Effects of Nitrate Assimilation in Leaves and Roots on Biomass Allocation and Drought Stress Responses in Poplar Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Gas Exchange and Chlorophyll Fluorescence Measurements
2.3. Chlorophyll Content, Microanatomy, and Nitrogen and Carbon Content
2.4. Malondialdehyde (MDA), Nitrate Reductase Activity (NR) and Free Proline
2.5. Biomass Allocation, Soluble Sugar and Starch
2.6. Statistical Analysis
3. Results
3.1. Growth and Biomass Allocation Changes under Water and Nitrogen Availabilities
3.2. Nitrogen Assimilation and Leaf Nitrogen Status
3.3. Leaf Ecophysiological Traits
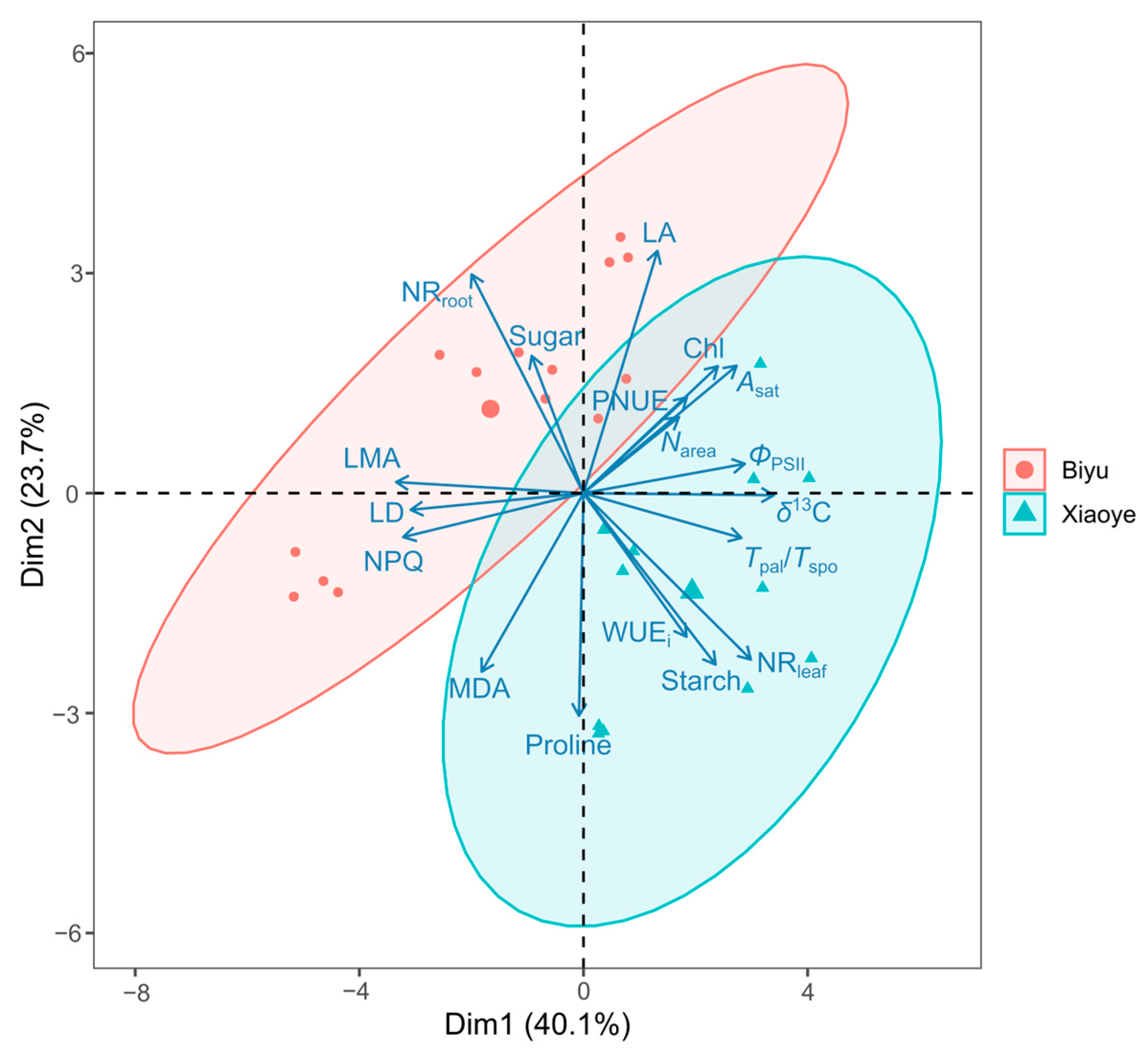
3.4. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weiner, J. Allocation, plasticity and allometry in plants. Perspect. Plant Ecol. Evol. Syst. 2004, 6, 207–215. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Yang, X.; Gao, R.; Hou, X.; Huo, L.; Huang, Z.; Cornelissen, J.H.C. Allometry rather than abiotic drivers explains biomass allocation among leaves, stems and roots of Artemisia across a large environmental gradient in China. J. Ecol. 2021, 109, 1026–1040. [Google Scholar] [CrossRef]
- Ågren, G.I.; Ingestad, T. Root: Shoot ratio as a balance between nitrogen productivity and photosynthesis. Plant Cell Environ. 1987, 10, 579–586. [Google Scholar] [CrossRef]
- Feng, H.; Guo, J.; Peng, C.; Kneeshaw, D.; Roberge, G.; Pan, C.; Ma, X.; Zhou, D.; Wang, W. Nitrogen addition promotes terrestrial plants to allocate more biomass to aboveground organs: A global meta-analysis. Global Chang. Biol. 2023, 29, 3970–3989. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Thornley, J.H.M. A balanced quantitative model for root: Shoot ratios in vegetative plants. Ann. Bot. 1972, 36, 431–441. [Google Scholar] [CrossRef]
- Müller, I.; Schmid, B.; Weiner, J. The effect of nutrient availability on biomass allocation patterns in 27 species of herbaceous plants. Perspect. Plant Ecol. Evol. Syst. 2000, 3, 115–127. [Google Scholar] [CrossRef]
- Coleman, J.S.; McConnaughay, K.D.M.; Ackerly, D.D. Interpreting phenotypic variation in plants. Trends Ecol. Evol. 1994, 9, 187–191. [Google Scholar] [CrossRef]
- Niklas, K.J.; Enquist, B.J. Canonical rules for plant organ biomass partitioning and annual allocation. Am. J. Bot. 2002, 89, 812–819. [Google Scholar] [CrossRef]
- Enquist, B.J.; Niklas, K.J. Global allocation rules for patterns of biomass partitioning in seed plants. Science 2002, 295, 1517–1520. [Google Scholar] [CrossRef]
- Smith-Martin, C.M.; Xu, X.; Medvigy, D.; Schnitzer, S.A.; Powers, J.S. Allometric scaling laws linking biomass and rooting depth vary across ontogeny and functional groups in tropical dry forest lianas and trees. New Phytol. 2019, 226, 639–640. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Jagodzinski, A.M.; Ruiz-Peinado, R.; Kuyah, S.; Luo, Y.; Oleksyn, J.; Usoltsev, V.A.; Buckley, T.N.; Reich, P.B.; Sack, L. How does biomass distribution change with size and differ among species? An analysis for 1200 plant species from five continents. New Phytol. 2015, 208, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D. OPT-ing out: Root–shoot dynamics are caused by local resource capture and biomass allocation, not optimal partitioning. Plant Cell Environ. 2023, 46, 3023–3039. [Google Scholar] [CrossRef] [PubMed]
- Puglielli, G.; Laanisto, L.; Poorter, H.; Niinemets, Ü. Global patterns of biomass allocation in woody species with different tolerances of shade and drought: Evidence for multiple strategies. New Phytol. 2021, 229, 308–322. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Zhao, Y.; Wang, W.; Gao, T.; Zong, Y. Response of drought on water and nitrogen utilization and carbohydrate distribution of Populus × euramericana ‘Biyu’ cuttings. Arid Zone Res. 2022, 39, 893–899. [Google Scholar]
- Gao, T.; Shang, J.; Song, L.; Wang, W. Responses of leaf photosynthetic and anatomical characteristics in Populus simonii cuttings to drought and re-watering. Sci. Soil Water Conserv. 2021, 19, 18–26. [Google Scholar]
- Fahrenkrog, A.M.; Neves, L.G.; Resende Jr, M.F.R.; Dervinis, C.; Davenport, R.; Barbazuk, W.B.; Kirst, M. Population genomics of the eastern cottonwood (Populus deltoides). Ecol. Evol. 2017, 7, 9426–9440. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.Z.; Zhao, X.; Pan, W.; Zhang, J.F.; Li, B.L.; Zhang, D.Q. Phenotypic variation among five provenances of Populus simonii in northern China. For. Stud. China 2011, 13, 97–103. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Minocha, R.; Martinez, G.; Lyons, B.; Long, S. Development of a standardized methodology for quantifying total chlorophyll and carotenoids from foliage of hardwood and conifer tree species. Can. J. For. Res. 2009, 39, 849–861. [Google Scholar] [CrossRef]
- Sáez-Plaza, P.; Navas, M.J.; Wybraniec, S.; Michałowski, T.; Asuero, A.G. An overview of the kjeldahl method of nitrogen determination. Part II. sample preparation, working scale, instrumental finish, and quality control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Landhäusser, S.M.; Chow, P.S.; Dickman, L.T.; Furze, M.E.; Kuhlman, I.; Schmid, S.; Wiesenbauer, J.; Wild, B.; Gleixner, G.; Hartmann, H.; et al. Standardized protocols and procedures can precisely and accurately quantify non-structural carbohydrates. Tree Physiol. 2018, 38, 1764–1778. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org (accessed on 16 June 2023).
- Weiner, J.; Du, Y.-L.; Zhao, Y.-M.; Li, F.-M. Allometry and yield stability of cereals. Front. Plant Sci. 2021, 12, 681490. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.; Raven, J.A. Root or shoot nitrate assimilation in terrestrial vascular plants—Does it matter? Plant Soil 2022, 476, 31–62. [Google Scholar] [CrossRef]
- Rennenberg, H.; Wildhagen, H.; Ehlting, B. Nitrogen nutrition of poplar trees. Plant Biol. 2010, 12, 275–291. [Google Scholar] [CrossRef]
- Miller, A.J.; Cramer, M.D. Root nitrogen acquisition and assimilation. Plant Soil 2005, 274, 1–36. [Google Scholar] [CrossRef]
- Andrews, M. The partitioning of nitrate assimilation between root and shoot of higher plants. Plant Cell Environ. 1986, 9, 511–519. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Campbell, W.H. Nitrate reductase structure, function and regulation: Bridging the gap between biochemistry and physiology. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 277–303. [Google Scholar] [CrossRef]
- Shi, H.; Ma, W.; Song, J.; Lu, M.; Rahman, S.U.; Bui, T.T.X.; Vu, D.D.; Zheng, H.; Wang, J.; Zhang, Y. Physiological and transcriptional responses of Catalpa bungei to drought stress under sufficient- and deficient-nitrogen conditions. Tree Physiol. 2017, 37, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, E.E.; Huber, S.C. Regulation of photosynthesis by end-product accumulation in leaves of plants storing starch, sucrose, and hexose sugars. Plant Physiol. 1992, 99, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
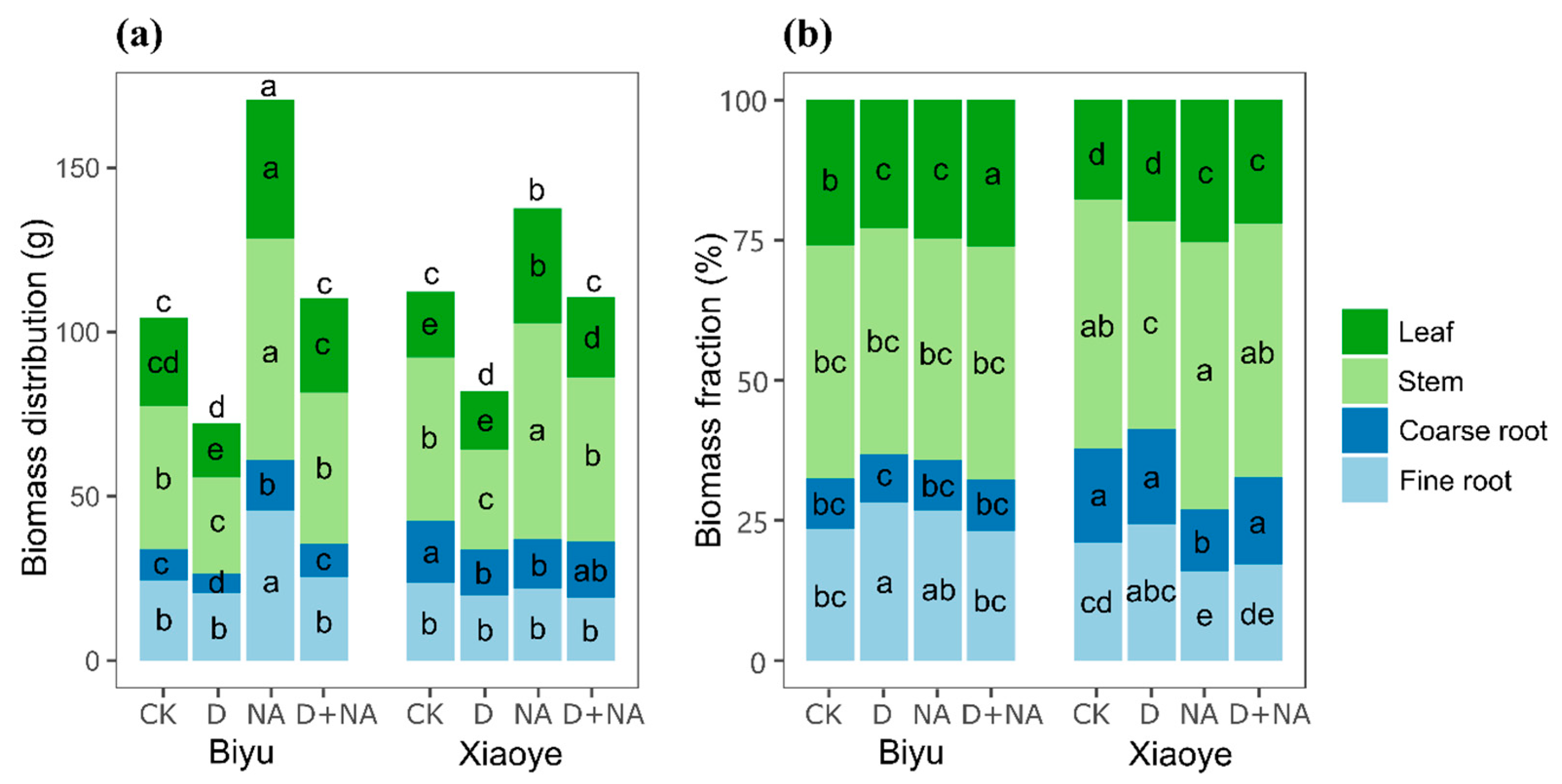
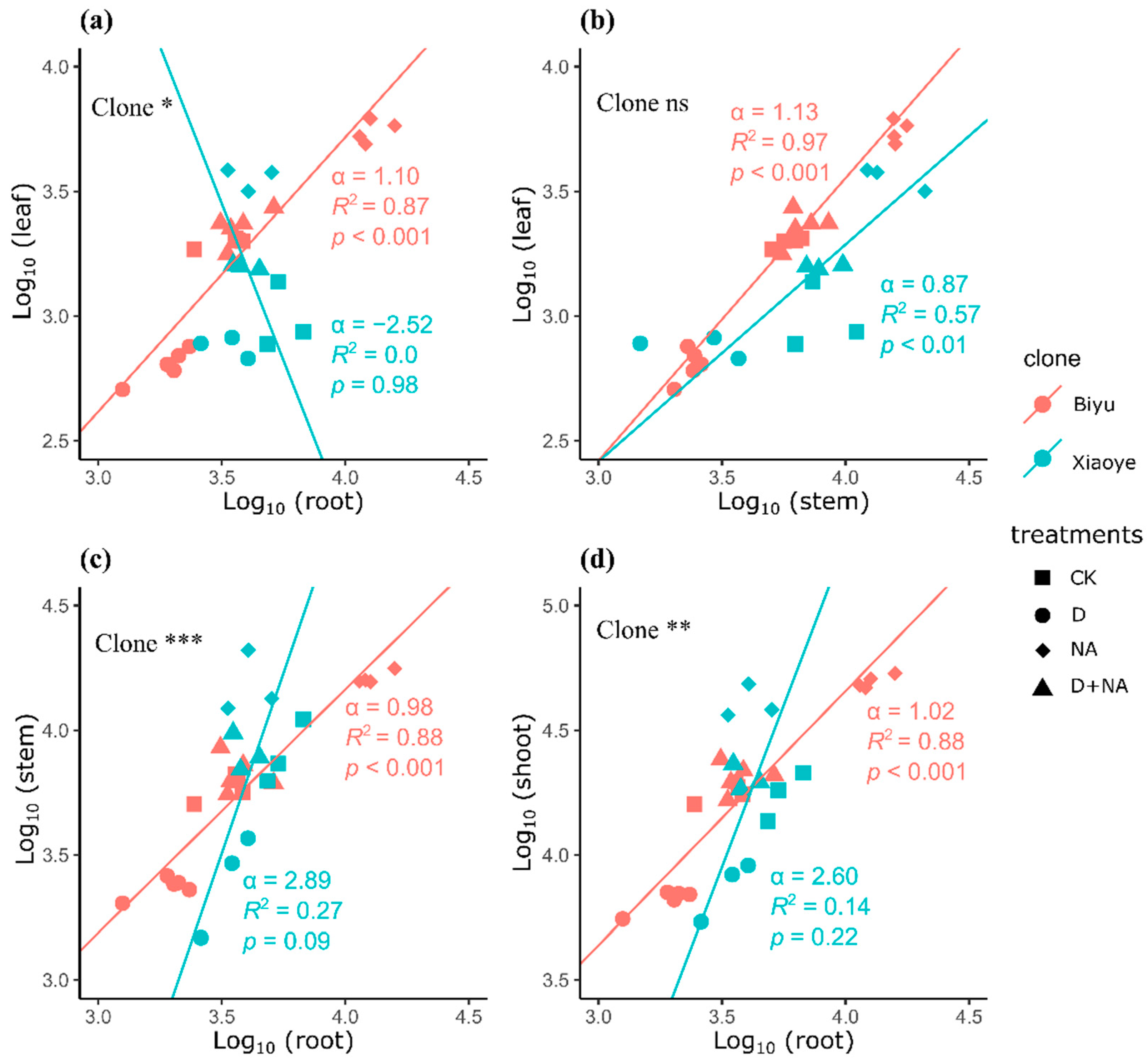
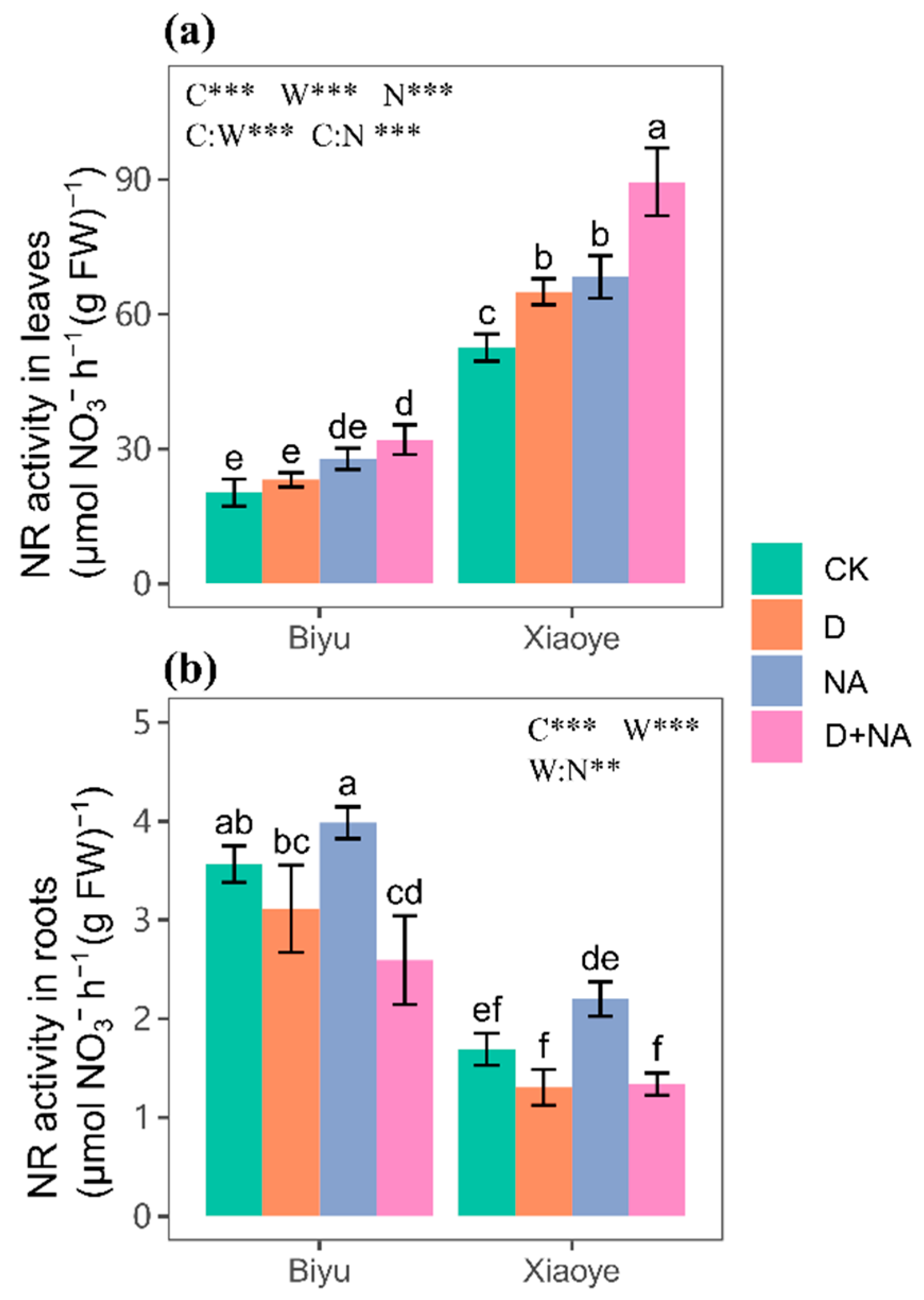
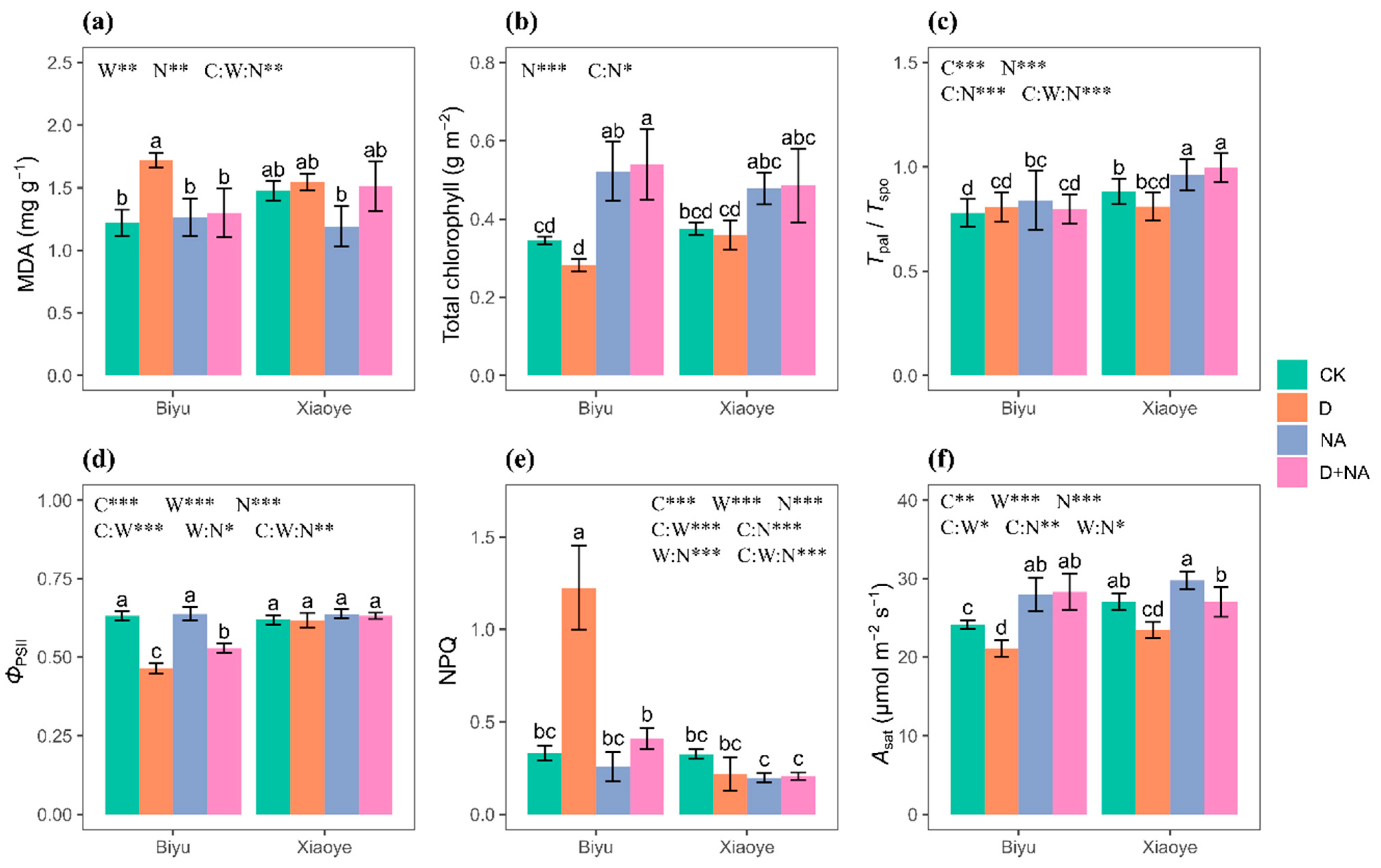


| Biyu | Xiaoye | Results from Analysis of Variance | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CK | D | NA | D + NA | CK | D | NA | D + NA | C | W | N | C:W | C:N | W:N | C:W:N | |
| LA (cm2) | 151 ± 9 b | 69 ± 6 e | 178 ± 6 a | 117 ± 8 c | 124 ± 9 c | 64 ± 4 e | 159 ± 9 b | 87 ± 6 d | *** | *** | *** | 0.34 | * | 0.12 | *** |
| LN (mg g−1) | 11.9 ± 0.7 d | 12.4 ± 0.6 d | 16.8 ± 0.7 bc | 19.3 ± 1.3 a | 15.0 ± 0.8 c | 15.5 ± 0.7 c | 18.9 ± 0.9 ab | 19.6 ± 0.6 a | *** | *** | ** | 0.15 | ** | 0.07 | 0.18 |
| Narea (g m−2) | 0.89 ± 0.06 c | 0.89 ± 0.04 c | 1.05 ± 0.05 b | 1.18 ± 0.08 a | 0.95 ± 0.05 bc | 1.01 ± 0.04 bc | 1.04 ± 0.05 b | 1.02 ± 0.03 bc | 0.95 | * | *** | 0.19 | *** | 0.26 | * |
| C/N | 32 ± 5 a | 28 ± 3 ab | 23 ± 2 ab | 20 ± 0.6 b | 26 ± 2 ab | 24 ± 2 ab | 22 ± 10 ab | 21 ± 2 b | 0.08 | 0.11 | *** | 0.44 | 0.14 | 0.99 | 0.97 |
| LMA (g m−2) | 74.4 ± 3 a | 71.5 ± 2 b | 62.6 ± 3 d | 61.4 ± 3 de | 63.6 ± 1 c | 64.8 ± 2 b | 55.1 ± 3 e | 52.1 ± 5 e | *** | 0.18 | *** | 0.62 | 0.90 | 0.76 | 0.20 |
| LD (g cm−3) | 0.30 ± 0.01 a | 0.30 ± 0.01 a | 0.26 ± 0.02 bc | 0.27 ± 0.01 bc | 0.29 ± 0.01 ab | 0.28 ± 0.01 ab | 0.26 ± 0.02 bc | 0.24 ± 0.02 c | ** | 0.18 | *** | 0.15 | 0.94 | 0.94 | 0.75 |
| qP | 0.86 ± 0.02 a | 0.71 ± 0.00 c | 0.85 ± 0.02 a | 0.69 ± 0.01 c | 0.81 ± 0.02 b | 0.82 ± 0.02 b | 0.84 ± 0.03 ab | 0.83 ± 0.01 ab | *** | *** | 0.27 | *** | ** | 0.24 | 0.96 |
| ETR (μmol e m−2 s−1) | 74 ± 2 a | 54 ± 2 c | 75 ± 3 a | 62 ± 2 b | 72 ± 2 a | 73 ± 1 a | 74 ± 2 a | 74 ± 1 a | *** | *** | *** | *** | * | * | *** |
| Free Proline (mg g−1) | 42 ± 0.9 d | 52 ± 2 bc | 40 ± 2 d | 51 ± 4 bc | 45 ± 3 cd | 66 ± 4 a | 45 ± 4 cd | 54 ± 2 b | *** | *** | ** | 0.06 | 0.06 | * | * |
| PNUE (μmol g−1 s−1) | 27.4 ± 2 a | 23.8 ± 1 b | 26.6 ± 1 ab | 24.0 ± 2 b | 28.4 ± 1 a | 23.3 ± 1 b | 28.6 ± 1 a | 26.5 ± 1 ab | * | *** | 0.26 | 0.65 | 0.07 | 0.09 | 0.35 |
| WUEi (μmol mmol−1) | 3.39 ± 0.26 cd | 3.16 ± 0.21 d | 3.30 ± 0.22 cd | 3.35 ± 0.21 cd | 4.32 ± 0.34 a | 4.49 ± 0.42 a | 4.15 ± 0.18 ab | 3.76 ± 0.25 bc | *** | 0.16 | * | 0.74 | ** | 0.37 | ** |
| δ13C (%) | −30.5 ± 0.2 cd | −30.8 ± 0.4 d | −30.0 ± 0.4 abc | −29.9 ± 0.4 abc | −30.3 ± 0.3 bcd | −29.9 ± 0.4 abc | −29.3 ± 0.3 ab | −29.3 ± 0.2 a | *** | 0.84 | *** | 0.27 | 0.94 | 0.72 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Shang, J.; Ræbild, A.; Gao, T.; Xie, Q. Effects of Nitrate Assimilation in Leaves and Roots on Biomass Allocation and Drought Stress Responses in Poplar Seedlings. Forests 2024, 15, 779. https://doi.org/10.3390/f15050779
Wang W, Shang J, Ræbild A, Gao T, Xie Q. Effects of Nitrate Assimilation in Leaves and Roots on Biomass Allocation and Drought Stress Responses in Poplar Seedlings. Forests. 2024; 15(5):779. https://doi.org/10.3390/f15050779
Chicago/Turabian StyleWang, Weifeng, Jiazhou Shang, Anders Ræbild, Tianhui Gao, and Qihao Xie. 2024. "Effects of Nitrate Assimilation in Leaves and Roots on Biomass Allocation and Drought Stress Responses in Poplar Seedlings" Forests 15, no. 5: 779. https://doi.org/10.3390/f15050779
APA StyleWang, W., Shang, J., Ræbild, A., Gao, T., & Xie, Q. (2024). Effects of Nitrate Assimilation in Leaves and Roots on Biomass Allocation and Drought Stress Responses in Poplar Seedlings. Forests, 15(5), 779. https://doi.org/10.3390/f15050779





