Altitudinal Variation in Species Diversity, Distribution, and Regeneration Status of a Secondary Picea Forest in Guandi Mountain, Northern China
Abstract
1. Introduction
2. Materials and Methods
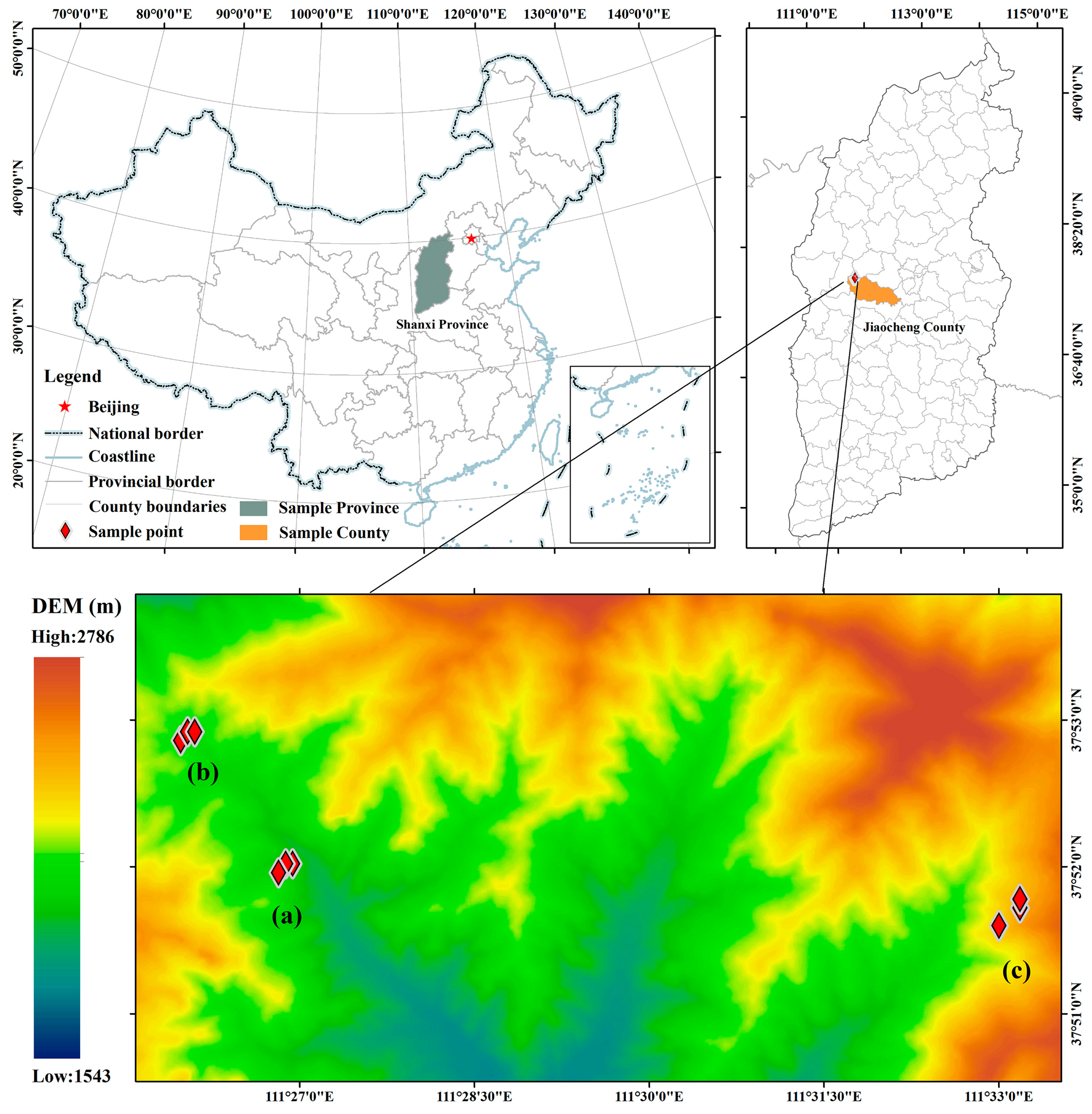
2.1. Study Area
2.2. Field Sampling and Measurements
2.3. Data Collection and Analysis
- (1)
- IVI of tree layer = (relative density + relative dominance + relative frequency)/3;
- (2)
- IVI of shrub and herb layer = (relative density + relative dominance + relative coverage)/3.
- (1)
- The Shannon diversity index:
- (2)
- Simpson’s dominance index:
- (3)
- Pielou’s evenness index:
- (4)
- The Margalef richness index:
- (5)
- Fisher’s alpha:
3. Results
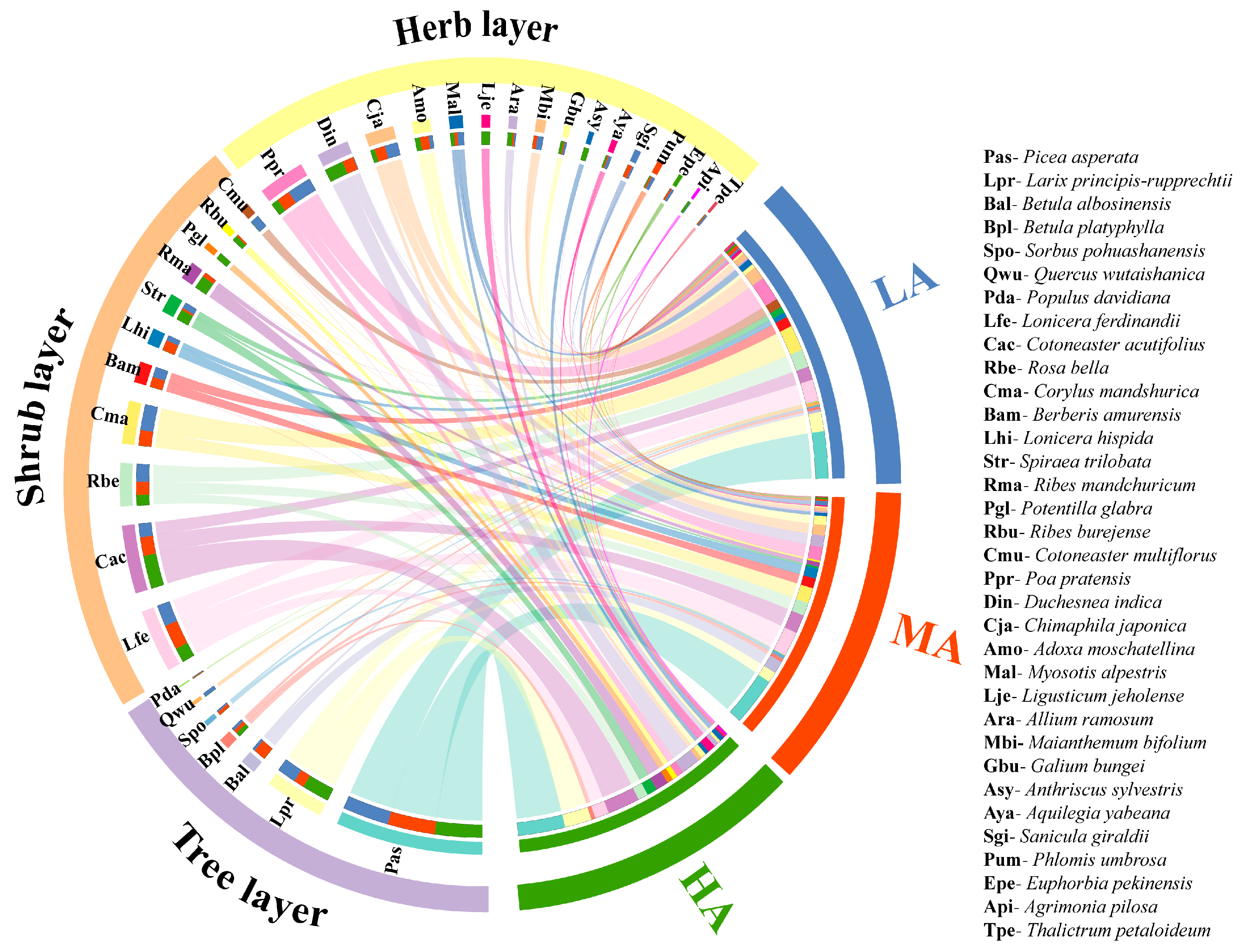
3.1. Distribution of Species along Altitudes
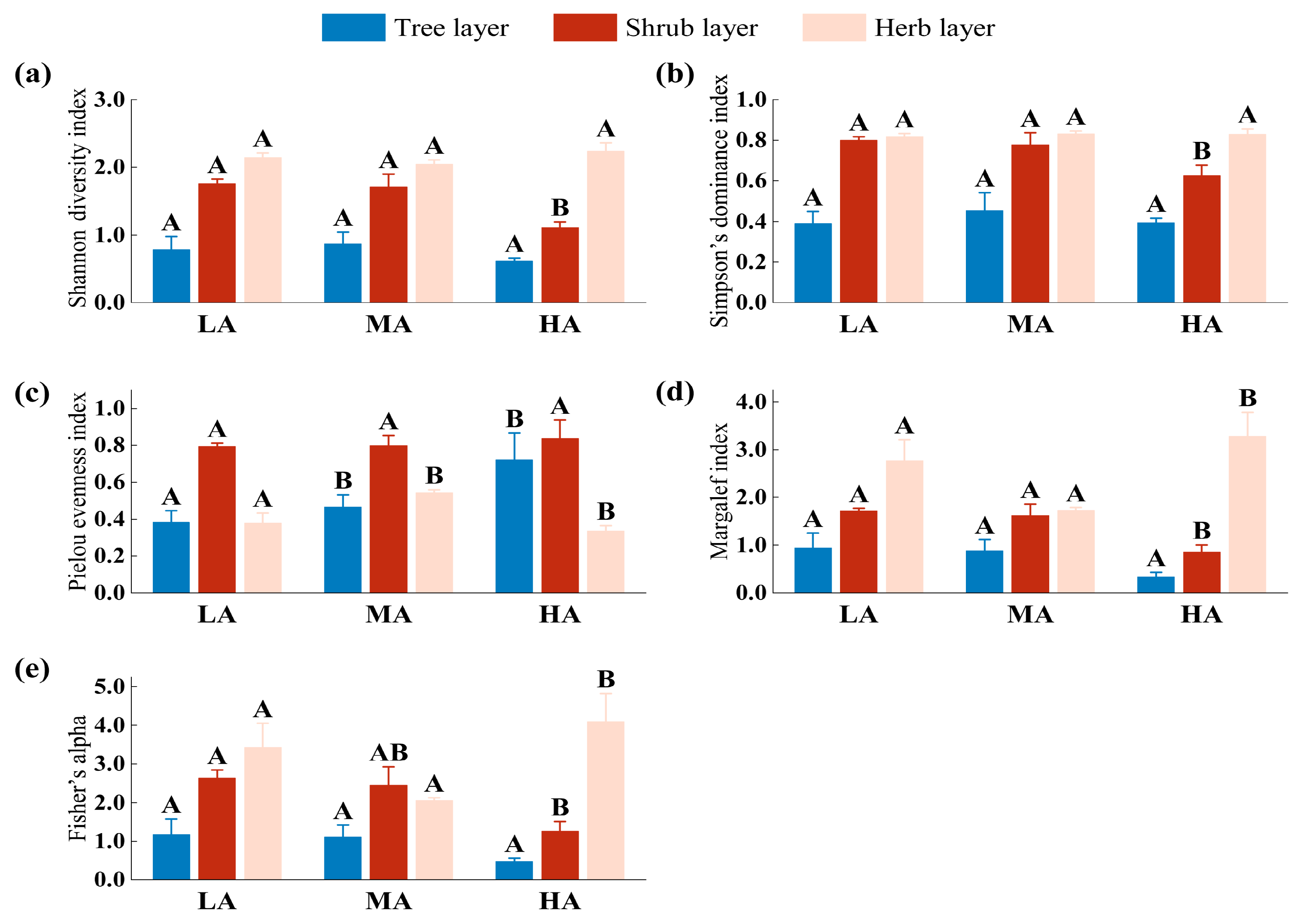
3.2. Analysis of Species Diversity along Altitudes
3.3. Forest Tree Regeneration Status along Altitudes
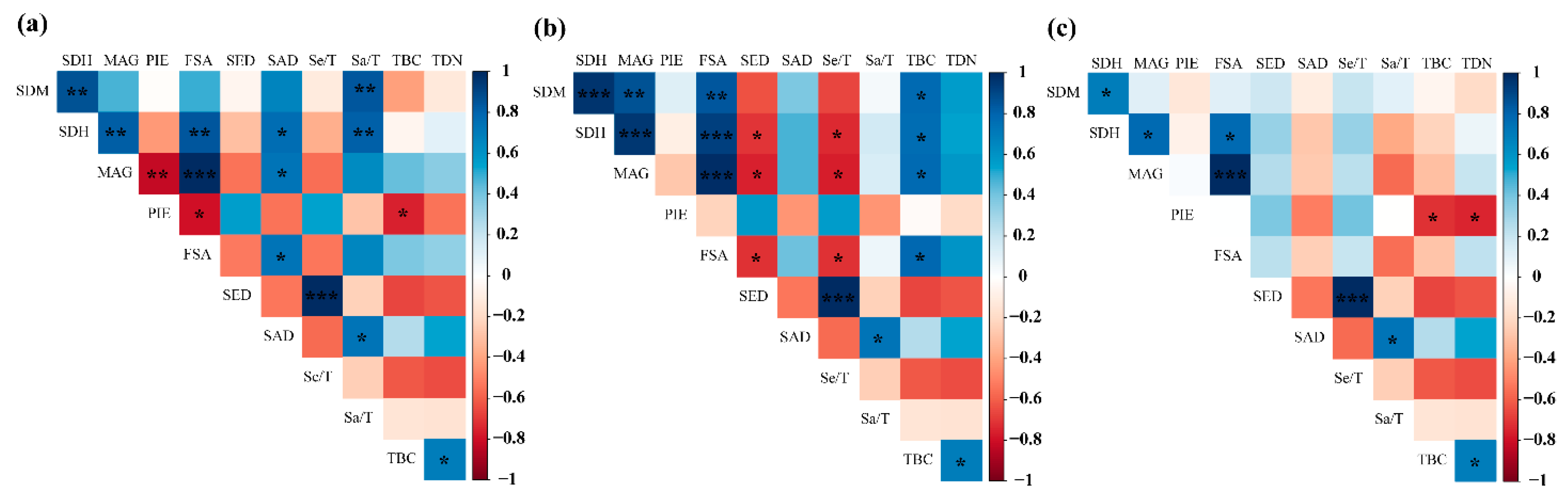
3.4. Pearson Correlation Analysis of Species Diversity Indices and Regeneration Parameters
3.5. Role of Disturbance and Environmental Variables
4. Discussion
4.1. Composition Characteristics, Diversity, and Distribution of Species along Altitudes
4.2. Regeneration Status of the Secondary Picea Forest along Altitudes
4.3. Relationship between Species Diversity Index and Regeneration Parameters
4.4. Role of Anthropogenic and Environmental Factors on Species Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Clark, J.S.; McLachlan, J.S. Stability of forest biodiversity. Nature 2003, 423, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Negi, V.S.; Pathak, R.; Rawal, R.S.; Bhatt, I.D.; Sharma, S. Long-term ecological monitoring on forest ecosystems in Indian Himalayan Region: Criteria and indicator approach. Ecol. Indic. 2019, 102, 374–381. [Google Scholar] [CrossRef]
- Lohbeck, M.; Albers, P.; Boels, L.E.; Bongers, F.; Morel, S.; Sinclair, F.; Smith-Dumont, E. Drivers of farmer-managed natural regeneration in the Sahel. Lessons for restoration. Sci. Rep. 2020, 10, 15038. [Google Scholar] [CrossRef]
- Paul, C.; Hanley, N.; Meyer, S.T.; Fürst, C.; Weisser, W.W.; Knoke, T. On the functional relationship between biodiversity and economic value. Sci. Adv. 2020, 6, eaax7712. [Google Scholar] [CrossRef]
- Guo, X.; Coops, N.C.; Tompalski, P. Regional mapping of vegetation structure for biodiversity monitoring using airborne lidar data. Ecol. Inform. 2017, 38, 50–61. [Google Scholar] [CrossRef]
- Guo, Y.; Schöb, C.; Ma, W. Increasing water availability and facilitation weaken biodiversity–biomass relationships in shrublands. Ecology 2019, 100, e02624. [Google Scholar] [CrossRef] [PubMed]
- Depauw, L.; Perring, M.P.; Landuyt, D. Light availability and land-use history drive biodiversity and functional changes in forest herb layer communities. J. Ecol. 2020, 108, 1411–1425. [Google Scholar] [CrossRef]
- Kharkwal, G.; Mehrotra, P.; Rawat, Y.S.; Pangtey, Y.P.S. Phytodiversity and growth form in relation to altitudinal gradient in the Central Himalayan (Kumaun) region of India. Curr. Sci. 2005, 89, 873–878. [Google Scholar] [CrossRef]
- Sansevero, J.B.B.; Prieto, P.V.; de Moraes, L.F.D.; Rodrigues, P.J.P. Natural regeneration in plantations of native trees in Lowland Brazilian Atlantic Forest: Community structure, diversity, and dispersal syndromes. Restor. Ecol. 2011, 19, 379–389. [Google Scholar] [CrossRef]
- Montfort, F.; Nourtier, M.; Grinand, C. Regeneration capacities of woody species biodiversity and soil properties in Miombo woodland after slash-and-burn agriculture in Mozambique. For. Ecol. Manag. 2021, 488, 119039. [Google Scholar] [CrossRef]
- Rahman, M.; Khan, M.; Roy, B.; Fardusi, M. Assessment of natural regeneration status and diversity of tree species in the biodiversity conservation areas of Northeastern Bangladesh. J. For. Res. 2011, 22, 551–559. [Google Scholar] [CrossRef]
- Good, N.F.; Good, R.E. Population dynamics of tree seedlings and saplings in a Mature Eastern Hardwood. Forest 1972, 99, 172–178. [Google Scholar] [CrossRef]
- Deb, P.; Sundriyal, R.C. Tree regeneration and seedling survival patterns in old-growth lowland tropical rainforest in Namdapha National Park, north-east India. For. Ecol. Manag. 2008, 255, 3995–4006. [Google Scholar] [CrossRef]
- Trevor Caughlin, T.; Peña-Domene, M.; Martínez-Garza, C. Demographic costs and benefits of natural regeneration during tropical forest restoration. Ecol. Lett. 2019, 22, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.B.; Chun, J.H.; Song, H.K.; Cho, H.J. Altitudinal patterns of plant species richness on the Baekdudaegan Mountains, South Korea: Mid-domain effect, area, climate, and Rapoport’s rule. Ecol. Res. 2013, 28, 67–79. [Google Scholar] [CrossRef]
- Jactel, H.; Bauhus, J.; Boberg, J. Tree diversity drives forest stand resistance to natural disturbances. Curr. For. Rep. 2017, 3, 223–243. [Google Scholar] [CrossRef]
- Sterner, R.W.; Ribic, C.A.; Schatz, G.E. Testing for life historical changes in spatial patterns of four tropical tree species. J. Ecol. 1986, 3, 621–633. [Google Scholar] [CrossRef]
- Bennett, N.J.; Roth, R.; Klain, S.C.; Chan, K.; Christie, P.; Clark, D.A.; Cullman, G.; Curran, D.; Durbin, T.J.; Epstein, G.; et al. Conservation social science: Understanding and integrating human dimensions to improve conservation. Biol. Conserv. 2017, 205, 93–108. [Google Scholar] [CrossRef]
- Jiang, Y.; Kang, M.; Zhu, Y.; Xu, G. Plant biodiversity patterns on Helan mountain, China. Acta Oecologica 2007, 32, 125–133. [Google Scholar] [CrossRef]
- Wang, W.; He, Z.; Du, J.; Ma, D.; Zhao, P. Altitudinal patterns of species richness and flowering phenology in herbaceous community in Qilian Mountains of China. Int. J. Biometeorol. 2022, 66, 741–751. [Google Scholar] [CrossRef]
- Jiang, Z.; Ma, K.; Liu, H.; Tang, Z. A trait-based approach reveals the importance of biotic filter for elevational herb richness pattern. J. Biogeogr. 2018, 45, 2288–2298. [Google Scholar] [CrossRef]
- O’Brien, E. Water-energy dynamics, climate, and prediction of woody plant species richness: An interim general model. J. Biogeogr. 1998, 25, 379–398. [Google Scholar] [CrossRef]
- Currie, D.J.; Mittelbach, G.G.; Cornell, H.V. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 2004, 7, 1121–1134. [Google Scholar] [CrossRef]
- Letten, A.D.; Ashcroft, M.B.; Keith, D.A. The importance of temporal climate variability for spatial patterns in plant diversity. Ecography 2013, 36, 1341–1349. [Google Scholar] [CrossRef]
- Tang, R.; Li, S.; Lang, X.; Huang, X.; Su, J. Rare species contribute greater to ecosystem multifunctionality in a subtropical forest than common species due to their functional diversity. For. Ecol. Manag. 2023, 538, 120981. [Google Scholar] [CrossRef]
- Huang, X.; Sun, X.; Jiang, Y.; Xue, F.; Cui, M.; Zhao, S.; Kang, M. The Radial Growth of Picea wilsonii Was More Restricted by Precipitation Due to Climate Warming on Mt. Guandi, China. Forests 2021, 12, 1602. [Google Scholar] [CrossRef]
- Zhang, J.T.; Song, N.; Fan, L. Evaluation of nine distance-based measures of functional diversity applied to forest communities. Ann. For. Res. 2013, 56, 43–52. [Google Scholar] [CrossRef]
- Xu, M.; Ma, L.; Jia, Y.; Liu, M. Integrating the effects of latitude and altitude on the spatial differentiation of plant community diversity in a mountainous ecosystem in China. PLoS ONE 2017, 12, e0174231. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Nan, H. Abiotic and biotic drivers of species diversity in understory layers of cold temperate coniferous forests in North China. J. For. Res. 2019, 30, 2213–2225. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, Y. Distributional patterns of species diversity of main plant communities along altitudinal gradient in secondary forest region, Guandi Mountain, China. J. For. Res. 2006, 17, 111–115. [Google Scholar] [CrossRef]
- Zhou, X.; Fu, L.; Sharma, R.P. Generalized or general mixed-effect modelling of tree morality of Larix gmelinii subsp. principis-rupprechtii in Northern China. J. For. Res. 2021, 32, 2447–2458. (In Chinese) [Google Scholar] [CrossRef]
- Haq, S.M.; Calixto, E.S.; Rashid, I.; Srivastava, G.; Khuroo, A.A. Tree diversity, distribution and regeneration in major forest types along an extensive elevational gradient in Indian Himalaya: Implications for sustainable forest management. For. Ecol. Manag. 2022, 506, 119968. [Google Scholar] [CrossRef]
- Gairola, S.; Sharma, C.M.; Ghildiyal, S.K.; Suyal, S. Regeneration dynamics of dominant tree species along an altitudinal gradient in moist temperate valley slopes of the Garhwal Himalaya. J. For. Res. 2012, 23, 53–63. [Google Scholar] [CrossRef]
- Haq, S.M.; Rashid, I.; Khuroo, A.A. Anthropogenic disturbances alter community structure in the forests of Kashmir Himalaya. Trop. Ecol. 2019, 60, 6–15. [Google Scholar] [CrossRef]
- Sagar, R.; Raghubanshi, A.; Singh, S. Tree species composition, dispersion and diversity along a disturbance gradient in a dry tropical forest region of India. For. Ecol. Manag. 2003, 186, 61–71. [Google Scholar] [CrossRef]
- Yao, Z.; Shi, L.; He, Y. Grazing intensity, duration, and grassland type determine the relationship between soil microbial diversity and ecosystem multifunctionality in Chinese grasslands: A meta-analysis. Ecol. Indic. 2023, 154, 110801. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, J.; Wu, Y. The Influence of Tourism on the Plant Diversity of the Eight Streams of Guandi Mountain. S. China Agric. 2018, 12, 92–95. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, M.; Kang, X.; Meng, J.; Zhang, L. Distribution patterns and associations of dominant tree species in a mixed coniferous-broadleaf forest in the Changbai Mountains. J. Mt. Sci. 2015, 12, 659–670. [Google Scholar] [CrossRef]
- Zhang, M. Spatial association and optimum adjacent distribution of trees in a mixed coniferous-broadleaf forest in northeastern China. Appl. Ecol. Environ. Res. 2017, 15, 1551–1564. [Google Scholar] [CrossRef]
- Sajad, S.; Haq, S.M.; Yaqoob, U.; Calixto, E.S.; Hassan, M. Tree composition and standing biomass in forests of the northern part of Kashmir Himalaya. Vegetos 2021, 34, 857–866. [Google Scholar] [CrossRef]
- Kim, T.K. Understanding one-way ANOVA using conceptual figures. Korean J. Anesthesiol. 2017, 70, 22–26. [Google Scholar] [CrossRef] [PubMed]
- R Core Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 20 July 2022).
- Van Den Wollenberg, A.L. Redundancy analysis an alternative for canonical correlation analysis. Psychometrika 1977, 42, 207–219. [Google Scholar] [CrossRef]
- Ewald, J. The influence of coniferous canopies on understorey vegetation and soils in mountain forests of the northern Calcareous Alps. Appl. Veg. Sci. 2000, 3, 123–134. [Google Scholar] [CrossRef]
- Franklin, J.; Andrade, R.; Daniels, M.L. Geographical ecology of dry forest tree communities in the West Indies. J. Biogeogr. 2018, 45, 1168–1181. [Google Scholar] [CrossRef]
- Jin, Y.; Li, J.; Liu, C. Precipitation reduction alters herbaceous community structure and composition in a savanna. J. Veg. Sci. 2019, 30, 821–831. [Google Scholar] [CrossRef]
- Utaile, Y.U.; Helsen, K.; Aydagnehum, S.G. Typology of the woody plant communities of the Ethiopian Nech Sar National Park and an assessment of vegetation-environment relations and human disturbance impacts. Plant Ecol. Evol. 2020, 153, 33–44. [Google Scholar] [CrossRef]
- Ni, X.; Guo, W.; Liu, T. Long-Term effects of altered precipitation patterns on alpine vegetation species composition on the Qinghai-Tibet Plateau. Forests 2022, 14, 47. [Google Scholar] [CrossRef]
- Ming, A.; Yang, Y.; Liu, S. A decade of close-to-nature transformation alters species composition and increases plant community diversity in two coniferous plantations. Front. Plant Sci. 2020, 11, 1141. [Google Scholar] [CrossRef]
- Muche, M.; Molla, E.; Rewald, B.; Tsegay, B.A. Diversity and composition of farm plantation tree/shrub species along altitudinal gradients in North-eastern Ethiopia: Implication for conservation. Heliyon 2022, 8, e09048. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, J.; Gao, H.; Xu, W.; Dong, M.; Shen, G.; Zhou, X. Interspecific plant competition increases soil labile organic carbon and nitrogen contents. For. Ecol. Manag. 2020, 462, 117991. [Google Scholar] [CrossRef]
- Huang, W.; Pohjonen, V.; Johansson, S.; Nashanda, M.; Katigula, M.I.L.; Luukkanen, O. Species diversity, forest structure and species composition in Tanzanian tropical forests. For. Ecol. Manag. 2003, 173, 11–24. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, W.; Wu, N. Shrub island effects on a high-altitude forest cutover in the eastern Tibetan Plateau. Ann. For. Sci. 2011, 68, 1127. [Google Scholar] [CrossRef][Green Version]
- Liu, B. Vertical patterns in plant diversity and their relations with environmental factors on the southern slope of the Tianshan Mountains (middle section) in Xinjiang (China). J. Mt. Sci. 2017, 14, 742–757. [Google Scholar] [CrossRef]
- Wen, B.; Zhang, X.; Yang, Z. Influence of tourist disturbance on soil properties, plant communities, and surface water quality in the Tianchi scenic area of Xinjiang, China. J. Arid Land 2016, 8, 304–313. [Google Scholar] [CrossRef]
- Cui, W.; Zheng, X.X. Spatial heterogeneity in tree diversity and forest structure of evergreen broadleaf forests in Southern China along an altitudinal gradient. Forests 2016, 7, 216. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, H.; Qu, M.; Yang, S.; Wang, W.; Zhao, W. The interactions among herbaceous diversity, edaphic factors, and topography under typical afforestation in the transition zone between the Qinghai–Tibet Plateau and Loess Plateau. J. Plant Interact. 2021, 16, 75–82. [Google Scholar] [CrossRef]
- Chen, T.G.; Zhang, J.T.; Shangguan, T.L.; Zhang, F.; Wang, Q.X. The study of diversity in Shenweigou of Guandi Mountain, Shanxi Province. Acta Bot. Boreali-Occident. Sin. 2000, 20, 638–646. (In Chinese) [Google Scholar] [CrossRef]
- McCain, C.M. Could temperature and water availability drive elevational species richness patterns? A global case study for bats. Glob. Ecol. Biogeogr. 2007, 16, 1–13. [Google Scholar] [CrossRef]
- Khumbongmayum, A.D.; Khan, M.L.; Tripathi, R.S. Survival and growth of seedlings of a few tree species in the four sacred groves of Manipur, Northeast India. Curr. Sci. 2005, 88, 1781–1788. [Google Scholar] [CrossRef]
- Rahman, I.U.; Afzal, A.; Iqbal, Z.; Bussmann, R.W.; Alsamadany, H.; Calixto, E.S.; Ijaz, F. Ecological gradients hosting plant communities in Himalayan subalpine pastures: Application of multivariate approaches to identify indicator species. Ecol. Inform. 2020, 60, 101162. [Google Scholar] [CrossRef]
- Jantz, P.; Goetz, S.; Laporte, N. Carbon stock corridors to mitigate climate change and promote biodiversity in the tropics. Nature Clim. Chang. 2014, 4, 138–142. [Google Scholar] [CrossRef]
- Rawat, D.S.; Dash, S.S.; Sinha, B.K.; Kumar, V.; Banerjee, A.; Singh, P. Community structure and regeneration status of tree species in Eastern Himalaya: A case study from Neora Valley National Park, West Bengal, India. Taiwania 2018, 63, 16–24. [Google Scholar] [CrossRef]
- Gebeyehu, G.; Soromessa, T.; Bekele, T.; Teketay, D. Species composition, stand structure, and regeneration status of tree species in dry Afromontane forests of Awi Zone, northwestern Ethiopia. Ecosyst. Health Sustain. 2019, 5, 199–215. [Google Scholar] [CrossRef]
- Wang, X.; Ye, J.; Li, B.; Zhang, J.; Lin, F.; Hao, Z. Spatial distributions of species in an old-growth temperate forest, northeastern China. Can. J. For. Res. 2010, 40, 1011–1019. [Google Scholar] [CrossRef]
- Kang, X.; Yang, H.; Guo, Z.; Xu, M.; Zhao, H. Species interactions in spruce–fir mixed stands and implications for enrichment planting in the Changbai Mountains, China. Mt. Res. Dev. 2012, 32, 187–196. [Google Scholar] [CrossRef]
- Bharathi, S.; Devi Prasad, A.G. Diversity, population structure and regeneration status of arboreal species in the four sacred groves of Kushalnagar, Karnataka. J. For. Res. 2017, 28, 357–370. [Google Scholar] [CrossRef]
- Malik, Z.A.; Bhatt, A.B. Regeneration status of tree species and survival of their seedlings in Kedarnath Wildlife Sanctuary and its adjoining areas in Western Himalaya, India. Trop. Ecol. 2016, 57, 677–690. [Google Scholar]
- Moktan, M.R.; Gratzer, G.; Richards, W.H. Regeneration of mixed conifer forests under group tree selection harvest management in western Bhutan Himalayas. For. Ecol. Manag. 2009, 257, 2121–2132. [Google Scholar] [CrossRef]
- Lorimer, C.G.; Chapman, J.W.; Lambert, W.D. Tall understorey vegetation as a factor in the poor development of oak seedlings beneath mature stands. J. Ecol. 1994, 82, 227. [Google Scholar] [CrossRef]
- Sagar, R.; Singh, J.S. Structure, diversity, and regeneration of tropical dry deciduous forest of northern India. Biodivers. Conserv. 2005, 14, 935–959. [Google Scholar] [CrossRef]
- Vetaas, O.R. The effect of environmental factors on the regeneration of Quercus semecarpifolia Sm. in central Himalaya, Nepal. Plant Ecol. 2000, 146, 137–144. [Google Scholar] [CrossRef]
- Belden, A.C.; Pallardy, S.G. Successional trends and apparent Acer saccharum regeneration failure in an oak-hickory forest in central Missouri, USA. Plant Ecol. 2009, 204, 305–322. [Google Scholar] [CrossRef]
- Schipper, A.M.; Posthuma, L.; de Zwart, D.; Huijbregts, M.A.J. Deriving field-based species sensitivity distributions (f-SSDs) from stacked species distribution models (S-SDMs). Environ. Sci. Technol. 2014, 48, 14464–14471. [Google Scholar] [CrossRef] [PubMed]
- Gotore, T.; Ndagurwa, H.G.T.; Kativu, S. Woody plant assemblage and the structure of miombo woodland along a disturbance gradient in Hurungwe, Zambezi Valley, Zimbabwe. J. For. Res. 2021, 32, 1867–1877. [Google Scholar] [CrossRef]
- Pausas, J.G.; Austin, M.P. Patterns of plant species richness in relation to different environments: An appraisal. J. Veg. Sci. 2001, 12, 153–166. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, Y.H.; Dong, G. Altitudinal patterns of taxonomic, phylogenetic and functional diversity of forest communities in Mount Guandi, Shanxi, China. Chin. J. Plant Ecol. 2019, 43, 762–773. Available online: http://www.plant-ecology.com (accessed on 1 July 2023). (In Chinese).
- Qian, H.; Hao, Z.; Zhang, J. Phylogenetic structure and phylogenetic diversity of angiosperm assemblages in forests along an elevational gradient in Changbaishan, China. J. Plant Ecol. 2014, 7, 154–165. [Google Scholar] [CrossRef]
- Sanchez, M.; Pedroni, F.; Eisenlohr, P.V.; Oliveira-Filho, A.T. Changes in tree community composition and structure of Atlantic rain forest on a slope of the Serra do Mar range, southeastern Brazil, from near sea level to 1000 m of altitude. Flora 2013, 208, 184–196. [Google Scholar] [CrossRef]
- Myers-Smith, I.H.; Hik, D.S. Climate warming as a driver of tundra shrubline advance. J. Ecol. 2018, 106, 547–560. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.M.; Ilyas, M.; Alqarawi, A.A.; Ahmad, Z.; Abd_Allah, E.F. Plant species and communities assessment in interaction with edaphic and topographic factors; an ecological study of the mount Eelum District Swat, Pakistan. Saudi J. Biol. Sci. 2017, 24, 778–786. [Google Scholar] [CrossRef]
- Silva Pedro, M.; Rammer, W.; Seidl, R. A disturbance-induced increase in tree species diversity facilitates forest productivity. Landsc. Ecol. 2016, 31, 989–1004. [Google Scholar] [CrossRef]
- Oldfield, C.; Peterson, C. Woody species composition, diversity, and recovery six years after wind disturbance and salvage logging of a southern Appalachian forest. Forests 2019, 10, 129. [Google Scholar] [CrossRef]
- Viljur, M.; Abella, S.R.; Adámek, M. The effect of natural disturbances on forest biodiversity: An ecological synthesis. Biol. Rev. 2022, 97, 1930–1947. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Zhao, F.Q. Impact of logging intensity on the spatial distribution and association of dominant tree species in a broadleaved Korean pine mixed forest. Acta Ecol. Sin. 2015, 35, 0046–0055. (In Chinese) [Google Scholar] [CrossRef][Green Version]
- Ding, Y.; Zang, R.; Lu, X.; Huang, J. The Impacts of selective logging and clear-cutting on woody plant diversity after years of natural recovery in a tropical montane rain forest, South China. Sci. Total Environ. 2017, 579, 1683–1691. [Google Scholar] [CrossRef]
- Xue, Y.; Cheng, A.; Li, S. The influence of environmental and species diversity on the survival of shrubs within subtropical forests. Biodiversity 2023, 31, 23–33. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, B.; Zhang, X. Grazing alters the relationships between species diversity and biomass during community succession in a semiarid grassland. Sci. Total Environ. 2023, 887, 164155. [Google Scholar] [CrossRef]

| Interference Intensity | Interference Level | Interference Category | ||
|---|---|---|---|---|
| Cutting | Grazing | Traveling | ||
| No/Low | 0 | Relative pile density < 0.1 | Livestock carrying capacity 0.5 to 1.5 sheep·ha−1·a−1 | Traces of plant loss are identified after trampling by tourists; there are dense bare patches within the transect, with widespread livestock feces visible; there are many types and quantities of garbage. |
| Moderate | 1 | 0.1 ≤ Relative pile density ≤ 0.2 | Livestock carrying capacity 1.5 to 2.5 sheep·ha−1·a−1 | The density of trampling marks decreases and is nearly nonexistent; scattered bare spots within the sample strip are not very noticeable, with occasional occurrences of livestock feces; there is a relatively small amount of garbage. |
| High | 2 | Relative pile density > 0.2 | Livestock carrying capacity 2.5 to 4.5 sheep·ha−1·a−1 | No obvious bare spots, livestock feces are hardly visible, no tourist garbage. |
| Altitude Gradient/m | Plot /NO. | Forest Stand Factor | Terrain Factor | Climate Factor | Interference Factor | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relative Stand Density | Relative Canopy Density | Average Height/m | Average DBH/cm | Aspect/° | Slope | MAT/°C | MAP/mm | Cutting | Grazing | Traveling | ||
| LA (1850–1950) | L1 | 0.16 | 0.11 | 24.11 | 24.63 | 180 | 0.23 | 4.1 | 674 | 1 | 1 | 0 |
| L2 | 0.13 | 0.12 | 25.31 | 27.05 | 180 | 0.24 | 4.1 | 674 | 1 | 1 | 1 | |
| L3 | 0.14 | 0.11 | 19.56 | 23.44 | 181 | 0.24 | 4.2 | 674 | 0 | 1 | 0 | |
| MA (2050–2150) | M4 | 0.10 | 0.11 | 21.79 | 26.41 | 298 | 0.35 | 4.1 | 670 | 2 | 0 | 1 |
| M5 | 0.08 | 0.12 | 24.02 | 29.49 | 226 | 0.35 | 4.1 | 669 | 1 | 0 | 1 | |
| M6 | 0.11 | 0.11 | 18.04 | 25.07 | 312 | 0.35 | 4.1 | 669 | 1 | 0 | 1 | |
| HA (2250–2350) | H7 | 0.09 | 0.11 | 15.24 | 24.31 | 230 | 0.26 | 5.6 | 662 | 2 | 1 | 1 |
| H8 | 0.10 | 0.10 | 17.82 | 23.14 | 90 | 0.30 | 5.5 | 659 | 2 | 1 | 2 | |
| H9 | 0.10 | 0.10 | 15.60 | 20.36 | 300 | 0.26 | 5.5 | 660 | 2 | 1 | 2 | |
| Altitude Gradient/m | Plot/NO. | SED/ (Plant·ha) | SAD/ (Plant·ha) | Se/At | Sa/At | TBC/ (m2·ha) | TDN/ (Plant·ha) |
|---|---|---|---|---|---|---|---|
| LA (1850–1950) | L1 | 20 | 88 | 0.02 | 0.10 | 30.13 | 844 |
| L2 | 12 | 48 | 0.02 | 0.07 | 33.13 | 732 | |
| L3 | 56 | 92 | 0.09 | 0.14 | 24.58 | 656 | |
| MA (2050–2150) | M4 | 92 | 44 | 0.20 | 0.10 | 25.15 | 460 |
| M5 | 28 | 52 | 0.08 | 0.14 | 28.40 | 368 | |
| M6 | 52 | 104 | 0.11 | 0.22 | 20.73 | 472 | |
| HA (2250–2350) | H7 | 116 | 36 | 0.32 | 0.10 | 16.86 | 364 |
| H8 | 96 | 36 | 0.21 | 0.08 | 17.51 | 456 | |
| H9 | 240 | 32 | 0.69 | 0.09 | 19.90 | 348 |
| Altitude Gradient/m | Species | SED/ (Plant·ha) | SAD/ (Plant·ha) | TDN/ (Plant·ha) | Se/At | Sa/At |
|---|---|---|---|---|---|---|
| LA (1850–1950) | Picea asperata | 8 | 132 | 1796 | 0.004 | 0.07 |
| Larix principis-rupprechtii | 4 | 8 | 400 | 0.01 | 0.02 | |
| Betula platyphylla | 4 | 16 | 20 | 0.20 | 0.80 | |
| Quercus wutaishansea | 24 | 32 | 8 | 3 | 4 | |
| MA (2050–2150) | Picea asperata | 48 | 32 | 1080 | 0.04 | 0.03 |
| Larix principis-rupprechtii | 8 | 8 | 116 | 0.07 | 0.07 | |
| Betula albosinensis | 116 | 156 | 52 | 2.23 | 3 | |
| HA (2250–2350) | Picea asperata | 436 | 100 | 736 | 0.59 | 0.136 |
| Larix principis-rupprechtii | 16 | 4 | 408 | 0.04 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Liu, Z.; Yang, Z.; Shen, H.; Wang, J.; Wu, X. Altitudinal Variation in Species Diversity, Distribution, and Regeneration Status of a Secondary Picea Forest in Guandi Mountain, Northern China. Forests 2024, 15, 771. https://doi.org/10.3390/f15050771
Zhang M, Liu Z, Yang Z, Shen H, Wang J, Wu X. Altitudinal Variation in Species Diversity, Distribution, and Regeneration Status of a Secondary Picea Forest in Guandi Mountain, Northern China. Forests. 2024; 15(5):771. https://doi.org/10.3390/f15050771
Chicago/Turabian StyleZhang, Mengtao, Zhuoyue Liu, Zhaohui Yang, Huanhuan Shen, Jin Wang, and Xiaogang Wu. 2024. "Altitudinal Variation in Species Diversity, Distribution, and Regeneration Status of a Secondary Picea Forest in Guandi Mountain, Northern China" Forests 15, no. 5: 771. https://doi.org/10.3390/f15050771
APA StyleZhang, M., Liu, Z., Yang, Z., Shen, H., Wang, J., & Wu, X. (2024). Altitudinal Variation in Species Diversity, Distribution, and Regeneration Status of a Secondary Picea Forest in Guandi Mountain, Northern China. Forests, 15(5), 771. https://doi.org/10.3390/f15050771








