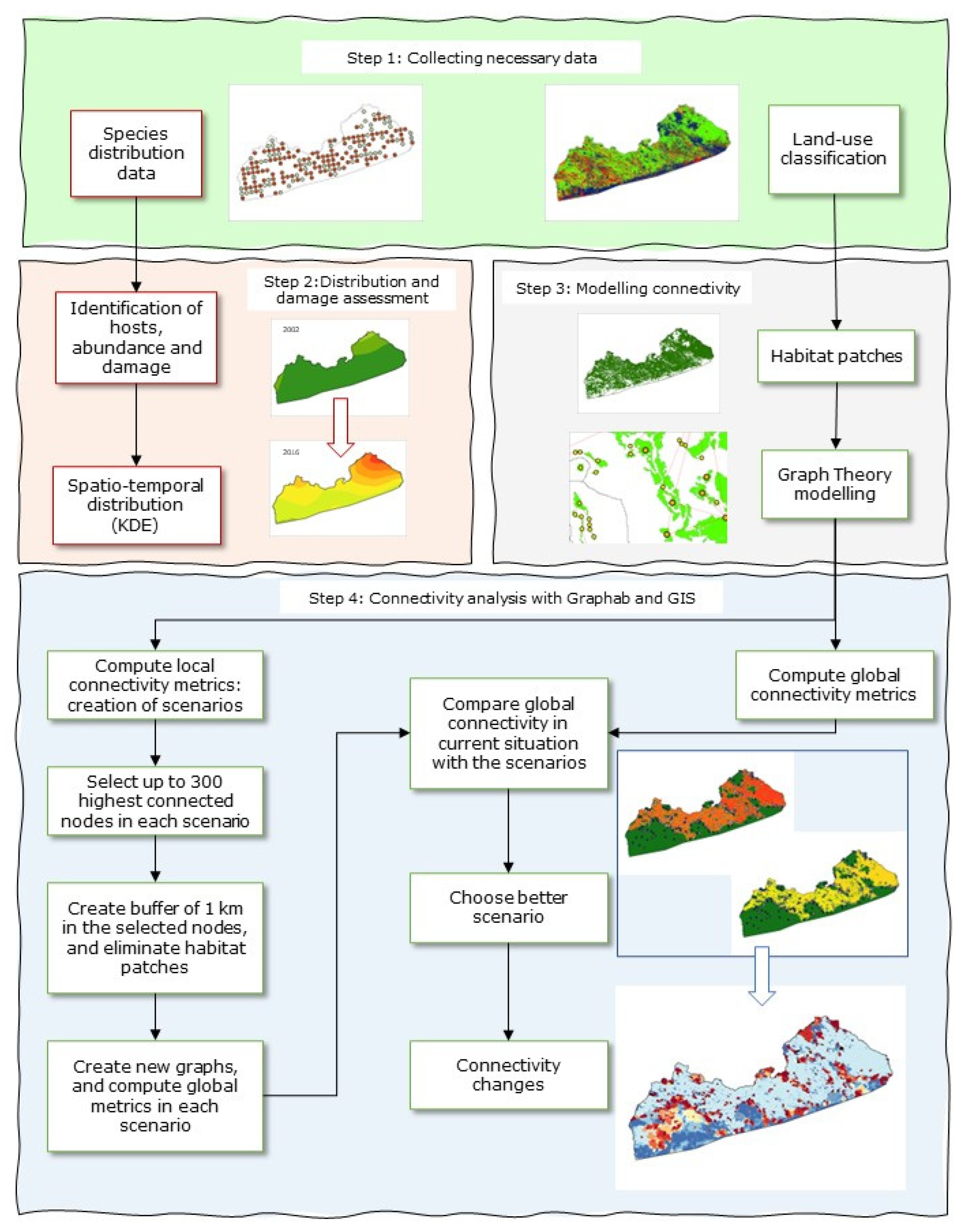
2.2. Data Acquisition and Processing
2.2.1. Land-Use Data
2.2.2. Species Distribution Data
2.3. Temporal Distribution of CC
2.4. Connectivity Analysis: Landscape Network of CC
2.5. Connectivity Metrics Selection
2.6. Proposals to Reduce the Connectivity
2.7. Statistical Analysis
3. Results
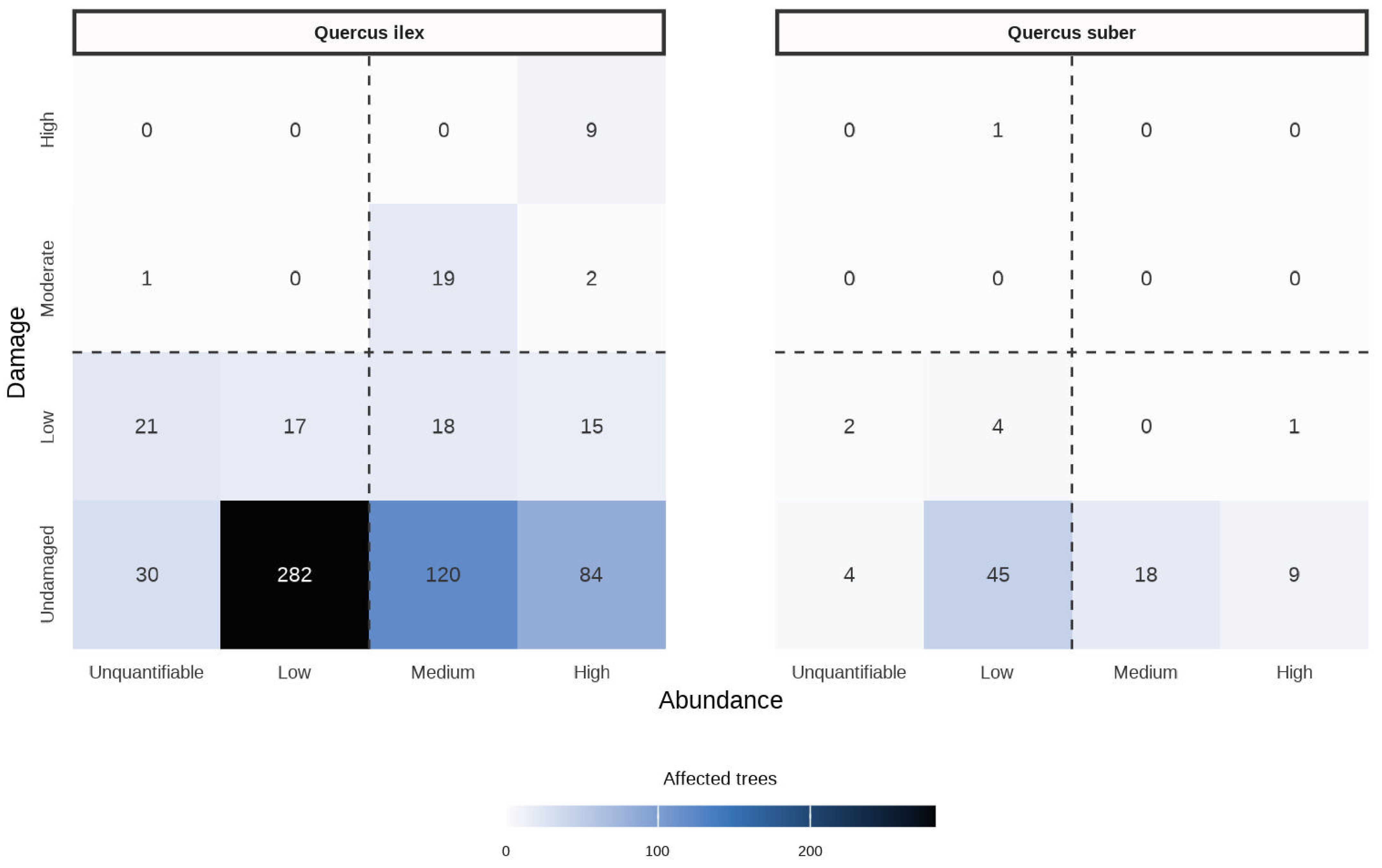
3.1. Abundance of and Damage by the CC
3.2. Spatial-Temporal Pattern Change
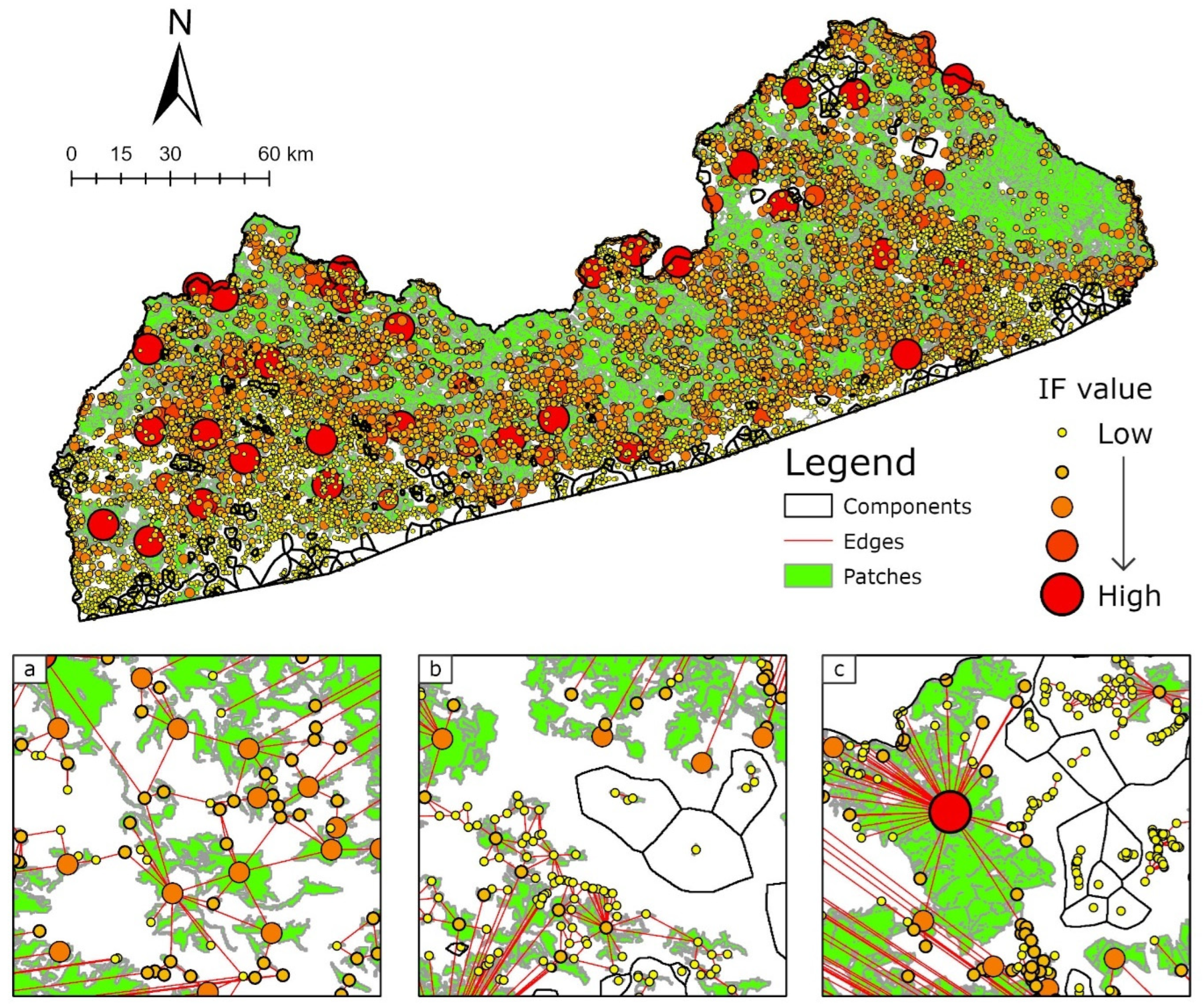
3.3. Impact of Graphs in Global Connectivity
3.4. Best Control Graph for CC
4. Discussion
4.1. Cerambyx “Complex” Damage and Mortality
4.2. Spatial-Temporal Pattern Change
4.3. Connectivity Analysis
4.4. Effectiveness of Local Metrics to Reduce Global Connectivity
4.5. Practical Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreno, G.; Pulido, F.J. The Functioning, Management and Persistence of Dehesas. Agrofor. Eur. 2008, 6, 127–160. [Google Scholar] [CrossRef]
- Ramírez-Hernández, A.; Micó, E.; Marcos-García, M.d.l.Á.; Brustel, H.; Galante, E. The “Dehesa”, a Key Ecosystem in Maintaining the Diversity of Mediterranean Saproxylic Insects (Coleoptera and Diptera: Syrphidae). Biodivers. Conserv. 2014, 23, 2069–2086. [Google Scholar] [CrossRef]
- Duque-Lazo, J.; Navarro-Cerrillo, R.M. What to Save, the Host or the Pest? The Spatial Distribution of Xylophage Insects within the Mediterranean Oak Woodlands of Southwestern Spain. For. Ecol. Manag. 2017, 392, 90–104. [Google Scholar] [CrossRef]
- Hernández-Lambraño, R.E.; de la Cruz, D.R.; Sánchez-Agudo, J.Á. Spatial Oak Decline Models to Inform Conservation Planning in the Central-Western Iberian Peninsula. For. Ecol. Manag. 2019, 441, 115–126. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Roques, A.; Battisti, A. Forest Insects and Climate Change. Curr. For. Rep. 2018, 4, 35–50. [Google Scholar] [CrossRef]
- Logan, J.A.; Régnière, J.; Powell, J.A. Assessing the Impacts of Global Warming on Forest Pest Dynamics. Front. Ecol. Environ. 2003, 1, 130–137. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Zugasti-Martínez, C.; Mendiola-Díaz, F.J.; De-Juan-Murillo, J.M.; Sánchez-González, Á.; Conejo-Rodríguez, Y.; Ponce-Escudero, F.; Fernández-Moreno, F. Larval Assemblages of Large Saproxylic Cerambycids in Iberian Oak Forests: Wood Quality and Host Preference Shape Resource Partitioning. Popul. Ecol. 2017, 59, 315–328. [Google Scholar] [CrossRef]
- Calle, C.V. Evaluación del Grado de Afectación por Larvas de “Cerambyx Welensii” Küster 1846 (Col., Cerambycidae) Del Arbolado de Las Dehesas (“Quercus Suber”, L. y “Q. Rotundifolia”, Lam.) En Extremadura y Su Relación Con Algunas Variables Importantes Del Ecosistema. Ph.D. Thesis, Universidad de Extremadura, Badajoz, Spain, 2013. [Google Scholar]
- Sánchez-Cuesta, R.; Ruiz-Gómez, F.J.; Duque-Lazo, J.; González-Moreno, P.; Navarro-Cerrillo, R.M. The Environmental Drivers Influencing Spatio-Temporal Dynamics of Oak Defoliation and Mortality in Dehesas of Southern Spain. For. Ecol. Manag. 2021, 485, 118946. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Mendiola-Diaz, F.J.; Sánchez-González, Á. Dispersal Differences of a Pest and a Protected Cerambyx Species (Coleoptera: Cerambycidae) in Oak Open Woodlands: A Mark–Recapture Comparative Study. Ecol. Entomol. 2017, 42, 18–32. [Google Scholar] [CrossRef]
- McGarigal, K.; Marks, B.J. Spatial Pattern Analysis Program for Quantifying Landscape Structure; Department of Agriculture, Forest Service, Pacific NorthWest Research Station: Portland, OR, USA, 1995.
- Rivas, C.A.; Guerrero-Casado, J.; Navarro-Cerrillo, R.M. Deforestation and Fragmentation Trends of Seasonal Dry Tropical Forest in Ecuador: Impact on Conservation. For. Ecosyst. 2021, 8, 46. [Google Scholar] [CrossRef]
- Fahrig, L.; Arroyo-Rodríguez, V.; Bennett, J.R.; Boucher-Lalonde, V.; Cazetta, E.; Currie, D.J.; Eigenbrod, F.; Ford, A.T.; Harrison, S.P.; Jaeger, J.A.G.; et al. Is Habitat Fragmentation Bad for Biodiversity? Biol. Conserv. 2019, 230, 179–186. [Google Scholar] [CrossRef]
- Hermosilla, T.; Wulder, M.; White, J.C.; Bolton, D. Impact of Time on Interpretations of Forest Fragmentation: Three-Decades of Fragmentation Dynamics over Canada. Remote Sens. Environ. 2018, 222, 65–77. [Google Scholar] [CrossRef]
- Asbjornsen, H.; Ashton, M.S.; Vogt, D.J.; Palacios, S. Effects of Habitat Fragmentation on the Buffering Capacity of Edge Environments in a Seasonally Dry Tropical Oak Forest Ecosystem in Oaxaca, Mexico. Agric. Ecosyst. Environ. 2004, 103, 481–495. [Google Scholar] [CrossRef]
- Haddad, N.M.; Gonzalez, A.; Brudvig, L.A.; Burt, M.A.; Levey, D.J.; Department, E.I.D. Experimental Evidence Does Not Support the Habitat Amount Hypothesis. Ecography 2015, 40, 48–55. [Google Scholar] [CrossRef]
- Taylor, P.D.; Fahrig, L.; Henein, K.; Merriam, G. Connectivity Is a Vital Element of Landscape Structure. Oikos 1993, 68, 571–573. [Google Scholar] [CrossRef]
- Koh, I.; Rowe, H.I.; Holland, J.D. Graph and Circuit Theory Connectivity Models of Conservation Biological Control Agents. Ecol. Appl. 2013, 23, 1554–1573. [Google Scholar] [CrossRef]
- Hamilton, G.S.; Mather, P.B.; Wilson, J.C. Habitat Heterogeneity Influences Connectivity in a Spatially Structured Pest Population. J. Appl. Ecol. 2006, 43, 219–226. [Google Scholar] [CrossRef]
- Brown, J.H.; Kodric-Brown, A. Turnover Rates in Insular Biogeography: Effect of Immigration on Extinction. Ecology 1977, 58, 445–449. [Google Scholar] [CrossRef]
- Minor, E.S.; Urban, D.L. A Graph-Theory Framework for Evaluating Landscape Connectivity and Conservation Planning. Conserv. Biol. 2008, 22, 297–307. [Google Scholar] [CrossRef]
- Pascual-Hortal, L.; Saura, S. Comparison and Development of New Graph-Based Landscape Connectivity Indices: Towards the Priorization of Habitat Patches and Corridors for Conservation. Landsc. Ecol. 2006, 21, 959–967. [Google Scholar] [CrossRef]
- Urban, D.; Keitt, T. Landscape Connectivity: A Graph-Theoretic Perspective. Ecology 2001, 82, 1205–1218. [Google Scholar] [CrossRef]
- Calabrese, J.M.; Fagan, W.F. A Comparison-Shopper ’ s Guide to Connectivity Metrics. Front. Ecol. Environ. 2004, 2, 529–536. [Google Scholar] [CrossRef]
- Bodin, Ö.; Saura, S. Ranking Individual Habitat Patches as Connectivity Providers: Integrating Network Analysis and Patch Removal Experiments. Ecol. Modell. 2010, 221, 2393–2405. [Google Scholar] [CrossRef]
- Rayfield, B.; Fortin, M.J.; Fall, A. Connectivity for Conservation: A Framework to Classify Network Measures. Ecology 2011, 92, 847–858. [Google Scholar] [CrossRef]
- Ceia, R.S.; Ramos, J.A. Birds as predators of cork and holm oak pests. Agrofor. Syst. 2016, 90, 159–176. [Google Scholar] [CrossRef]
- Kenis, M.; Hilszczanski, J. Natural enemies of Cerambycidae and Buprestidae infesting living trees. In Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis; Lieutier, F., Day, K.R., Battisti, A., Grégoire, J.C., Evans, H.F., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 475–498. [Google Scholar]
- Sanz, J.J. Experimentally increased insectivorous bird density results in a reduction of caterpillar density and leaf damage to Pyrenean oak. Ecol. Res. 2001, 16, 387–394. [Google Scholar] [CrossRef]
- East, M.L.; Perrins, C.M. The effect of nestboxes on breeding populations of birds in broadleaved temperate woodlands. Ibis 1988, 130, 393–401. [Google Scholar] [CrossRef]
- Gurrutxaga, M.; Lozano, P.J. Efectos de La Fragmentación de Hábitats y Pérdida de Conectividad Ecológica Dentro de La Dinámica Territorial. Rev. Geogr. 2006, 16, 35–54. [Google Scholar]
- Bereczki, K.; Ódor, P.; Csóka, G.; Mag, Z.; Báldi, A. Effects of forest heterogeneity on the efficiency of caterpillar control service provided by birds in temperate oak forests. For. Ecol. Manag. 2014, 327, 96–105. [Google Scholar] [CrossRef]
- Gómez-Zotano, J.; Alcántara-Manzanares, J.; Olmedo-Cobo, J.A.; Martínez-Ibarra, E. La Sistematización Del Clima Mediterráneo: Identificación, Clasificación y Caracterización Climática de Andalucía (España). Rev. Geogr. Norte Gd. 2015, 61, 161–180. [Google Scholar] [CrossRef]
- Junta de Andalucía. Sistema de Información de Ocupación Del Suelo de España En Andalucía; Junta de Andalucía: Sevilla, Spain, 2018. [Google Scholar]
- Duque-Lazo, J.; Navarro-Cerrillo, R.M.; Ruíz-Gómez, F.J. Assessment of the Future Stability of Cork Oak (Quercus Suber L.) Afforestation under Climate Change Scenarios in Southwest Spain. For. Ecol. Manag. 2018, 409, 444–456. [Google Scholar] [CrossRef]
- Junta de Andalucía. Manual Para El Establecimiento y La Evaluación de Las Parcelas de RED SEDA y La RED DE PINSAPO, Consejería de Medio Ambiente y Ordenación del Territorio, 2014.
- Tiberi, R.; Branco, M.; Bracalini, M.; Croci, F.; Panzavolta, T. Cork oak pests: A review of insect damage and management. Ann. For. Sci. 2016, 73, 219–232. [Google Scholar] [CrossRef]
- International Co-operative Programme on Assessment, & Monitoring of Air Pollution Effects on Forests. Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; Federal Research Centre for Forestry and Forest Products, 1988. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation, R Package Version 1.0.10. 2022. Available online: https://cran.r-project.org/src/contrib/Archive/dplyr/dplyr_1.0.10.tar.gz (accessed on 29 February 2024).
- Nenadic, O.; Greenacre, M. Correspondence Analysis in R, with Two- and Three-Dimensional Graphics: The ca Package. J. Stat. Softw. 2007, 20, 1–13. [Google Scholar]
- Silverman, B.W. Density Estimation for Statistics and Data Analysis; Routledge: New York, NY, USA, 1986. [Google Scholar]
- Baddeley, A.; Rubak, E.; Turner, R. Spatial Point Patterns: Methodology and Applications with R; Chapman and Hall/CRC Press: London, UK, 2015. [Google Scholar]
- Hijmans, R.J. Terra: Spatial Data Analysis, R Package Version 1.7-3. 2023. Available online: https://www.rdocumentation.org/packages/terra/versions/1.7-3 (accessed on 29 February 2024).
- Hernangómez, D. Tidyterra: Tidyverse Methods and Ggplot2 Helpers for Terra Objects, Version 0.3.1. 2023. Available online: https://dieghernan.github.io/tidyterra/news/index.html (accessed on 29 February 2024).
- Pohlert, T. PMCMRplus: Calculate Pairwise Multiple Comparisons of Mean Rank Sums Extended, R Package Version 1.9.6. 2022. Available online: https://CRAN.R-project.org/package=PMCMRplus (accessed on 30 August 2023).
- Foltête, J.C.; Vuidel, G.; Savary, P.; Clauzel, C.; Sahraoui, Y.; Girardet, X.; Bourgeois, M. Graphab: An Application for Modeling and Managing Ecological Habitat Networks. Softw. Impacts 2021, 8, 100065. [Google Scholar] [CrossRef]
- Foltête, J.C.; Clauzel, C.; Vuidel, G. A Software Tool Dedicated to the Modelling of Landscape Networks. Environ. Model. Softw. 2012, 38, 316–327. [Google Scholar] [CrossRef]
- Foltête, J.C.; Clauzel, C.; Vuidel, G.; Tournant, P. Integrating Graph-Based Connectivity Metrics into Species Distribution Models. Landsc. Ecol. 2012, 27, 557–569. [Google Scholar] [CrossRef]
- Clauzel, C.; Foltête, J.-C.; Girardet, X.; Vuidel, G. User Manual Graphab 2.6. 2020. Available online: https://sourcesup.renater.fr/www/graphab/download/manual-2.6-en.pdf (accessed on 29 February 2024).
- Minor, E.S.; Urban, D.L. Graph Theory as a Proxy for Spatially Explicit Population Models in Conservation Planning. Ecol. Appl. 2007, 17, 1771–1782. [Google Scholar] [CrossRef]
- Saura, S.; Pascual-Hortal, L. A New Habitat Availability Index to Integrate Connectivity in Landscape Conservation Planning: Comparison with Existing Indices and Application to a Case Study. Landsc. Urban Plan. 2007, 83, 91–103. [Google Scholar] [CrossRef]
- Girardet, X.; Conruyt-Rogeon, G.; Foltête, J.C. Does Regional Landscape Connectivity Influence the Location of Roe Deer Roadkill Hotspots? Eur. J. Wildl. Res. 2015, 61, 731–742. [Google Scholar] [CrossRef]
- McRae, B.H.; Dickson, B.G.; Keitt, T.H.; Shah, V.B. Using Circuit Theory to Model Connectivity in Ecology, Evolution, and Conservation. Ecology 2008, 89, 2712–2724. [Google Scholar] [CrossRef]
- Foltête, J.C.; Girardet, X.; Clauzel, C. A Methodological Framework for the Use of Landscape Graphs in Land-Use Planning. Landsc. Urban Plan. 2014, 124, 140–150. [Google Scholar] [CrossRef]
- Sahraoui, Y.; Foltête, J.C.; Clauzel, C. A Multi-Species Approach for a Assessing the Impact of Land-Cover Changes on Landscape Connectivity. Landsc. Ecol. 2017, 32, 1819–1835. [Google Scholar] [CrossRef]
- Keeley, A.T.; Beier, P.; Jenness, J.S. Connectivity Metrics for Conservation Planning and Monitoring. Biol. Conserv. 2021, 255, 109008. [Google Scholar] [CrossRef]
- Tarabon, S.; Bergès, L.; Dutoit, T.; Isselin-Nondebeu, F. Environmental Impact Assessment of Development Projects Improved by Merging Species Distribution and Habitat Connectivity Modelling. J. Environ. Manag. 2019, 241, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Mimet, A.; Clauzel, C.; Foltête, J.C. Locating Wildlife Crossings for Multispecies Connectivity across Linear Infrastructures. Landsc. Ecol. 2016, 31, 1955–1973. [Google Scholar] [CrossRef]
- Alonso, C.L. Arrendajo—Garrulus Glandarius (Linnaeus, 1758). In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Morales, M.B., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2016; Available online: http://www.vertebradosibericos.org/ (accessed on 15 January 2022).
- Han, Q.; Keeffe, G. Mapping the Flow of Forest Migration through the City under Climate Change. Urban Plan. 2019, 4, 139–151. [Google Scholar] [CrossRef]
- Han, Q.; Keeffe, G. Stepping Stones: Assessing the Permeability of Urban Greenspaces to Climate-Driven Migration of Trees. Smart Sustain. Built Environ. 2020, 9, 246–257. [Google Scholar] [CrossRef]
- Sekercioglu, C.H. Forest Fragmentation Hits Insectivorous Birds Hard. Dir. Sci. 2002, 1, 62–64. [Google Scholar] [CrossRef]
- Sánchez-Osorio, I.; López-Pantoja, G.; Paramio, A.M.; Lencina, J.L.; Gallego, D.; Domínguez, L. Field Attraction of Cerambyx Welensii to Fermentation Odors and Host Monoterpenes. J. Pest Sci. 2016, 89, 59–68. [Google Scholar] [CrossRef]
- Sánchez-Osorio, I.; Tapias, R.; Domínguez, L.; López-Pantoja, G.; González, M.D.M. Electroantennographic Responses of Cerambyx Welensii Küster to Host-Related Volatiles. Forests 2021, 12, 1168. [Google Scholar] [CrossRef]
- Sánchez-Osorio, I.; Domínguez, L.; López-Pantoja, G.; Tapias, R. Antennal Response of Prinobius Myardi to Synthetic Tree Volatiles. Silva Fenn. 2015, 49, 1–8. [Google Scholar] [CrossRef]
- López-Pantoja, G.; Sánchez-Osorio, I.; Dominguez, L. Cerambícidos Xilófagos de Encinas y Alcornoques: Estudio Bioecológico y Control de Poblaciones. CIDEU 2006, 39–44. [Google Scholar]
- Domínguez, L.; López-Pantoja, G.; Cremades, D.; Paramio, A.; Hidalgo, P.J.; Sánchez-Osorio, I. Incidence of Large Wood Borers in the Conservation of Dehesa Islands Forests in Southwestern Spain. Forests 2022, 13, 413. [Google Scholar] [CrossRef]
- Tubé, A.; Jana, U.; de Toni, A.; Woodward, S.; Schopf, A.; Netherer, S.; Angelstam, P.; Mudgal, S.; Sonigo, P. Disturbances of EU Forests Caused by Biotic Agents; European Commision: 2012. Available online: https://www.researchgate.net/publication/363892531_Disturbances_of_EU_forests_caused_by_biotic_agents (accessed on 29 February 2024).
- López-Pantoja, G.; Nevado, L.D.; Sánchez-Osorio, I. Mark-Recapture Estimates of the Survival and Recapture Rates of Cerambyx Welensii Küster (Coleoptera Cerambycidae) in a Cork Oak Dehesa in Huelva (Spain). Cent. Eur. J. Biol. 2008, 3, 431–441. [Google Scholar] [CrossRef]
- López-Pantoja, G.; Dominguez, L.; Sánchez-Osorio, I. Analysis of Prinobius Myardi Mulsant Population Dynamics in a Mediterranean Cork Oak Stand. Ann. Société Entomol. Fr. 2011, 47, 260–268. [Google Scholar] [CrossRef]
- Shibata, E. Adult Populations of the Sugi Bark Borer, Semanotus Japonicus Lacordaire (Coleoptera: Cerambycidae), in Japanese Cedar Stands: Population Parameters, Dispersal, and Spatial Distribution. Res. Popul. Ecol. 1986, 28, 253–266. [Google Scholar] [CrossRef]
- Williams, D.; Li, G.; Gao, R. Tracking Movements of Individual Anoplophora Glabripennis (Coleoptera: Cerambycidae) Adults: Application of Harmonic Radar. Environ. Entomol. 2004, 33, 644–649. [Google Scholar] [CrossRef]
- Kenis, M.; Hurley, B.P.; Colombari, F.; Lawson, S.; Sun, J.; Wilcken, C.; Weeks, C.; Sathyapala, S. Guide to the Classical Biological Control of Insect Pests in Planted and Natural Forests; Bulletin: FAO Forestry Paper; No.182; FAO: Rome, Italy, 2019. [Google Scholar]
- Iwona, M.; Marek, P.; Katarzyna, W.; Edward, B.; Julia, S. Use of a Genetically Informed Population Viability Analysis to Evaluate Management Options Por Polish Populations of Endangered Beetle Cerambyx Cerdo L.(1758)(Coleoptera, Cerambycidae). J. Insect Conserv. 2018, 22, 69–83. [Google Scholar] [CrossRef]
- Boesing, A.L.; Nichols, E.; Metzger, J.P. Effects of Landscape Structure on Avian-Mediated Insect Pest Control Services: A Review. Landsc. Ecol. 2017, 32, 931–944. [Google Scholar] [CrossRef]
- Saura, S.; Lidón, R. A Common Currency for the Different Ways in Which Patches and Links Can Contribute to Habitat Availability and Connectivity in the Landscape. Ecography 2010, 33, 523–537. [Google Scholar] [CrossRef]
- Préau, C.; Grandjean, F.; Sellier, Y.; Gailledrat, M.; Bertrand, R.; Isselin-Nondedeu, F. Habitat Patches for Newts in the Face of Climate Change: Local Scale Assessment Combining Niche Modelling and Graph Theory. Sci. Rep. 2020, 10, 3570. [Google Scholar] [CrossRef]
- Railsback, S.F.; Johnson, M.D. Effects of Land Use on Bird Populations and Pest Control Services on Coffee Farms. Proc. Natl. Acad. Sci. USA 2014, 111, 6109–6114. [Google Scholar] [CrossRef]
- Morán-López, T.; Alonso, C.L.; Díaz, M. Landscape Effects on Jay Foraging Behavior Decrease Acorn Dispersal Services in Dehesas. Acta Oecol. 2015, 69, 52–64. [Google Scholar] [CrossRef]
- Kurek, P.; Dobrowolska, D.; Wiatrowska, B. Dispersal Distance and Burial Mode of Acorns in Eurasian Jays Garrulus Glandarius in European Temperate Forests. Acta Ornithol. 2019, 53, 155–162. [Google Scholar] [CrossRef]
- Canelo, T.; Gaytán, Á.; Pérez-Izquierdo, C.; Bonal, R. Effects of Longer Droughts on Holm Oak Quercus Ilex l. Acorn Pests: Consequences for Infestation Rates, Seed Biomass and Embryo Survival. Diversity 2021, 13, 110. [Google Scholar] [CrossRef]
- López-Sánchez, A.; Perea, R.; Dirzo, R.; Roig, S. Livestock vs. Wild Ungulate Management in the Conservation of Mediterranean Dehesas: Implications for Oak Regeneration. For. Ecol. Manag. 2016, 362, 99–106. [Google Scholar] [CrossRef]



| Land Use | Definition | Area (ha) | %Area Total | Cost | Type |
|---|---|---|---|---|---|
| CC habitat | Forest formations with at least 5% of Quercus ilex or Quercus suber | 982,815 | 46.37 | 1 | Habitat |
| Other dehesas | Savana-like ecosystem characterized by the presence of scattered trees (Q. ilex and Q. suber not included here) | 90,150 | 4.25 | 10 | Suitable |
| Other forest | Other forest structures including deciduous, coniferous, and mixed forests | 284,873 | 13.44 | 10 | Suitable |
| Afforestation | Anthropically established forest by plantation in previous agricultural lands | 3669 | 0.17 | 100 | Neutral |
| Shrubland | Forestry land formed by bushes and shrubs in absence of trees | 131,345 | 6.20 | 100 | Neutral |
| Pastures | Includes all herbaceous grazing formations | 110,992 | 5.24 | 100 | Neutral |
| Agriculture | Land under cultivation for aliments production (citrus plants, herbaceous crops, vineyards, etc.) | 359,129 | 16.94 | 100 | Neutral |
| Other use | Includes urban uses, water bodies, bare soil, roads, and other anthropogenic uses | 156,264 | 7.37 | 1000 | Unfavourable |
| Connectivity Metric | Computing Level | Description | Formula | Ecological Significance |
|---|---|---|---|---|
| Flux (F) [23,51] | Global | Global: sum of potential dispersions from all patches | A high flux between patches indicates a large number of dispersing individuals | |
| Number of Components (NC) [23] | Global | Number of components in the graph | Comparing the number of components in different situations | |
| Probability of Connectivity (PC) [52] | Global | Sum of products of capacity of all pairs of patches weighted by their interaction probability, divided by the square of the area of the study zone | Indicates the probability that two animals randomly placed within the landscape fall into habitat areas that are reachable from each other (interconnected) given a set of N habitat patches and the weighted connections among them | |
| Connectivity Correlation (Ccor) [21] | Local | Ratio between the degree of the node i and the degree of its neighbouring patches j | Indicates compartmentalization or presence of sub-networks which may reduce the spread of the potentially cascading effects of a disturbance | |
| Current Flow (CF) [53,54] | Local | Sum of currents passing through the focal patch | Indicates patches with higher probability of movement for a random walker | |
| Interaction Flux (IF) [55,56] | Local | Sum of products of the focal patch capacity with all the other patches, weighted by their interaction probability | Indicates the potential accessibility of any habitat point (i.e., pixel) to the overall network |
| S1 | S2 | S3 | |
|---|---|---|---|
| Local metric | IF | Ccor | CF |
| Min patch size | 25.90 | 3.96 | 3.96 |
| Max patch size | 375,115 | 375,115 | 375,115 |
| Mean patch size | 3034.40 | 2949.50 | 2977 |
| Median patch size | 119.40 | 84.6 | 49.10 |
| Std. Dev | 29,228.62 | 29,231.70 | 29,234 |
| Graph Characteristic | Current | Variation | ||
|---|---|---|---|---|
| S1 | S2 | S3 | ||
| NL | 23,767 | 22,635 (−4.8%) | 20,346 (−14.4%) | 22,420 (−5.7%) |
| NN | 16,300 | 15,924 (−2.3%) | 14,602 (−10.4%) | 15,734 (−3.5%) |
| NC | 289 | 335 (+15.9%) | 401 (+38.7%) | 383 (+35.5%) |
| Largest Patch Index | 17.70 | 16.93 (−4.3%) | 17.58 (−0.7%) | 17.63 (−0.4%) |
| Total habitat area (km2) | 10,156.20 | 9410.08 (−7.3%) | 9614.61 (−5.3%) | 9611.51 (−5.4%) |
| PC | 2.4116 × 10−2 | 1.4931 × 10−2 (−38.1%) | 2.3375 × 10−2 (−3.1%) | 1.5124 × 10−2 (−37.3%) |
| F | 2.0949 × 1013 | 1.6635 × 1013 (−20.6%) | 1.9179 × 1013 (−8.4%) | 1.8643 × 1013 (−11%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cidre-González, A.; Rivas, C.A.; Navarro-Cerrillo, R.M. Fragmentation and Connectivity in dehesa Ecosystems Associated with Cerambyx spp. Dispersion and Control: A Graph-Theory Approach. Forests 2024, 15, 648. https://doi.org/10.3390/f15040648
Cidre-González A, Rivas CA, Navarro-Cerrillo RM. Fragmentation and Connectivity in dehesa Ecosystems Associated with Cerambyx spp. Dispersion and Control: A Graph-Theory Approach. Forests. 2024; 15(4):648. https://doi.org/10.3390/f15040648
Chicago/Turabian StyleCidre-González, Adrián, Carlos A. Rivas, and Rafael M. Navarro-Cerrillo. 2024. "Fragmentation and Connectivity in dehesa Ecosystems Associated with Cerambyx spp. Dispersion and Control: A Graph-Theory Approach" Forests 15, no. 4: 648. https://doi.org/10.3390/f15040648






