Influence of Picea Abies Logs on the Distribution of Vascular Plants in Old-Growth Spruce Forests
Abstract
1. Introduction
- What is the size of the influence zone of a decaying fallen log on the ground vegetation structure?
- Is the homogeneity of vascular plant cover altered by the presence of deadwood?
- How do vascular plant species occurrence and shoot density change with a distance from decaying logs?
- Does (and how does) the homogeneity of vascular plant cover in the influence zone of deadwood change with an increased decay stage?
2. Materials and Methods
2.1. Experimental Sites Description
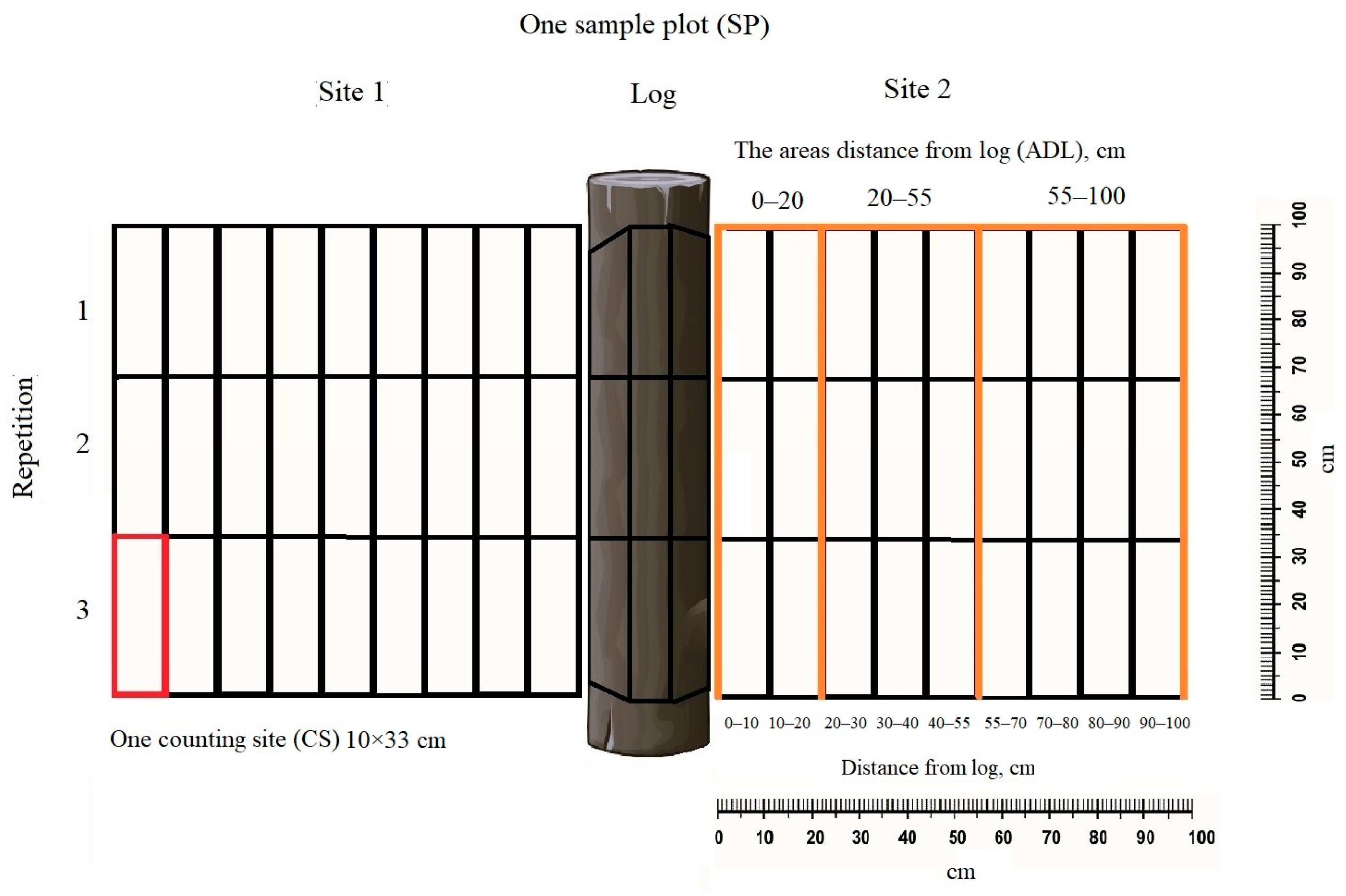
2.2. Data Collection
2.3. Data Analysis
- 0—indicates that the species is absent,
- 1—the presence of single individuals,
- 2—the rare occurrence with insignificant coverage (maximum 5%),
- 3—the rare occurrence with few individuals, but minimal coverage is 20%, or minimal occurrence is 20%, but maximal coverage is 7%.
- 4—the minimal occurrence is 20%, the projective coverage in the interval of 7%–20%.
- 5—the minimal occurrence is 50%, and the minimal projective coverage is 20%.
- 6—the minimal occurrence is 75%, and the minimal projective coverage is 30%.
3. Results
3.1. The Impact of P. abies Fallen Logs on the Horizontal Distribution Vascular Plants
3.2. The Impact of P. abies Fallen Logs on the Homogeneity of Vascular Plant Cover
3.3. Increasing the Log Decay Class Reduces the Heterogeneity of Vascular Plant Cover
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Mosseler, A.; Lynds, J.A.; Major, J.E. Old-Growth Forests of the Acadian Forest Region. Environ. Rev. 2003, 11, 47–77. [Google Scholar] [CrossRef]
- Puettmann, K.; Messier, C.; Coates, K. A Critique of Silviculture: Managing for Complexity; Bibliovault OAI Repository, The University of Chicago Press: Chicago, IL, USA, 2009. [Google Scholar]
- Storozhenko, V.G. Features of the Horizontal Structure of Forests of Spruce Formations in the European Taiga of Russia. Leśn. Zhurnal 2022, 2, 39–49. (In Russian) [Google Scholar] [CrossRef]
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.V.; Lattin, J.D.; Anderson, N.H.; Cline, S.P.; Aumen, N.G.; Sedell, J.R.; et al. Ecology of Coarse Woody Debris in Temperate Ecosystems. In Advances in Ecological Research; MacFadyen, A., Ford, E.D., Eds.; Academic Press: Orlando, FL, USA, 1986; pp. 133–302. [Google Scholar]
- Stokland, J.N.; Siitonen, J.; Jonsson, B.G. Biodiversity in Dead Wood; Cambridge University Press: Cambridge, UK, 2012; ISBN 9780521888738. [Google Scholar]
- Franklin, J.F.; Spies, T.A.; Van Pelt, R.; Carey, A.B.; Thornburgh, D.A.; Berg, D.R.; Lindenmayer, D.B.; Harmon, M.E.; Keeton, W.S.; Shaw, D.C.; et al. Disturbances and Structural Development of Natural Forest Ecosystems with Silvicultural Implications, Using Douglas-Fir Forests as an Example. For. Ecol. Manag. 2002, 155, 399–423. [Google Scholar] [CrossRef]
- Aakala, T. Temporal Variability of Deadwood Volume and Quality in Boreal Old-Growth Forests. Silva Fenn. 2011, 45, 81. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Global Forest Resources Assessment Update 2005: Terms and Definitions; Forest Resources Assessment Programme, Working Papers 83/E; FAO: Rome, Italy, 2004. [Google Scholar]
- Dittrich, S.; Jacob, M.; Bade, C.; Leuschner, C.; Hauck, M. The Significance of Deadwood for Total Bryophyte, Lichen, and Vascular Plant Diversity in an Old-Growth Spruce Forest. Plant Ecol. 2014, 215, 1123–1137. [Google Scholar] [CrossRef]
- Martin, M.; Fenton, N.J.; Morin, H. Tree-Related Microhabitats and Deadwood Dynamics Form a Diverse and Constantly Changing Mosaic of Habitats in Boreal Old-Growth Forests. Ecol. Indic. 2021, 128, 107813. [Google Scholar] [CrossRef]
- Larrieu, L.; Courbaud, B.; Drénou, C.; Goulard, M.; Bütler, R.; Kozák, D.; Kraus, D.; Krumm, F.; Lachat, T.; Müller, J.; et al. Perspectives: Key Factors Determining the Presence of Tree-Related Microhabitats: A Synthesis of Potential Factors at Site, Stand and Tree Scales, with Perspectives for Further Research. For. Ecol. Manag. 2022, 515, 120–235. [Google Scholar] [CrossRef]
- White, N. The Importance of Wood-Decay Fungi in Forest Ecosystems. In Fungal Biotechnology in Agricultural, Food and Environmental Applications; Marcel Dekker Inc.: New York, NY, USA, 2003. [Google Scholar]
- Ódor, P.; Heilmann-Clausen, J.; Christensen, M.; Aude, E.; van Dort, K.W.; Piltaver, A.; Siller, I.; Veerkamp, M.T.; Walleyn, R.; Standovár, T.; et al. Diversity of Dead Wood Inhabiting Fungi and Bryophytes in Semi-Natural Beech Forests in Europe. Biol. Conserv. 2006, 131, 58–71. [Google Scholar] [CrossRef]
- Baldrian, P.; Zrůstová, P.; Tláskal, V.; Davidová, A.; Merhautová, V.; Vrška, T. Fungi Associated with Decomposing Deadwood in a Natural Beech-Dominated Forest. Fungal Ecol. 2016, 23, 109–122. [Google Scholar] [CrossRef]
- Müller, M.M.; Varama, M.; Heinonen, J.; Hallaksela, A.-M. Influence of Insects on the Diversity of Fungi in Decaying Spruce Wood in Managed and Natural Forests. For. Ecol. Manag. 2002, 166, 165–181. [Google Scholar] [CrossRef]
- Déchêne, A.D.; Buddle, C.M. Decomposing Logs Increase Oribatid Mite Assemblage Diversity in Mixedwood Boreal Forest. Biodivers. Conserv. 2010, 19, 237–256. [Google Scholar] [CrossRef]
- Jonsson, B.; Kruys, N.; Ranius, T. Ecology of Species Living on Dead Wood—Lessons for Dead Wood Management. Silva Fenn. 2005, 39, 390. [Google Scholar] [CrossRef]
- Staniaszek-Kik, M.; Szczepanska, K. Epixylic Lichen Biota in the Polish Part of the Karkonosze Mts (West Sudety Mts). Fragm. Florist. Geobot. Pol. 2012, 19, 137–151. [Google Scholar]
- Staniaszek-kik, M.; Žarowiec, J.; Chmura, D. The Effect of Forest Management Practices on Deadwood Resources and Structure in Protected and Managed Montane Forests during Tree-Stand Reconstruction after Dieback of Norway Spruce. Balt. For. 2019, 25, 249–256. [Google Scholar] [CrossRef]
- Crites, S.; Dale, M.R. Diversity and Abundance of Bryophytes, Lichens, and Fungi in Relation to Woody Substrate and Successional Stage in Aspen Mixedwood Boreal Forests. Can. J. Bot. 1998, 76, 641–651. [Google Scholar] [CrossRef]
- Kumar, P.; Chen, H.Y.H.; Thomas, S.C.; Shahi, C. Effects of Coarse Woody Debris on Plant and Lichen Species Composition in Boreal Forests. J. Veg. Sci. 2017, 28, 389–400. [Google Scholar] [CrossRef]
- Kumar, P.; Chen, H.Y.H.; Thomas, S.C.; Shahi, C. Epixylic Vegetation Abundance, Diversity, and Composition Vary with Coarse Woody Debris Decay Class and Substrate Species in Boreal Forest. Can. J. For. Res. 2018, 48, 399–411. [Google Scholar] [CrossRef]
- Chećko, E.; Jaroszewicz, B.; Olejniczak, K.; Kwiatkowska-Falińska, A.J. The Importance of Coarse Woody Debris for Vascular Plants in Temperate Mixed Deciduous Forests. Can. J. For. Res. 2015, 45, 1154–1163. [Google Scholar] [CrossRef]
- Chmura, D.; Żarnowiec, J.; Staniaszek-Kik, M. Interactions between Plant Traits and Environmental Factors within and among Montane Forest Belts: A Study of Vascular Species Colonising Decaying Logs. For. Ecol. Manag. 2016, 379, 216–225. [Google Scholar] [CrossRef]
- Unar, P.; Daněk, P.; Adam, D.; Paločková, L.; Holík, J. Can Deadwood Be Preferred to Soil? Vascular Plants on Decaying Logs in Different Forest Types in Central Europe. Eur. J. For. Res. 2023, 143, 379–391. [Google Scholar] [CrossRef]
- Kuuluvainen, T. Gap Disturbance, Ground Microtopography, and the Regeneration Dynamics of Boreal Coniferous Forests in Finland: A Review. Ann. Zool. Fenn. 1994, 31, 35–51. [Google Scholar]
- Nakagawa, M.; Kurahashi, A.; Kaji, M.; Hogetsu, T. The Effects of Selection Cutting on Regeneration of Picea jezoensis and Abies sachalinensis in the Sub-Boreal Forests of Hokkaido, Northern Japan. For. Ecol. Manag. 2001, 146, 15–23. [Google Scholar] [CrossRef]
- Zielonka, T.; Piatek, G. Norway Spruce Regeneration on Decaying Logs in Subalpine Forests in the Tatra National Park. Pol. Bot. J. 2001, 46, 251–260. [Google Scholar]
- Mori, A.; Mizumachi, E.; Osono, T.; Doi, Y. Substrate-Associated Seedling Recruitment and Establishment of Major Conifer Species in an Old-Growth Subalpine Forest in Central Japan. For. Ecol. Manag. 2004, 196, 287–297. [Google Scholar] [CrossRef]
- Zielonka, T. When Does Dead Wood Turn into a Substrate for Spruce Replacement? J. Veg. Sci. 2006, 17, 739–746. [Google Scholar] [CrossRef]
- Bobkova, K.S.; Bessonov, I.M. Natural regeneration in the Middle Taiga Spruce Forests of the European Northeast. Rus. J. For. Sci. 2009, 5, 10–16. (In Russian) [Google Scholar]
- Bače, R.; Svoboda, M.; Pouska, V.; Janda, P.; Červenka, J. Natural Regeneration in Central-European Subalpine Spruce Forests: Which Logs Are Suitable for Seedling Recruitment? For. Ecol. Manag. 2012, 266, 254–262. [Google Scholar] [CrossRef]
- Cervenka, J.; Bače, R.; Svoboda, M. Stand-Replacing Disturbance Does Not Directly Alter the Succession of Norway Spruce Regeneration on Dead Wood. J. For. Sci. 2014, 60, 417–424. [Google Scholar] [CrossRef]
- Orman, O.; Adamus, M.; Szewczyk, J. Regeneration Processes on Coarse Woody Debris in Mixed Forests: Do Tree Germinants and Seedlings Have Species-specific Responses When Grown on Coarse Woody Debris? J. Ecol. 2016, 104, 1809–1818. [Google Scholar] [CrossRef]
- Baier, R.; Ettl, R.; Hahn, C.; Göttlein, A. Early Development and Nutrition of Norway Spruce (Picea abies (L.) Karst.) Seedlings on Different Seedbeds in the Bavarian Limestone Alps—A Bioassay. Ann. For. Sci. 2006, 63, 339–348. [Google Scholar] [CrossRef]
- Dyrenkov, S.A. Demographic Features of Norway Spruce and Siberian Spruce. In Population Ecology; INION: Moscow, Russia, 1988; pp. 216–217. (In Russian) [Google Scholar]
- Franklin, J.; Van Pelt, R. Spatial Aspects of Structural Complexity in Old-Growth Forests. J. For. 2004, 102, 22–27. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Wallenius, T.H.; Kauhanen, H.; Aakala, T.; Mikkola, K.; Demidova, N.; Ogibin, B. Episodic, Patchy Disturbances Characterize an Old-Growth Picea abies Dominated Forest Landscape in Northeastern Europe. For. Ecol. Manag. 2014, 320, 96–103. [Google Scholar] [CrossRef]
- Ulanova, N.G. The Effects of Windthrow on Forests at Different Spatial Scales: A Review. For. Ecol. Manag. 2000, 135, 155–167. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Kalmari, R. Regeneration Microsites of Picea abies Seedlings in a Windthrow Area of a Boreal Old-Growth Forest in Southern Finland. Ann. Bot. Fenn. 2004, 40, 401–413. [Google Scholar]
- Radyukina, A.Y. The Deadwood Effect On The Sod-Podzolic Soil Properties. Rus. J. For. Sci. 2004, 4, 51–60. (In Russian) [Google Scholar]
- Safonov, M.A.; Ostapenko, A.V.; Uvarova, A.I. The Ecotopes Specifics Formed by Woody Debris in the Southern Ural Forest Ecosystems. Mod. Prob. Sci. Educ. 2017, 6, 236. Available online: https://science-education.ru/en/article/view?id=27053&ysclid=lwc0121h3s622037121 (accessed on 15 May 2024). (In Russian).
- Błońska, E.; Kacprzyk, M.; Spólnik, A. Effect of Deadwood of Different Tree Species in Various Stages of Decomposition on Biochemical Soil Properties and Carbon Storage. Ecol. Res. 2017, 32, 193–203. [Google Scholar] [CrossRef]
- Peršoh, D.; Borken, W. Impact of Woody Debris of Different Tree Species on the Microbial Activity and Community of an Underlying Organic Horizon. Soil. Biol. Biochem. 2017, 115, 516–525. [Google Scholar] [CrossRef]
- Minnich, C.; Peršoh, D.; Poll, C.; Borken, W. Changes in Chemical and Microbial Soil Parameters Following 8 Years of Deadwood Decay: An Experiment with Logs of 13 Tree Species in 30 Forests. Ecosystems 2021, 24, 955–967. [Google Scholar] [CrossRef]
- Gonzalez-Polo, M.; Fernández-Souto, A.; Austin, A.T. Coarse Woody Debris Stimulates Soil Enzymatic Activity and Litter Decomposition in an Old-Growth Temperate Forest of Patagonia, Argentina. Ecosystems 2013, 16, 1025–1038. [Google Scholar] [CrossRef]
- Sokolova, G.G. The influence of terrain altitude, slope exposure and slope degree on plant spatial distiribution. Acta Biol. Sib. 2016, 2, 34. [Google Scholar] [CrossRef][Green Version]
- Romashkin, I.; Shorohova, E.; Kapitsa, E.; Galibina, N.; Nikerova, K. Substrate Quality Regulates Density Loss, Cellulose Degradation and Nitrogen Dynamics in Downed Woody Debris in a Boreal Forest. For. Ecol. Manag. 2021, 491, 119–143. [Google Scholar] [CrossRef]
- Laiho, R.; Prescott, C.E. Decay and Nutrient Dynamics of Coarse Woody Debris in Northern Coniferous Forests: A Synthesis. Can. J. For. Res. 2004, 34, 763–777. [Google Scholar] [CrossRef]
- Siitonen, J. Forest Management, Coarse Woody Debris and Saproxylic Organisms: Fennoscandian Boreal Forests as an Example. Eco. Bul. 2001, 49, 11–42. [Google Scholar]
- Lonsdale, D.; Pautasso, M.; Holdenrieder, O. Wood-Decaying Fungi in the Forest: Conservation Needs and Management Options. Eur. J. For. Res. 2008, 127, 1–22. [Google Scholar] [CrossRef]
- Lassauce, A.; Paillet, Y.; Jactel, H.; Bouget, C. Deadwood as a Surrogate for Forest Biodiversity: Meta-Analysis of Correlations between Deadwood Volume and Species Richness of Saproxylic Organisms. Ecol. Indic. 2011, 11, 1027–1039. [Google Scholar] [CrossRef]
- Hoppe, B.; Kahl, T.; Karasch, P.; Wubet, T.; Bauhus, J.; Buscot, F.; Krüger, D. Network Analysis Reveals Ecological Links between N-Fixing Bacteria and Wood-Decaying Fungi. PLoS ONE 2014, 9, e88141. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, B.; Krüger, D.; Kahl, T.; Arnstadt, T.; Buscot, F.; Bauhus, J.; Wubet, T. A Pyrosequencing Insight into Sprawling Bacterial Diversity and Community Dynamics in Decaying Deadwood Logs of Fagus sylvatica and Picea abies. Sci. Rep. 2015, 5, 9456. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Emborg, J.; Hahn, K.; Mountford, E.P.; Ódor, P.; Standovár, T.; Rozenbergar, D.; Diaci, J.; Wijdeven, P.; Meyer, P.; et al. Wood-Inhabiting Fungi as Indicators of Nature Value in European Beech Forests. Monitoring and Indicators of Forest Biodiversity in Europe-from Ideas to Operationality. EFI Proc. 2005, 51, 229–237. [Google Scholar]
- Filipiak, M. Nutrient Dynamics in Decomposing Dead Wood in the Context of Wood Eater Requirements: The Ecological Stoichiometry of Saproxylophagous Insects. In Saproxylic Insects; Springer: Cham, Switzerland, 2018; pp. 429–469. [Google Scholar]
- Higashi, M.; Abe, T.; Burns, T. Carbonnitrogen Balance and Termite Ecology. Proc. R. Soc. B Biol. Sci. 1992, 249, 303–308. [Google Scholar]
- Lamour, A.; Termorshuizen, A.J.; Volker, D.; Jeger, M.J. Network Formation by Rhizomorphs of Armillaria Lutea in Natural Soil: Their Description and Ecological Significance. FEMS Micro. Ecol. 2007, 62, 222–232. [Google Scholar] [CrossRef]
- Oliveira Longa, C.; Francioli, D.; Gómez-Brandón, M.; Ascher-Jenull, J.; Bardelli, T.; Pietramellara, G.; Egli, M.; Sartori, G.; Insam, H. Culturable Fungi Associated with Wood Decay of Picea abies in Subalpine Forest Soils: A Field-Mesocosm Case Study. iForest Biogeosciences For. 2018, 11, 781–785. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Zackrisson, O.; Nilsson, M.-C.; Sellstedt, A. Quantifying Nitrogen-Fixation in Feather Moss Carpets of Boreal Forests. Nature 2002, 419, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Ininbergs, K.; Bay, G.; Rasmussen, U.; Wardle, D.A.; Nilsson, M.-C. Composition and Diversity of nifH Genes of Nitrogen-Fixing Cyanobacteria Associated with Boreal Forest Feather Mosses. N. Phytol. 2011, 192, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Purahong, W.; Arnstadt, T.; Kahl, T.; Bauhus, J.; Kellner, H.; Hofrichter, M.; Krüger, D.; Buscot, F.; Hoppe, B. Are Correlations between Deadwood Fungal Community Structure, Wood Physico-Chemical Properties and Lignin-Modifying Enzymes Stable across Different Geographical Regions? Fungal Ecol. 2016, 22, 98–105. [Google Scholar] [CrossRef]
- Söderström, L. Sequence of Bryophytes and Lichens in Relation to Substrate Variables of Decaying Coniferous Wood in Northern Sweden. Nord. J. Bot. 1988, 8, 89–97. [Google Scholar] [CrossRef]
- Caruso, A.; Rudolphi, J. Influence of Substrate Age and Quality on Species Diversity of Lichens and Bryophytes on Stumps. Bryologist 2009, 112, 520–531. [Google Scholar] [CrossRef]
- Rudolphi, J.; Gustafsson, L. Forests Regenerating after Clear-Cutting Function as Habitat for Bryophyte and Lichen Species of Conservation Concern. PLoS ONE 2011, 6, e18639. [Google Scholar] [CrossRef]
- Nowińska, R.; Urbanski, P.; Szewczyk, W. Species Diversity of Plants and Fungi on Logs of Fallen Trees of Different Species in Oak-Hornbeam Forests. Roc. Aka. Rol. w Poz. Bot. Stec. 2009, 13, 109–124. [Google Scholar]
- Unar, P.; Janík, D.; Adam, D.; Vymazalová, M. The colonization of decaying logs by vascular plants and the consequences of fallen logs for herb layer diversity in a lowland alluvial forest. Eur. J. For. Res. 2017, 136, 665–676. [Google Scholar] [CrossRef]
- Six, L.J.; Halpern, C.B. Substrate Effects on Distribution, Biomass Allocation, and Morphology of forest understory plants. Botany 2008, 86, 1133–1142. [Google Scholar] [CrossRef]
- Kushnevskaya, H.; Mirin, D.; Shorohova, E. Patterns of Epixylic Vegetation on Spruce Logs in Late-Successional Boreal Forests. For. Ecol. Manag. 2007, 250, 25–33. [Google Scholar] [CrossRef]
- Staniaszek-Kik, M.; Zarnowiec, J.; Chmura, D. Colonization Patterns of Vascular Plant Species on Decaying Logs of Fagus sylviatica L. in a Lower Mountain Forest Belt: A Case Study of the Sudeten Mountains (Southern Poland). Appl. Ecol. Environ. Res. 2014, 12, 601–613. [Google Scholar] [CrossRef]
- Skorokhodova, S.B. The climate of “Kivach” Reserve. Proc. Nat. Reserve Kivach. 2008, 4, 3–34. (In Russian) [Google Scholar]
- Fedorets, N.G.; Morozova, R.M.; Bakhmet, A.N.; Solodovnikov, A.N. The soils and soil cover of the Kivach Strict Nature Reserve. Proc. Kar. Cen. Sci. Rus. Acad. Sci. 2006, 10, 3–34. (In Russian) [Google Scholar]
- Shorohova, E.; Shorohov, A. Coarse woody debris dynamics and stores in the boreal virgin spruce forest. Ecol. Bull. 2001, 49, 129–135. [Google Scholar]
- Shorohova, E.; Kapitsa, A. Influence of the substrate and ecosystem attributes on the decomposition rates of coarse woody debris in European boreal forests. For. Ecol. Manag. 2014, 315, 173–184. [Google Scholar] [CrossRef]
- Tetioukhin, S.V.; Minayev, V.N.; Bogomolova, L.P. Forest Inventory: Reference Book for the North-Western Russia; Saint-Petersburg Forest Technical Academy Publishers: St Petersburg, Russia, 2004; 360p. (In Russian) [Google Scholar]
- Tretyakov, N.V.; Gorsky, P.V.; Samoilovich, G.G. Taxator’s Handbook: Tables for Forest Taxation; Forestry industry: Moscow, Russia, 1965; 459p. (In Russian) [Google Scholar]
- Ellenberg, H. Vegetation Ecology of Central Europe, 4th ed.; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Ellenberg, H. Zeigerwerte Der Gefässpflanzen Mitteleuropas; Goltze: Gottingen, Germany, 1976. [Google Scholar]
- Ellenberg, H.; Weber, H.E.; Dull, R.; Wirth, V.; Werner, W.; Paulisen, D. Zeigerwerte von Pflanzen in Mitteleuropa. Scri. Geob. 1992, 18, 9–166. [Google Scholar]
- Genikova, N.V.; Kryshen, A.M. Dynamica of Ground Cover in Piceeyum Myrtillosum in Northern Taiga During the First Years After Clear-Cutting. Botanicheskii Zhurnal. 2018, 103, 364–381. (In Russian) [Google Scholar] [CrossRef]
- Khan, K.; Hussain, A.; Jamil, M.A.; Duan, W.; Chen, L.; Khan, A. Alteration in Forest Soil Biogeochemistry through Coarse Wood Debris in Northeast China. Forests 2022, 13, 1861. [Google Scholar] [CrossRef]
- Lee, P.; Sturgess, K. The Effects of Logs, Stumps, and Root Throws on Understory Communities within 28-Year-Old Aspen-Dominated Boreal Forests. Can. J. Bot. 2001, 79, 905–916. [Google Scholar] [CrossRef]
- Kushnevskaya, E.V. Epixilic succession in Norway spruce forests of the Leningrad region. Botanicheskii Zhurnal. 2012, 7, 917–939. (In Russian) [Google Scholar]
- Harmon, M.E. The role of woody detritus in biogeochemical cycles: Past, present, and future. Biogeochemistry 2021, 154, 349–369. [Google Scholar] [CrossRef]
- Brant, J.B.; Sulzman, E.W.; Myrold, D.D. Microbial Community Utilization of Added Carbon Substrates in Response to Long-Term Carbon Input Manipulation. Soil. Biol. Biochem. 2006, 38, 2219–2232. [Google Scholar] [CrossRef]
- Perreault, L.; Forrester, J.A.; Mladenoff, D.J.; Gower, S.T. Linking deadwood and soil GHG fluxes in a second growth north temperate deciduous forest (Upper Midwest USA). Biogeochemistry 2021, 156, 177–194. [Google Scholar] [CrossRef]
- Noormets, A.; Epron, D.; Domec, J.C.; McNulty, S.G.; Fox, T.; Sun, G.; King, J.S. Effects of Forest Management on Productivity and Carbon Sequestration: A Review and Hypothesis. For. Ecol. Manag. 2015, 355, 124–140. [Google Scholar] [CrossRef]
- Spears, J.D.H.; Lajtha, K. The Imprint of Coarse Woody Debris on Soil Chemistry in the Western Oregon Cascades. Biogeochemistry 2004, 71, 163–175. [Google Scholar] [CrossRef]
- Bantle, A.; Borken, W.; Ellerbrock, R.H.; Schulze, E.D.; Weisser, W.W.; Matzner, E. Quantity and Quality of Dissolved Organic Carbon Released from Coarse Woody Debris of Different Tree Species in the Early Phase of Decomposition. For. Ecol. Manag. 2014, 329, 287–294. [Google Scholar] [CrossRef]
- Bantle, A.; Borken, W.; Matzner, E. Dissolved Nitrogen Release from Coarse Woody Debris of Different Tree Species in the Early Phase of Decomposition. For. Ecol. Manag. 2014, 334, 277–283. [Google Scholar] [CrossRef]

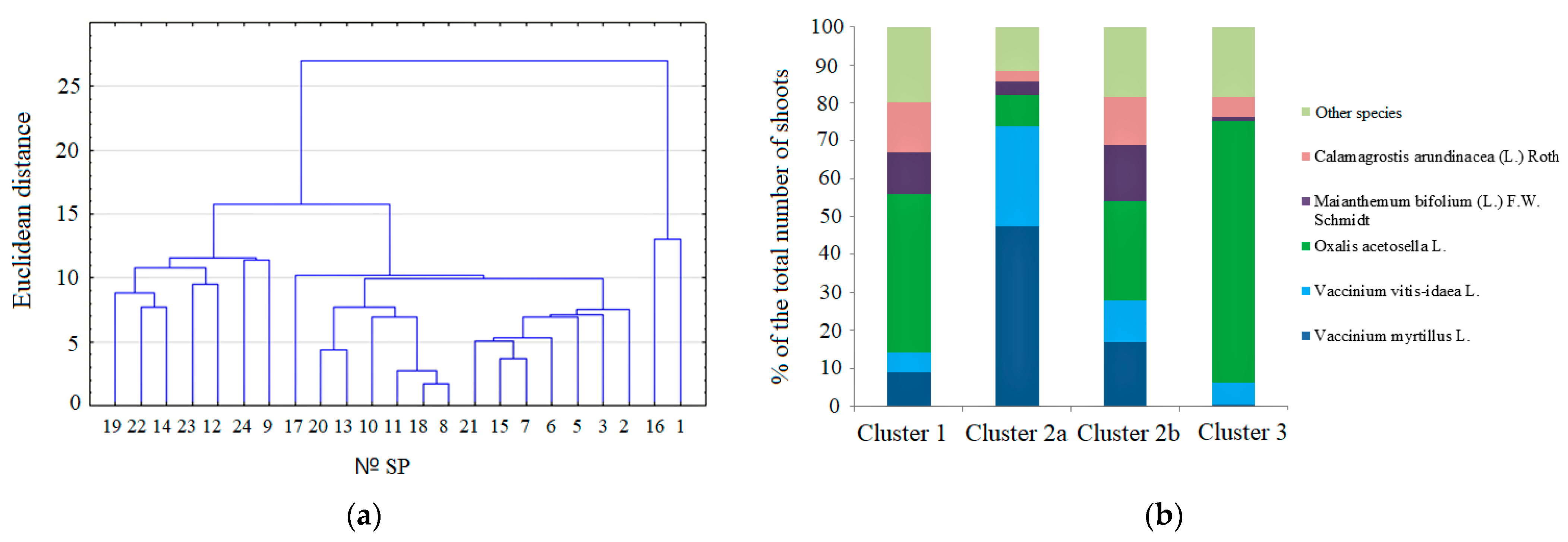



| Cluster | Microsite Type (Based on the Projective Cover of Species) | Predominance of Shoots of the Species on SP | № SP | Decay Class of the P. abies Logs |
|---|---|---|---|---|
| 1. | Small boreal grass type/ Parviherbosum (P) | Oxalis acetosella | 14 | 3 |
| 9, 19 | 5a | |||
| 12, 22, 23, 24 | 5б | |||
| 2a. | Blueberry type/ Myrtillosum (M) | Vaccinium myrtillus, Vaccinium vitis-idaea | 13 | 3 |
| 17, 18 | 4 | |||
| 8, 20 | 5 | |||
| 10, 11 | 5a | |||
| 2b. | Small boreal grass—blueberry type/ Oxalidoso-myrtillosum (OM) | Vaccinium myrtillus + Vaccinium vitis-idaea и Oxalis acetosella | 2, 3, 15 | 3 |
| 5, 6 | 4 | |||
| 7, 21 | 5a | |||
| 3. | Herbs and blueberry type/ Mixto-herboso-myrtillosum (HM) | Oxalis acetosella | 1 | 3 |
| 16 | 4 |
| Cluster | Microsite Type | Content in the Sub-Litter Soil Layer, % | Average Ellenberg Score (for Each SP) | |||||
|---|---|---|---|---|---|---|---|---|
| C | N | C/N | L | F | R | NS | ||
| 1. | P | 8.01 | 0.38 | 21.1 | 3.9 | 4.8 | 3.5 | 4.2 |
| 2a. | M | 8.77 | 0.28 | 31.3 | 4.4 | 3.3 | 2.2 | 2.5 |
| 2b. | OM | 4.63 | 0.2 | 23.2 | 3.9 | 4.6 | 3.0 | 3.4 |
| 3. | HM | 6.94 | 0.32 | 21.7 | 4.1 | 4.9 | 3.9 | 4.8 |
| Species | Microsite Type | |||||||
|---|---|---|---|---|---|---|---|---|
| P | M | OM | HM | |||||
| XS | S | XS | S | XS | S | XS | S | |
| Aconitum septentrionale Koelle | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Aegopodium podagraria L. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Angelica sylvestris L. | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 2 |
| Avenella flexuosa (L.) Drejer | 3 | 2 | 0 | 1 | 0 | 4 | 0 | 0 |
| Betula sp. | 3 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| Bromus inermis Leyss | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 4 |
| Calamagrostis arundinacea (L.) Roth | 3 | 5 | 0 | 2 | 3 | 4 | 4 | 4 |
| Carex sp. | 2 | 2 | 0 | 0 | 0 | 1 | 0 | 1 |
| Convallaria majalis L. | 4 | 4 | 3 | 2 | 3 | 3 | 0 | 5 |
| Equisetum sylvaticum L. | 0 | 1 | 0 | 0 | 0 | 0 | 5 | 3 |
| Fragaria vesca L. | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 4 |
| Galium album Mill. | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 3 |
| Geranium sylvaticum L. | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 |
| Goodyera repens (L.) R.Br. | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 |
| Gymnocarpium dryopteris (L.) Newm. | 3 | 3 | 0 | 0 | 4 | 3 | 0 | 3 |
| Lathyrus pratensis L. | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Linnaea borealis L. | 3 | 2 | 3 | 3 | 3 | 0 | 0 | 0 |
| Luzula pilosa (L.) Willd. | 3 | 4 | 0 | 2 | 4 | 4 | 3 | 2 |
| Lycopodium annotinum L. | 0 | 0 | 0 | 0 | 2 | 0 | 4 | 2 |
| Maianthemum bifolium (L.) F.W. Schmidt | 4 | 5 | 4 | 4 | 4 | 5 | 3 | 3 |
| Melampyrum pratense L. | 4 | 3 | 3 | 3 | 3 | 3 | 0 | 0 |
| Orthilia secunda (L.) House | 0 | 2 | 0 | 2 | 2 | 2 | 0 | 0 |
| Oxalis acetosella L. | 6 | 6 | 4 | 3 | 6 | 5 | 6 | 6 |
| Picea abies (L.) Karst | 6 | 3 | 5 | 3 | 6 | 2 | 6 | 2 |
| Platanthera bifolia (L.) Rich. | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Populus tremula L. | 3 | 2 | 3 | 2 | 0 | 2 | 0 | 0 |
| Pyrola rotundifolia L. | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rosa acicularis Lindl. | 3 | 2 | 0 | 1 | 0 | 0 | 0 | 3 |
| Rubus idaeus L. | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Rubus saxatilis L. | 5 | 4 | 0 | 2 | 3 | 3 | 3 | 4 |
| Solidago virgaurea L. | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 3 |
| Sorbus aucuparia L. | 3 | 2 | 0 | 2 | 2 | 1 | 3 | 2 |
| Trientalis europaea L. | 5 | 4 | 4 | 3 | 5 | 4 | 5 | 3 |
| Vaccinium myrtillus L. | 6 | 5 | 6 | 6 | 5 | 6 | 3 | 2 |
| Vaccinium vitis-idaea L. | 6 | 4 | 5 | 5 | 4 | 4 | 4 | 4 |
| Vicia cracca L. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Viola mirabilis L. | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 3 |
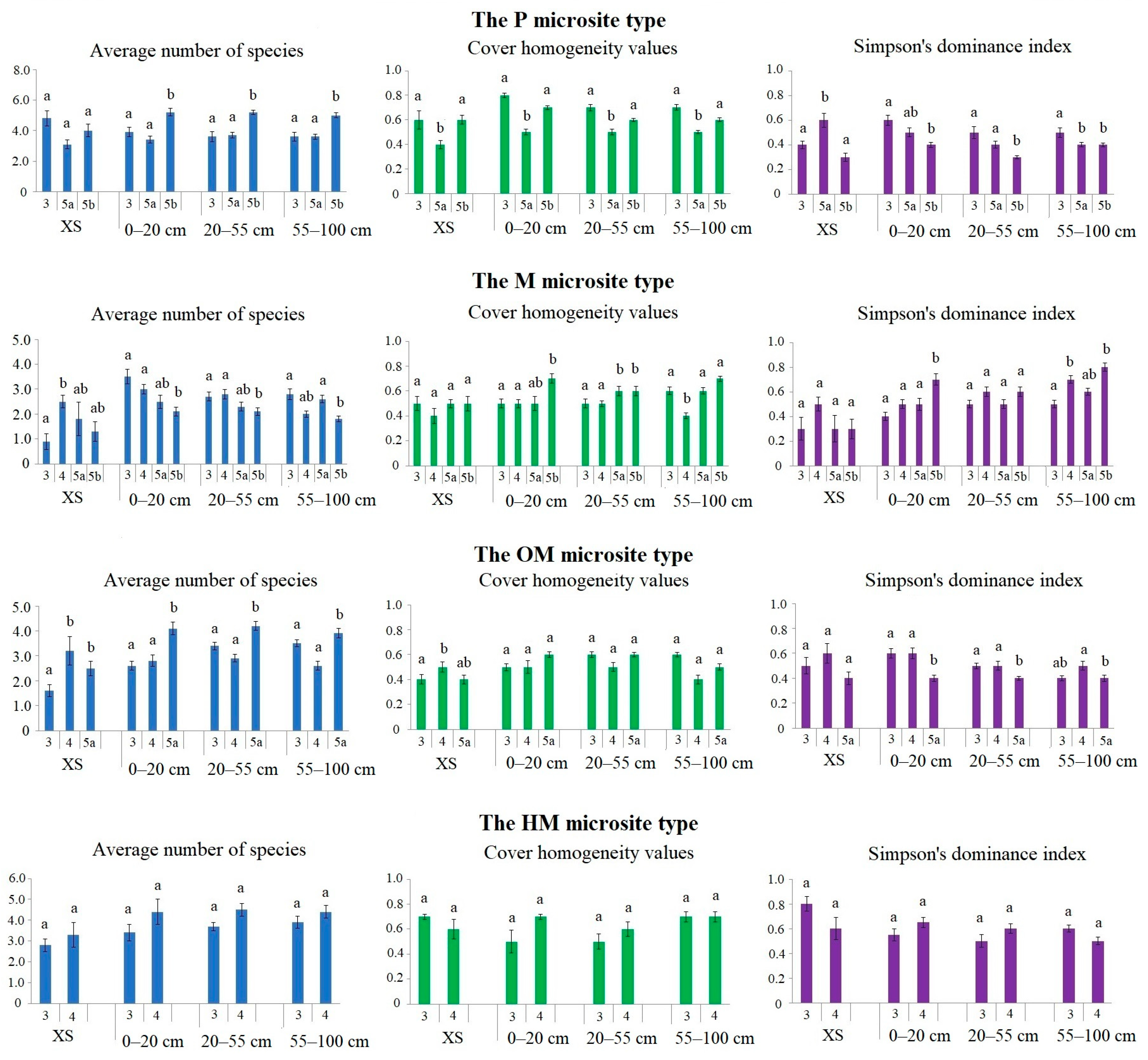
| The Microsite Type | Decay Class | Part of SP | Heterogeneity Parameters | |||
|---|---|---|---|---|---|---|
| Average Number of Species | Cover Homogeneity Values | Simpson’s Dominance Index | ||||
| HM | 3 | XS | 2.8 ± 0.3 a | 0.7 ± 0.02 a | 0.8 ± 0.06 a | |
| ADL, cm | 0–20 | 3.4 ± 0.4 a | 0.5 ± 0.09 a | 0.5 ± 0.05 b | ||
| 20–55 | 3.7 ± 0.2 a | 0.5 ± 0.06 a | 0.5 ± 0.05 б | |||
| 55–100 | 3.9 ± 0.3 a | 0.7 ± 0.04 a | 0.6 ± 0.03 ab | |||
| 4 | XS | 3.3 ± 0.6 a | 0.6 ± 0.08 a | 0.6 ± 0.09 ab | ||
| ADL, cm | 0–20 | 4.4 ± 0.6 a | 0.7 ± 0.02 a | 0.7 ± 0.04 a | ||
| 20–55 | 4.5 ± 0.3 a | 0.6 ± 0.06 a | 0.6 ± 0.04 ab | |||
| 55–100 | 4.4 ± 0.3 a | 0.7 ± 0.04 a | 0.5 ± 0.02 b | |||
| P | 3 | XS | 4.8 ± 0.5 a | 0.6 ± 0.07 a | 0.4 ± 0.03 a | |
| ADL, cm | 0–20 | 3.9 ± 0.3 a | 0.8 ± 0.02 b | 0.6 ± 0.04 a | ||
| 20–55 | 3.6 ± 0.3 a | 0.7 ± 0.03 ab | 0.5 ± 0.05 a | |||
| 55–100 | 3.6 ± 0.3 a | 0.7 ± 0.02 ab | 0.5 ± 0.04 a | |||
| 5a | XS | 3.1 ± 0.3 a | 0.4 ± 0.03 a | 0.6 ± 0.06 a | ||
| ADL, cm | 0–20 | 3.4 ± 0.2 a | 0.5 ± 0.02 ab | 0.5 ± 0.04 ab | ||
| 20–55 | 3.7 ± 0.2 a | 0.5 ± 0.02 b | 0.4 ± 0.03 b | |||
| 55–100 | 3.6 ± 0.2 a | 0.5 ± 0.01 b | 0.4 ± 0.02 b | |||
| 5b | XS | 4.0 ± 0.4 a | 0.6 ± 0.04 a | 0.3 ± 0.03 a | ||
| ADL, cm | 0–20 | 5.2 ± 0.2 a | 0.7 ± 0.02 a | 0.4 ± 0.02 a | ||
| 20–55 | 5.2 ± 0.1 a | 0.6 ± 0.01 a | 0.3 ± 0.01 a | |||
| 55–100 | 5.0 ± 0.2 a | 0.6 ± 0.01 a | 0.4 ± 0.02 a | |||
| OM | 3 | XS | 1.6 ± 0.2 a | 0.4 ± 0.04 a | 0.5 ± 0.06 ab | |
| ADL, cm | 0–20 | 2.6 ± 0.2 a | 0.5 ± 0.03 a | 0.6 ± 0.04 a | ||
| 20–55 | 3.4 ± 0.2 b | 0.6 ± 0.02 b | 0.5 ± 0.02 ab | |||
| 55–100 | 3.5 ± 0.1 b | 0.6 ± 0.02 b | 0.4 ± 0.02 b | |||
| 4 | XS | 3.2 ± 0.6 a | 0.5 ± 0.04 a | 0.6 ± 0.08 a | ||
| ADL, cm | 0–20 | 2.8 ± 0.2 a | 0.5 ± 0.05 a | 0.6 ± 0.04 a | ||
| 20–55 | 2.9 ± 0.2 a | 0.5 ± 0.04 a | 0.5 ± 0.03 a | |||
| 55–100 | 2.6 ± 0.2 a | 0.4 ± 0.04 a | 0.5 ± 0.04 a | |||
| 5a | XS | 2.5 ± 0.3 a | 0.4 ± 0.03 a | 0.4 ± 0.05 a | ||
| ADL, cm | 0–20 | 4.1 ± 0.3 b | 0.6 ± 0.02 a | 0.4 ± 0.02 a | ||
| 20–55 | 4.2 ± 0.2 b | 0.6 ± 0.02 a | 0.4± 0.01 a | |||
| 55–100 | 3.9 ± 0.2 b | 0.5 ± 0.02 a | 0.4 ± 0.02 a | |||
| M | 3 | XS | 0.9 ± 0.3 a | 0.5 ± 0.06 a | 0.3 ± 0.09 a | |
| ADL, cm | 0–20 | 3.5 ± 0.3 b | 0.5 ± 0.04 a | 0.4 ± 0.03 ab | ||
| 20–55 | 2.7 ± 0.2 b | 0.5 ± 0.03 a | 0.5 ± 0.03 ab | |||
| 55–100 | 2.8 ± 0.2 b | 0.6 ± 0.03 a | 0.5 ± 0.03 b | |||
| 4 | XS | 2.5 ± 0.2 a | 0.4 ± 0.06 a | 0.5 ± 0.05 a | ||
| ADL, cm | 0–20 | 3.0 ± 0.2 a | 0.5 ± 0.03 a | 0.5 ± 0.04 a | ||
| 20–55 | 2.8 ± 0.2 a | 0.5 ± 0.02 a | 0.6 ± 0.04 ab | |||
| 55–100 | 2.0 ± 0.1 a | 0.4 ± 0.02 a | 0.7 ± 0.03 b | |||
| 5a | XS | 1.8 ± 0.7 a | 0.5 ± 0.03 a | 0.3 ± 0.1 a | ||
| ADL, cm | 0–20 | 2.5 ± 0.3 a | 0.5 ± 0.06 a | 0.5 ± 0.05 ab | ||
| 20–55 | 2.3 ± 0.2 a | 0.6 ± 0.04 a | 0.5 ± 0.03 ab | |||
| 55–100 | 2.6 ± 0.2 a | 0.6 ± 0.02 a | 0.6 ± 0.03 b | |||
| 5b | XS | 1.3 ± 0.4 a | 0.5 ± 0.06 a | 0.3 ± 0.08 a | ||
| ADL, cm | 0–20 | 2.1 ± 0.2 a | 0.7 ± 0.04 a | 0.7 ± 0.04 b | ||
| 20–55 | 2.1 ± 0.2 a | 0.6 ± 0.04 a | 0.6 ± 0.04 b | |||
| 55–100 | 1.8 ± 0.1 a | 0.7 ± 0.02 a | 0.8 ± 0.03 b | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kikeeva, A.V.; Romashkin, I.V.; Nukolova, A.Y.; Fomina, E.V.; Kryshen, A.M. Influence of Picea Abies Logs on the Distribution of Vascular Plants in Old-Growth Spruce Forests. Forests 2024, 15, 884. https://doi.org/10.3390/f15050884
Kikeeva AV, Romashkin IV, Nukolova AY, Fomina EV, Kryshen AM. Influence of Picea Abies Logs on the Distribution of Vascular Plants in Old-Growth Spruce Forests. Forests. 2024; 15(5):884. https://doi.org/10.3390/f15050884
Chicago/Turabian StyleKikeeva, Anastasiya V., Ivan V. Romashkin, Anna Yu. Nukolova, Elena V. Fomina, and Alexandr M. Kryshen. 2024. "Influence of Picea Abies Logs on the Distribution of Vascular Plants in Old-Growth Spruce Forests" Forests 15, no. 5: 884. https://doi.org/10.3390/f15050884
APA StyleKikeeva, A. V., Romashkin, I. V., Nukolova, A. Y., Fomina, E. V., & Kryshen, A. M. (2024). Influence of Picea Abies Logs on the Distribution of Vascular Plants in Old-Growth Spruce Forests. Forests, 15(5), 884. https://doi.org/10.3390/f15050884






