Converting Low-Productivity Pasture to Well-Managed Pasture and Silvopastoral System Cause Relevant Changes in Soil Chemical and Microbiological Characteristics
Abstract
1. Introduction
2. Materials and Methods
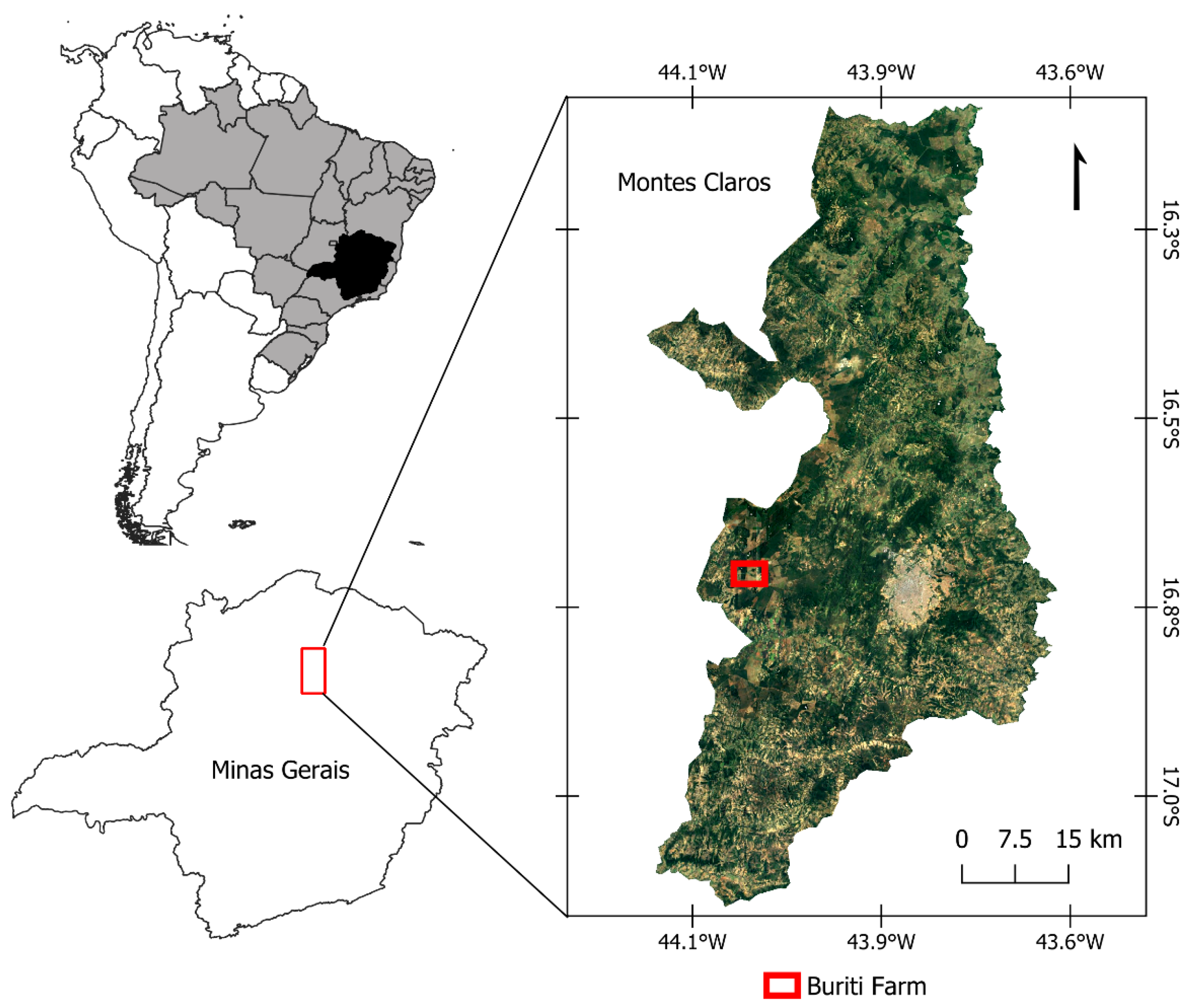
2.1. Site Description
2.2. Experimental Design
2.3. Determination of Soil Carbon and Nitrogen Stocks and Chemical Attributes
2.4. Determination of Soil Microbial Attributes
2.5. Statistical Analysis
3. Results
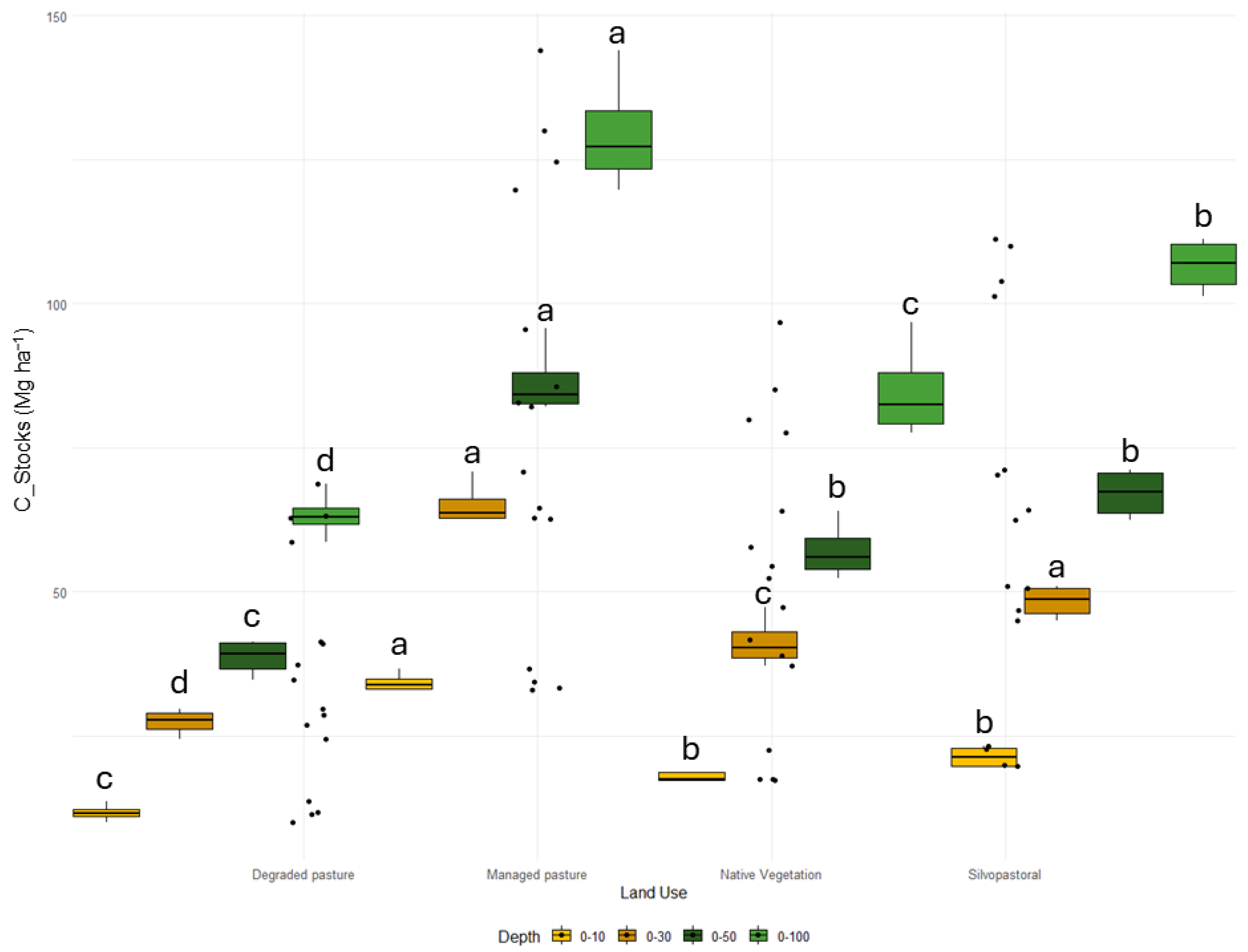
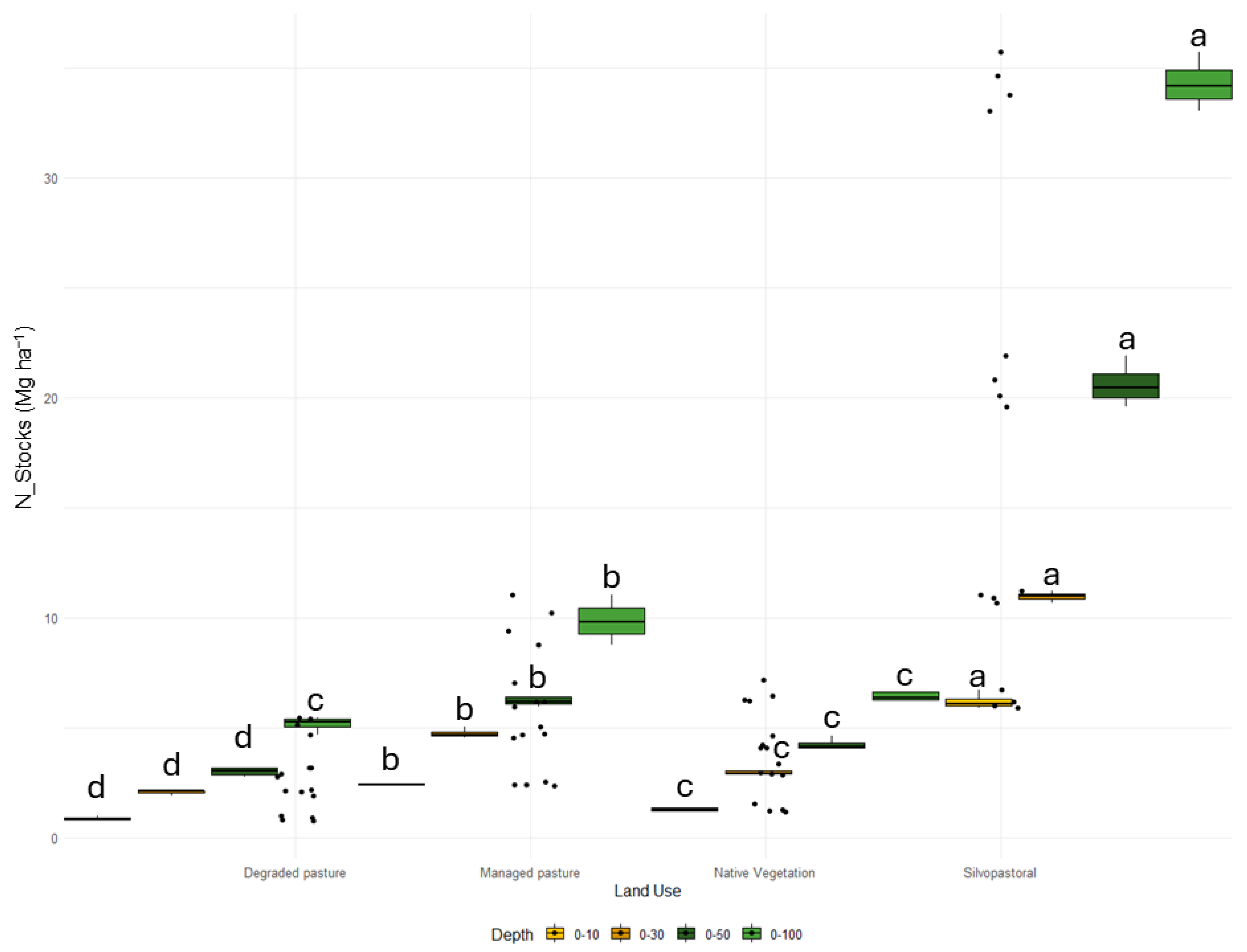
3.1. Soil Carbon and Nitrogen Stocks
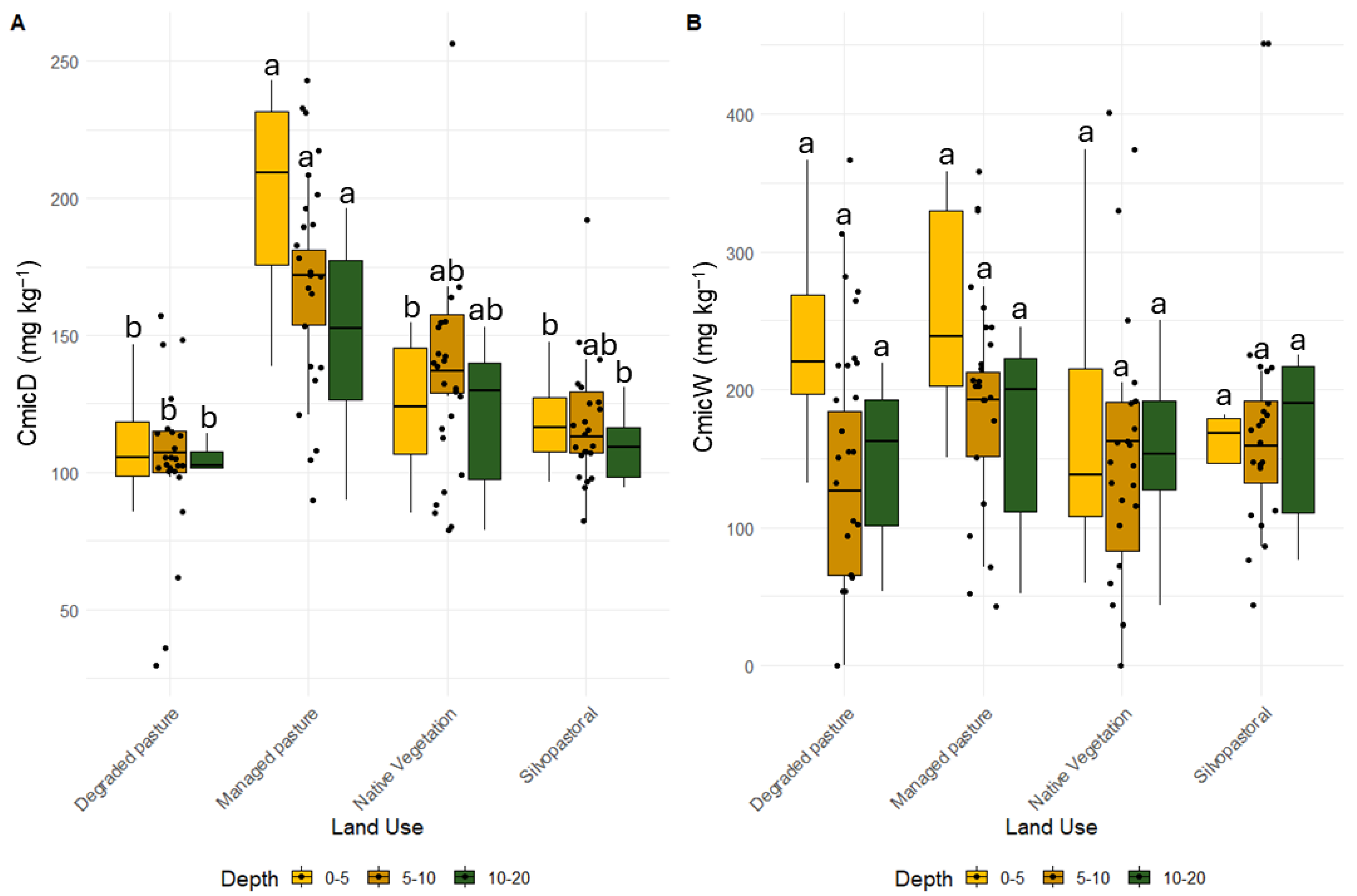
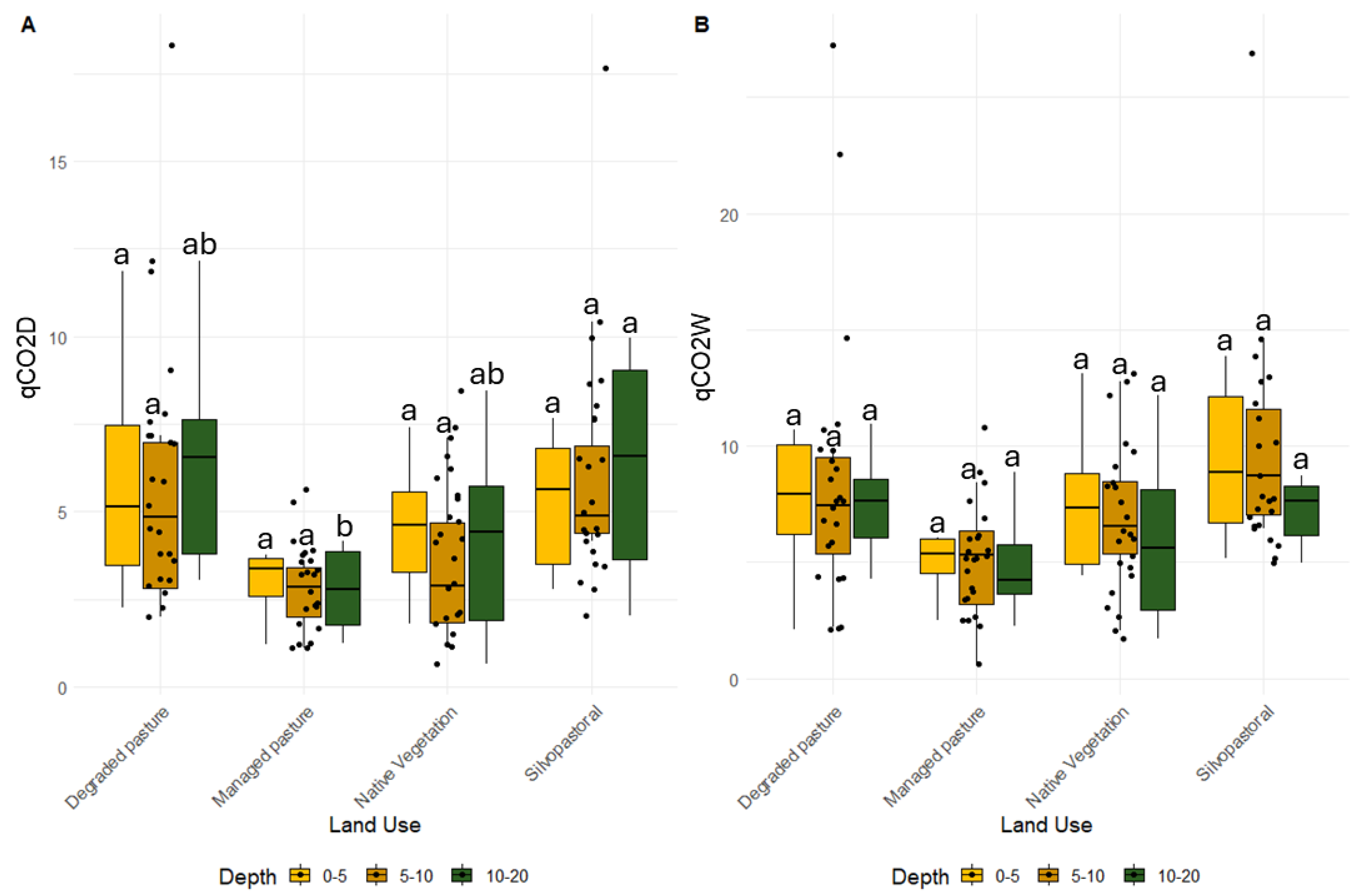
3.2. Soil Microbial Changes
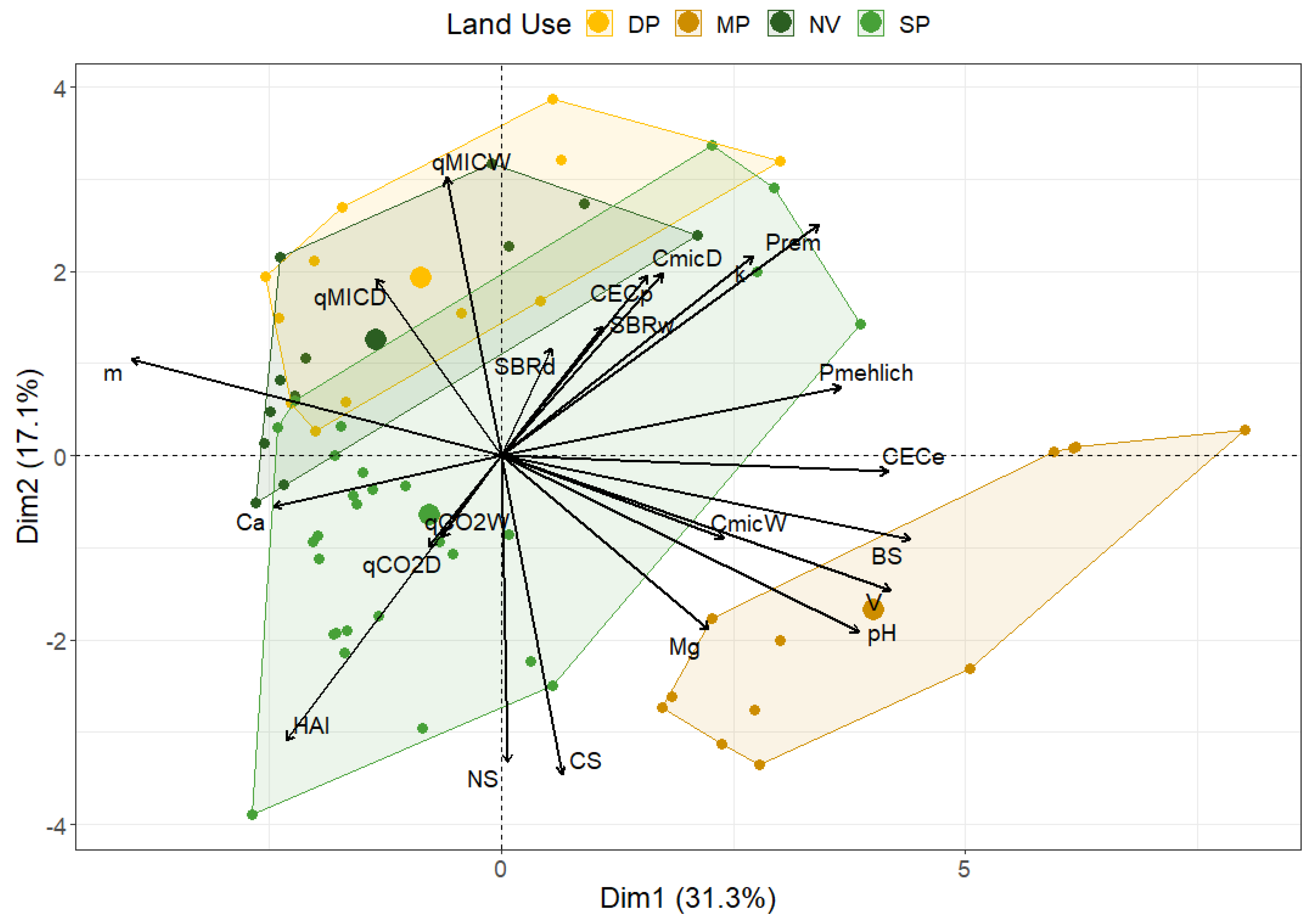
3.3. Exploring Multidimensional Relationships: Biplot PCA Analysis
4. Discussion
4.1. C and N Stocks under Different Land Use and Management Systems
4.2. Soil Microbiological Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2007: The Physical Science Basis: Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Francaviglia, R.; Almagro, M.; Vicente-Vicente, J.L. Conservation agriculture and soil organic carbon: Principles, processes, practices and policy options. Soil Syst. 2023, 7, 17. [Google Scholar] [CrossRef]
- Ghosh, S.; Das, A.; Mukherjee, S.; Dash, B.; Sahu, B.; Choudhury, S.R.; Manda, L.B. Agricultural management impacts on soil organic carbon storage. In Agricultural Soil Sustainability and Carbon Management; Meena, S.K., Ferreira, A.D.O., Meena, V.S., Rakshit, A., Shrestha, R.P., Rao, C.S., Siddique, K.H.M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 229–268. [Google Scholar] [CrossRef]
- Sarto, M.V.; Borges, W.L.; Bassegio, D.; Pires, C.A.; Rice, C.W.; Rosolem, C.A. Soil microbial community, enzyme activity, C and N stocks and soil aggregation as affected by land use and soil depth in a tropical climate region of Brazil. Arch. Microbiol. 2020, 202, 2809–2824. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Thompson, A.M.; Callister, S.J.; Tfaily, M.M.; Bell, S.L.; Hobbie, S.E.; Hofmockel, K.S. Dynamics of organic matter molecular composition under aerobic decomposition and their response to the nitrogen addition in grassland soils. Sci. Total Environ. 2022, 806, 150514. [Google Scholar] [CrossRef]
- Ramírez, P.B.; Calderón, F.J.; Fonte, S.J.; Santibáñez, F.; Bonilla, C.A. Spectral responses to labile organic carbon fractions as useful soil quality indicators across a climatic gradient. Ecol. Indic. 2020, 111, 106042. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Lee, S.A.; Kim, J.M.; Kim, Y.; Joa, J.H.; Kang, S.S.; Ahn, J.H.; Weon, H.Y. Different types of agricultural land use drive distinct soil bacterial communities. Sci. Rep. 2020, 10, 17418. [Google Scholar] [CrossRef] [PubMed]
- Ansari, J.; Bardhan, S.; Eivazi, F.; Anderson, S.H.; Mendis, S.S. Bacterial community diversity for three selected land use systems as affected by soil moisture regime. Appl. Soil Ecol. 2023, 192, 105100. [Google Scholar] [CrossRef]
- Caballero, C.B.; Biggs, T.W.; Vergopolan, N.; West, T.A.; Ruhoff, A. Transformation of Brazil’s biomes: The dynamics and fate of agriculture and pasture expansion into native vegetation. Sci. Total Environ. 2023, 896, 166323. [Google Scholar] [CrossRef]
- Teodoro, P.E.; Rossi, F.S.; Teodoro, L.P.R.; Santana, D.C.; Ratke, R.F.; Oliveira, I.C.; Silva, J.L.D.; Oliveira, J.L.G.; Silva, N.P.; Baio, F.H.R.; et al. Soil CO2 emissions under different land-use managements in Mato Grosso do Sul, Brazil. J. Clean. Prod. 2024, 434, 139983. [Google Scholar] [CrossRef]
- Alves, M.A.B.; Souza, A.P.; Almeida, F.T.; Hoshide, A.K.; Araújo, H.B.; Silva, A.F.; Carvalho, D.F. Effects of land use and cropping on soil erosion in agricultural frontier areas in the Cerrado-Amazon Ecotone, Brazil, using a rainfall simulator experiment. Sustainability 2023, 15, 4954. [Google Scholar] [CrossRef]
- Lisboa, F.J.G.; Chaer, G.M.; Fernandes, M.F.; Berbara, R.L.L.; Madari, B.E. The match between microbial community structure and soil properties is modulated by land use types and sample origin within an integrated agroecosystem. Soil Biol. Biochem. 2014, 78, 97–108. [Google Scholar] [CrossRef]
- Van Syoc, E.; Albeke, S.E.; Scasta, J.D.; Van Diepen, L.T. Quantifying the immediate response of the soil microbial community to different grazing intensities on irrigated pastures. Agric. Ecosyst. Environ. 2022, 326, 107805. [Google Scholar] [CrossRef] [PubMed]
- Seitz, T.J.; Schütte, U.M.; Drown, D.M. Unearthing shifts in microbial communities across a soil disturbance gradient. Front. Microbiol. 2022, 13, 781051. [Google Scholar] [CrossRef] [PubMed]
- Ologunde, O.H.; Bello, S.K.; Busari, M.A. Integrated agricultural system: A dynamic concept for improving soil quality. J. Saudi Soc. Agric. Sci. 2024, 1–9. [Google Scholar] [CrossRef]
- Reis, A.; Bechara, F.C.; Tres, D.R.; Trentin, B.E. Nucleation: Biocentric conception for the ecological restoration. Cienc. Florest. 2014, 24, 509–519. [Google Scholar] [CrossRef]
- Leite, M.F.A.; Liu, B.; Cardozo, E.G.; Silva, H.R.E.; Luz, R.L.; Muchavisoy, K.H.M.; Moraes, F.H.R.; Rousseau, G.X.; Kowalchuk, G.; Gehring, C.; et al. Microbiome resilience of Amazonian forests: Agroforest divergence to bacteria and secondary forest succession convergence to fungi. Glob. Change Biol. 2023, 29, 1314–1327. [Google Scholar] [CrossRef]
- Thottadi, B.P.; Singh, S.P. Climate-smart agriculture (CSA) adaptation, adaptation determinants and extension services synergies: A systematic review. Mitig. Adapt. Strateg. Glob. Change 2024, 29, 22. [Google Scholar] [CrossRef]
- Reis, J.C.; Kamoi, M.Y.T.; Michetti, M.; Wruck, F.J.; Rodrigues, R.A.R.; Farias Neto, A.L. Economic and environmental impacts of integrated systems adoption in Brazilian agriculture-forest frontier. Agrofor. Syst. 2023, 97, 847–863. [Google Scholar] [CrossRef]
- Santana, P.H.L.; Frazão, L.A.; Santos, L.D.F.; Fernandes, L.A.; Sampaio, R.A. Soil attributes and production of Eucalyptus in monoculture and silvopastoral systems in the north of Minas Gerais, Brazil. J. Agric. Sci. Technol. B 2016, 6, 361–370. [Google Scholar] [CrossRef][Green Version]
- Kunte, G.; Bhat, V. Deforestation, Climate Change and the Sustainability of Agriculture: A Review. J. Resour. Ecol. 2024, 15, 140–150. [Google Scholar] [CrossRef]
- Anghinoni, I.; Carvalho, P.D.F.; Costa, S.D.A. Abordagem sistêmica do solo em sistemas integrados de produção agrícola e pecuária no subtrópico brasileiro. Top. Cienc. Solo 2013, 8, 325–380. [Google Scholar]
- Schwab, N.; Schickhoff, U.; Fischer, E. Transition to agroforestry significantly improves soil quality: A case study in the central mid-hills of Nepal. Agric. Ecosyst. Environ. 2015, 205, 57–69. [Google Scholar] [CrossRef]
- Udawatta, R.P.; Kremer, R.J.; Nelson, K.A.; Jose, S.; Bardhan, S. Soil quality of a mature alley cropping agroforestry system in temperate North America. Commun. Soil Sci. Plant Anal. 2014, 45, 2539–2551. [Google Scholar] [CrossRef]
- INMET. Instituto Nacional de Meteorologia. 2018. Available online: http://www.inmet.gov.br/portal/ (accessed on 1 November 2023).
- Blake, G.R.; Hartge, K.H. Bulk density. In Methods of Soil Analysis. Part 1: Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 363–376. [Google Scholar]
- Embrapa. Manual de Métodos de Análise de Solos, 2nd ed.; Embrapa: Brasília, DF, Brazil, 1997. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- De-Polli, H.; Guerra, J.G.M. Determination of C of the Soil Microbial Biomass: Method of Fumigation-Extraction; Embrapa CNPAB: Seropédica, RJ, Brazil, 1998. [Google Scholar]
- Jenkinson, D.S.; Powlson, D.S. The effects of biocidal treatments on metabolism in soil—V: A method for measuring soil biomass. Soil Biol. Biochem. 1976, 8, 209–213. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol. Biochem. 1993, 25, 393–395. [Google Scholar] [CrossRef]
- Reis Junior, F.B.; Mendes, I.C. Biomassa Microbiana do Solo, 1st ed.; Embrapa Cerrados: Planaltina, DF, Brazil, 2007. [Google Scholar]
- Mendiburu, F.; Simon, R. Agricolae—Ten years of an Open source Statistical tool for experiments in breeding, agriculture and biology. PeerJ 2015, 3, e1404v1. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 3 December 2022).
- Freitas, I.C.; Ribeiro, J.M.; Araújo, N.C.A.; Santos, M.V.; Sampaio, R.A.; Fernandes, L.A.; Azevedo, A.M.; Feigl, B.J.; Cerri, C.E.; Frazão, L.A. Agrosilvopastoral systems and well-managed pastures increase soil carbon stocks in the Brazilian Cerrado. Rangel. Ecol. Manag. 2020, 73, 776–785. [Google Scholar] [CrossRef]
- Pinheiro, F.M.; Nair, P.R.; Nair, V.D.; Tonucci, R.G.; Venturin, R.P. Soil carbon stock and stability under Eucalyptus-based silvopasture and other land-use systems in the Cerrado biodiversity hotspot. J. Environ. Manag. 2021, 299, 113676. [Google Scholar] [CrossRef]
- Almeida, L.L.S.; Frazão, L.A.; Lessa, T.A.M.; Fernandes, L.A.; Veloso, Á.L.C.; Lana, A.M.Q.; Souza, I.A.; Pegoraro, R.F.; Ferreira, E.A. Soil carbon and nitrogen stocks and the quality of soil organic matter under silvopastoral systems in the Brazilian Cerrado. Soil Tillage Res. 2021, 205, 104785. [Google Scholar] [CrossRef]
- Frazão, L.A.; Cardoso, P.H.S.; Almeida Neta, M.N.; Mota, M.F.C.; Almeida, L.L.S.; Ribeiro, J.M.; Bicalho, T.F.; Feigl, B.J. Carbon and nitrogen stocks and organic matter fractions in the topsoil of traditional and agrisilvicultural systems in the Southeast of Brazil. Soil Res. 2021, 59, 794–805. [Google Scholar] [CrossRef]
- Freitas, I.C.; Alves, M.A.; Pena, A.N.L.; Ferreira, E.A.; Frazão, L.A. Changing the land use from degraded pasture into integrated farming systems enhance soil carbon stocks in the Cerrado biome. Acta Sci. Agron. 2024, 46, e63601. [Google Scholar] [CrossRef]
- Urquiaga, S.; Alves, B.J.R.; Jantalia, C.P.; Boddey, R.M. Variações nos estoques de carbono e emissões de gases de efeito estufa em solos das regiões tropicais e subtropicais do Brasil: Uma análise crítica. Inf. Agron. 2010, 130, 12–21. [Google Scholar]
- Abreu, L.H.G.; Freitas, I.C.; Santana, P.H.L.; Barbosa, D.L.A.; Santos, L.D.T.; Santos, M.V.; Santos, M.V.; Sanglard, D.A.; Frazão, L.A. Variation in soil carbon, nitrogen and microbial attributes within a silvopastoral system in the Brazilian Cerrado. Agrofor. Syst. 2020, 94, 2343–2353. [Google Scholar] [CrossRef]
- Macedo, M.C.M. Integração lavoura e pecuária: O estado da arte e inovações tecnológicas. Rev. Bras. Zootec. 2009, 38, 133–146. [Google Scholar] [CrossRef]
- Torres, I.F.; Bastida, F.; Hernandez, T.; Bombach, P.; Richnow, H.H.; Garcia, C. The role of lignin and cellulose in the carbon-cycling of degraded soils under semiarid climate and their relation to microbial biomass. Soil Biol. Biochem. 2014, 75, 152–160. [Google Scholar] [CrossRef]
- Olson, K.R.; Al-Kaisi, M.M. The importance of soil sampling depth for accurate account of soil organic carbon sequestration, storage, retention and loss. Catena 2015, 125, 33–37. [Google Scholar] [CrossRef]
- Tonucci, R.G.; Nair, V.D.; Nair, P.K.R.; Garcia, R. Grass vs. tree origin of soil organic carbon under different land-use systems in the Brazilian Cerrado. Plant Soil 2017, 419, 281–292. [Google Scholar] [CrossRef]
- Fontana, A.; Silva, C.F.D.; Pereira, M.G.; Loss, A.; Brito, R.J.D.; Benites, V.D.M. Avaliação dos compartimentos da matéria orgânica em área de Mata Atlântica. Acta Sci. Agron. 2011, 33, 545–550. [Google Scholar] [CrossRef]
- Smith, J.; Johnson, R.; Brown, L.; Davis, P. Estimation of Carbon Incorporation from Bovine Manure into Soil. J. Environ. Sci. 2023, 12, 123–135. [Google Scholar]
- Silva, A.S.D.; Colozzi Filho, A.; Nakatani, A.S.; Alves, S.J.; Andrade, D.D.S.; Guimarães, M.D.F. Microbial characteristics of soils under an integrated crop-livestock system. Rev. Bras. Cienc. Solo 2015, 39, 40–48. [Google Scholar] [CrossRef]
- Sousa, H.M.; Correa, A.R.; Silva, B.D.M.; Oliveira, S.D.S.; Campos, D.T.D.S.; Wruck, F.J. Dynamics of soil microbiological attributes in integrated crop–livestock systems in the Cerrado-Amazônia Ecotone. Rev. Caatinga 2020, 33, 09–20. [Google Scholar] [CrossRef]
- Reed, M.S.; Fazey, I.; Stringer, L.C.; Raymond, C.M.; Akhtar-Schuster, M.; Begni, G.; Bigas, H.; Brehm, S.; Briggs, J.; Bryce, R.; et al. Knowledge management for land degradation monitoring and assessment: An analysis of contemporary thinking. Land Degrad. Dev. 2013, 24, 307–322. [Google Scholar] [CrossRef]
- Oliveira, W.R.D.; Ramos, M.L.G.; Carvalho, A.M.; Coser, T.R.; Silva, A.M.M.; Lacerda, M.M.; Souza, K.W.; Marchão, R.L.; Vilela, L.; Pulrolnik, K. Dynamics of soil microbiological attributes under integrated production systems, continuous pasture, and native cerrado. Pesqui. Agropecu. Bras. 2016, 51, 1501–1510. [Google Scholar] [CrossRef]
- Stieven, A.C.; Oliveira, D.A.; Santos, J.O.; Wruck, F.J.; Campos, D.T.D.S. Impacts of integrated crop-livestock-forest on microbiological indicators of soil. Rev. Bras. Cienc. Agrar. 2014, 9, 53–58. [Google Scholar] [CrossRef][Green Version]
- Matos, N.M.; Ribeiro, F.P.; Gatto, A.; Bussinguer, A.P. Litter stock in three forest types in Cerrado at Distrito Federal. Floresta Ambient. 2017, 24, e00126215. [Google Scholar] [CrossRef]
- Gama-Rodrigues, A.C.; Barros, N.F.; Mendonça, E.S. Alterações edáficas sob plantios puros e misto de espécies florestais nativas do sudeste da Bahia, Brasil. Rev. Bras. Cienc. Solo 1999, 23, 581–592. [Google Scholar] [CrossRef]
- Lourente, E.R.P.; Silva, R.F.; Silva, D.A.; Marchetti, M.E.; Mercante, F.M. Macrofauna edáfica e sua interação com atributos químicos e físicos do solo sob diferentes sistemas de manejo. Acta Sci. Agron. 2007, 29, 17–22. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. Determination of ecophysiological maintenance carbon requirements of soil microorganisms in a dormant state. Biol. Fert. Soils 1985, 1, 81–89. [Google Scholar] [CrossRef]
- Moreira, F.M.S.; Siqueira, J.O. Microbiologia e Bioquímica do Solo, 2nd ed.; Ufla: Lavras, MG, Brazil, 2006. [Google Scholar]
- Alvarenga, M.I.N.; Siqueira, J.O.; Davide, A.C. Teor de carbono, biomassa microbiana, agregação e micorriza em solos de Cerrado com diferentes usos. Ciênc. Agrotec. 1999, 23, 617–625. [Google Scholar]
- Almeida, L.S.D.; Ferreira, V.A.S.; Fernandes, L.A.; Frazão, L.A.; Oliveira, A.L.G.; Sampaio, R.A. Indicadores de qualidade do solo em cultivos irrigados de cana-de-açúcar. Pesqui. Agropecu. Bras. 2016, 51, 1539–1547. [Google Scholar] [CrossRef][Green Version]
- Weerasekara, C.; Udawatta, R.P.; Jose, S.; Kremer, R.J.; Weerasekara, C. Soil quality differences in a row-crop watershed with agroforestry and grass buffers. Agrofor. Syst. 2016, 90, 829–838. [Google Scholar] [CrossRef]
- Cardinael, R.; Chevallier, T.; Barthès, B.G.; Saby, N.P.; Parent, T.; Dupraz, C.; Bernoux, M.; Chenu, C. Impact of alley cropping agroforestry on stocks, forms and spatial distribution of soil organic carbon—A case study in a Mediterranean context. Geoderma 2015, 259, 288–299. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, L.J.d.; Freitas, I.C.d.; Abreu, L.H.G.d.; Oliveira, D.M.d.S.; Barbosa, D.L.d.A.; Figueredo, C.C.; Camilotti, V.L.; Teixeira, M.V.; Frazão, L.A. Converting Low-Productivity Pasture to Well-Managed Pasture and Silvopastoral System Cause Relevant Changes in Soil Chemical and Microbiological Characteristics. Forests 2024, 15, 1029. https://doi.org/10.3390/f15061029
Silva LJd, Freitas ICd, Abreu LHGd, Oliveira DMdS, Barbosa DLdA, Figueredo CC, Camilotti VL, Teixeira MV, Frazão LA. Converting Low-Productivity Pasture to Well-Managed Pasture and Silvopastoral System Cause Relevant Changes in Soil Chemical and Microbiological Characteristics. Forests. 2024; 15(6):1029. https://doi.org/10.3390/f15061029
Chicago/Turabian StyleSilva, Libério Junio da, Igor Costa de Freitas, Luiz Henrique Gomes de Abreu, Dener Márcio da Silva Oliveira, Demerson Luiz de Almeida Barbosa, Cléber Cunha Figueredo, Vagner Luis Camilotti, Marcus Vinícius Teixeira, and Leidivan Almeida Frazão. 2024. "Converting Low-Productivity Pasture to Well-Managed Pasture and Silvopastoral System Cause Relevant Changes in Soil Chemical and Microbiological Characteristics" Forests 15, no. 6: 1029. https://doi.org/10.3390/f15061029
APA StyleSilva, L. J. d., Freitas, I. C. d., Abreu, L. H. G. d., Oliveira, D. M. d. S., Barbosa, D. L. d. A., Figueredo, C. C., Camilotti, V. L., Teixeira, M. V., & Frazão, L. A. (2024). Converting Low-Productivity Pasture to Well-Managed Pasture and Silvopastoral System Cause Relevant Changes in Soil Chemical and Microbiological Characteristics. Forests, 15(6), 1029. https://doi.org/10.3390/f15061029







