Enzyme Activity Stoichiometry Suggests That Fertilization, Especially Nitrogen Fertilization, Alleviates Nutrient Limitation of Soil Microorganisms in Moso Bamboo Forests
Abstract
1. Introduction
2. Materials and Methods
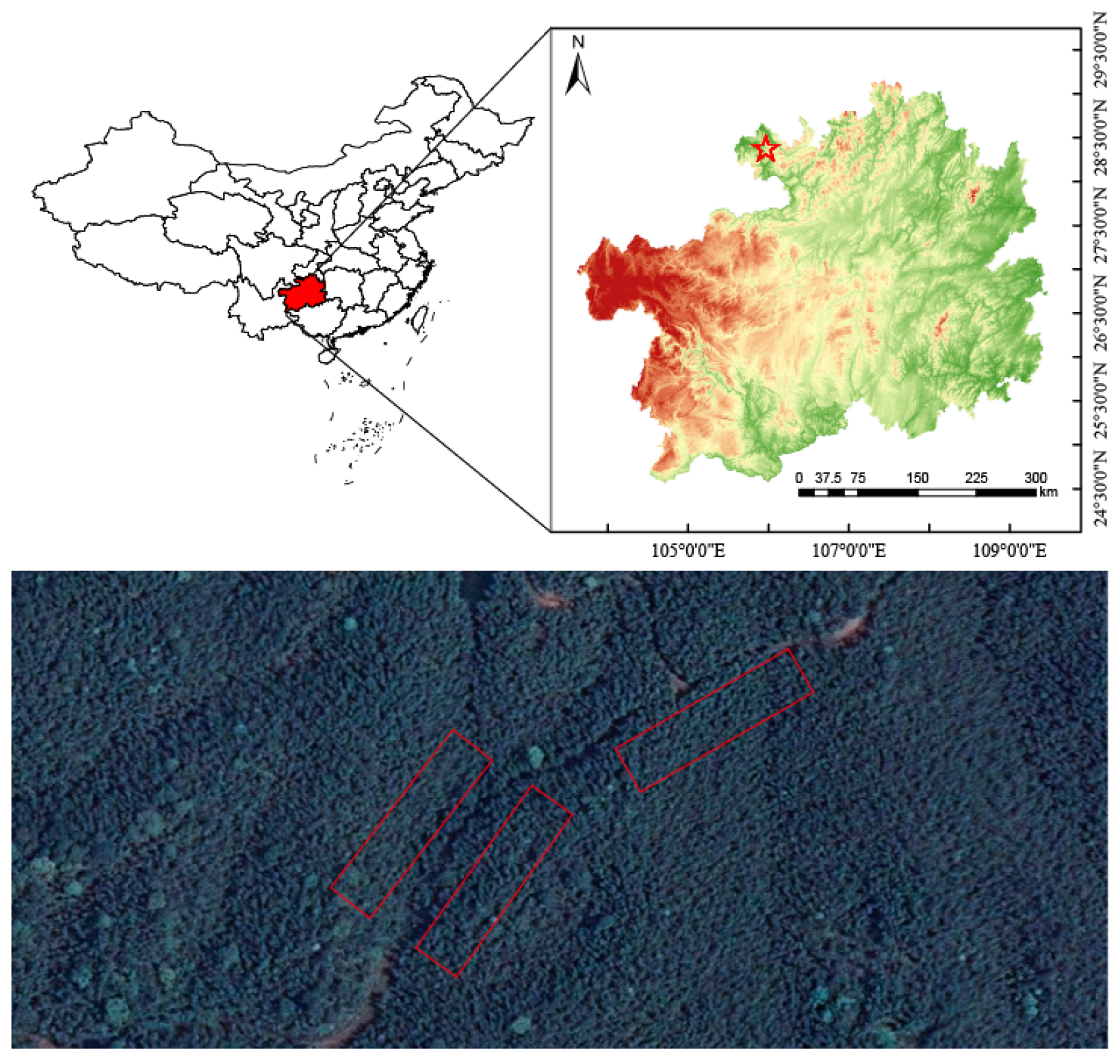
2.1. Study Site
2.2. Soil Sample Collection
2.2.1. Soil Chemical Properties
2.2.2. Microbial Biomass
2.2.3. Soil Extracellular Enzyme Activity
2.2.4. Enzyme Activity Stoichiometry
2.2.5. Enzyme Activity Vector Characterization
2.3. Data Analysis
3. Results
3.1. Changes in Soil Properties
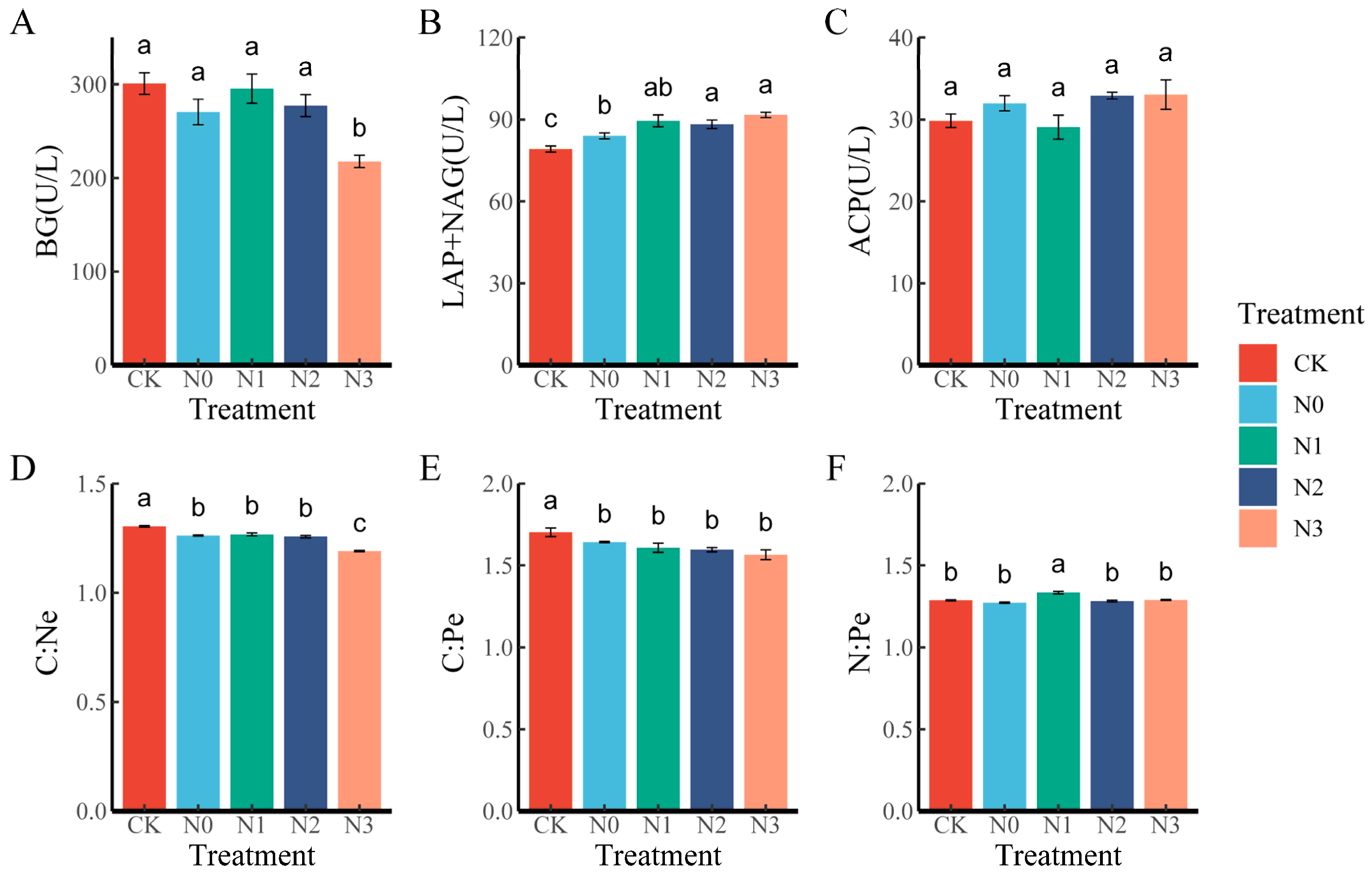
3.2. Changes in Extracellular Enzyme Activity and Stoichiometry
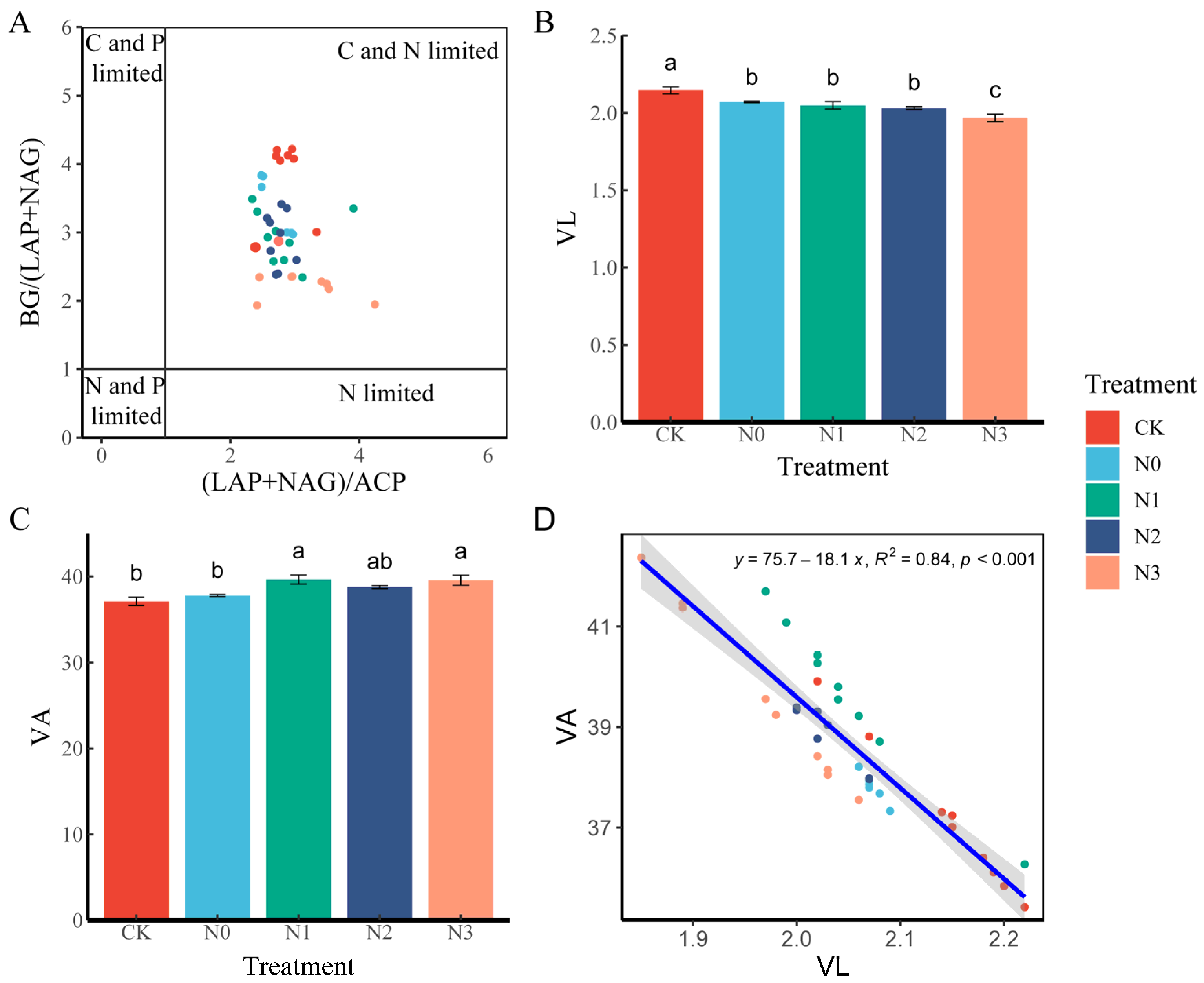
3.3. Changes in Microbial Nutrient Limitation
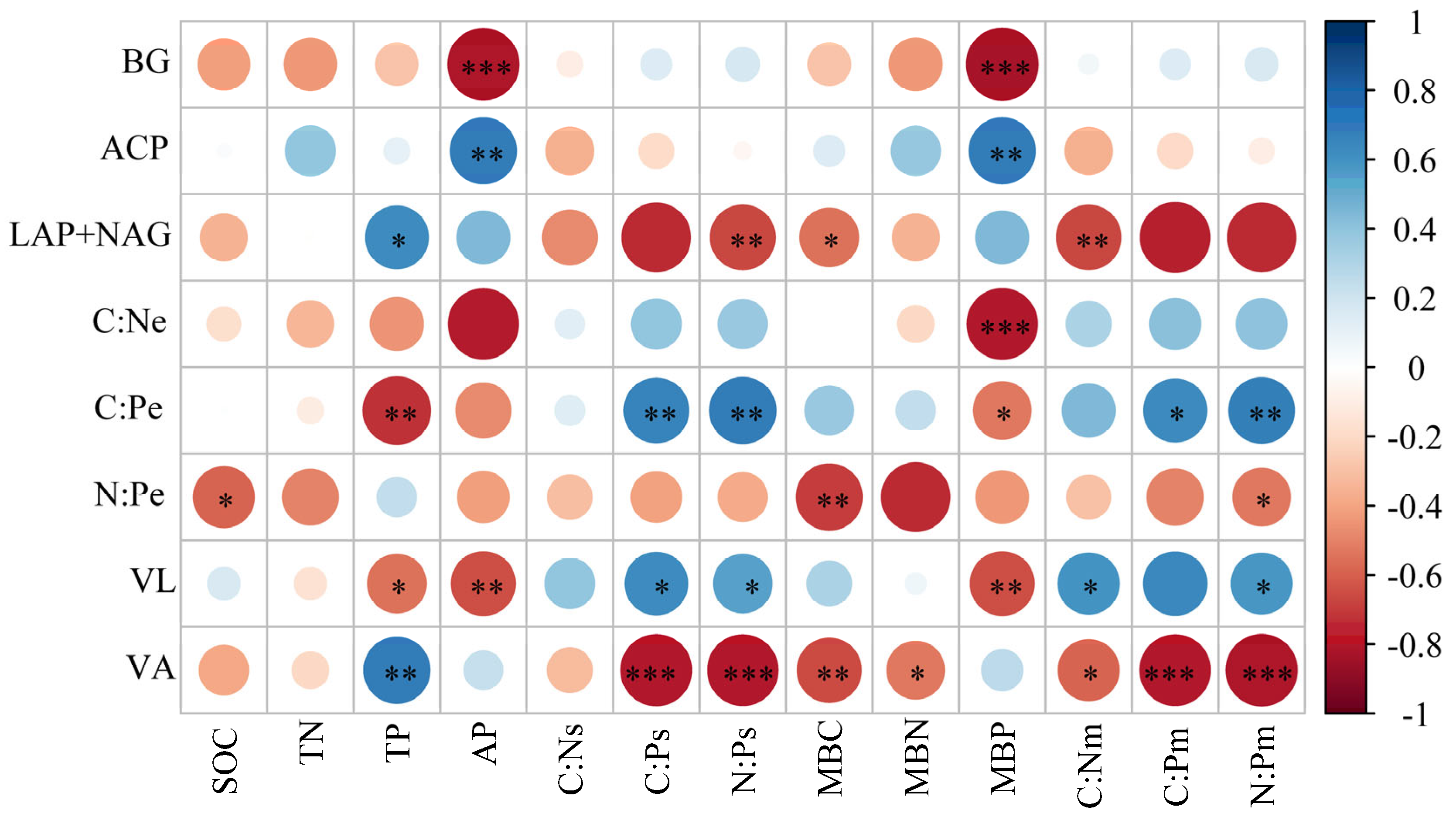
3.4. Environmental Factors Affecting Extracellular Enzyme Activity and Microbial Nutrient Limitation
4. Discussion
4.1. Extracellular Enzyme Activity and Stoichiometry in Response to N Application
4.2. Effects of Soil Microbial Nutrient Limitation in Fertilized Moso Bamboo Forests
4.3. Shortcomings of the Study and Future Prospects
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, Z. Bamboo and Rattan in the World; China Forest Publishing House: Beijng, China, 2002. [Google Scholar]
- Song, X.; Peng, C.; Ciais, P.; Li, Q.; Xiang, W.; Xiao, W.; Zhou, G.; Deng, L. Nitrogen Addition Increased CO2 Uptake More than Non-CO2 Greenhouse Gases Emissions in a Moso Bamboo Forest. Sci. Adv. 2020, 6, eaaw5790. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Li, Y. China’s Bamboo Resources in 2021. World Bamboo Ratt. 2023, 21, 100–103. [Google Scholar]
- Fu, L.; Su, J. Calculation of carbon sink of bamboo forest in China and its potential prediction. China For. Econ. 2023, 3, 96–102. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Zhang, Y.; Booth, T.; He, X. Changes of Carbon Stocks in Bamboo Stands in China during 100 Years. For. Ecol. Manag. 2009, 258, 1489–1496. [Google Scholar] [CrossRef]
- Zheng, Y.; Fan, S.; Guan, F.; Xia, W.; Wang, S.; Xiao, X. Strip Clearcutting Drives Vegetation Diversity and Composition in the Moso Bamboo Forests. For. Sci. 2022, 68, 27–36. [Google Scholar] [CrossRef]
- Zhao, J.; Cai, C. Physiological Integration Improves Nitrogen Use Efficiency of Moso Bamboo: An Isotopic (15N) Assessment. For. Ecol. Manag. 2023, 542, 121073. [Google Scholar] [CrossRef]
- Zhao, J.; Su, W.; Fan, S.; Cai, C.; Su, H.; Zeng, X. Ammonia Volatilization and Nitrogen Runoff Losses from Moso Bamboo Forests after Different Fertilization Practices. Can. J. For. Res. 2019, 49, 213–220. [Google Scholar] [CrossRef]
- Wu, P.; Wu, Q.; Huang, H.; Xie, L.; An, H.; Zhao, X.; Wang, F.; Gao, Z.; Zhang, R.; Bangura, K.; et al. Global Meta-Analysis and Three-Year Field Experiment Shows That Deep Placement of Fertilizer Can Enhance Crop Productivity and Decrease Gaseous Nitrogen Losses. Field Crops Res. 2024, 307, 109263. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, Q.; Yuan, X.; Zhang, Q.; Zhou, J.; Xu, M.; Sun, H.; Chen, L.; Gao, X.; Chen, Y. Soil Dissolved Organic Matter Quality and Bacterial Community Composition Regulate the Substrate-Binding Affinity of Hydrolytic Enzymes under Short-Term Nitrogen Addition. Geoderma 2024, 445, 116885. [Google Scholar] [CrossRef]
- Gong, H.; Li, J.; Liu, Z.; Hou, R.; Zhang, Y.; Xu, Y.; Zhu, W.; Yang, L.; Ouyang, Z. Linkages of Soil and Microbial Stoichiometry to Crop Nitrogen Use Efficiency: Evidence from a Long-Term Nitrogen Addition Experiment. CATENA 2024, 240, 107961. [Google Scholar] [CrossRef]
- Chu, H.; Ni, H.; Su, W.; Fan, S.; Long, Y.; Sun, Y. Enhanced Nitrogen Fertilizer Input Alters Soil Carbon Dynamics in Moso Bamboo Forests, Impacting Particulate Organic and Mineral-Associated Carbon Pools. Forests 2023, 14, 2460. [Google Scholar] [CrossRef]
- Gianfreda, L.; Ruggiero, P. Enzyme Activities in Soil. In Nucleic Acids and Proteins in Soil; Springer: Berlin/Heidelberg, Germany, 2006; pp. 257–311. [Google Scholar]
- Wyszkowska, J.; Borowik, A.; Kucharski, M.; Kucharski, J. Effect of Cadmium, Copper and Zinc on Plants, Soil Microorganisms and Soil Enzymes. J. Elem. 2013, 18, 769–796. [Google Scholar] [CrossRef]
- Cui, Y.; Fang, L.; Guo, X.; Wang, X.; Zhang, Y.; Li, P.; Zhang, X. Ecoenzymatic Stoichiometry and Microbial Nutrient Limitation in Rhizosphere Soil in the Arid Area of the Northern Loess Plateau, China. Soil Biol. Biochem. 2018, 116, 11–21. [Google Scholar] [CrossRef]
- Yang, L.; Jia, Y.; Li, Q.; Cui, H.; Lu, J.; Ma, J.; Xu, Z. Ecoenzymatic Stoichiometry in the Rhizosphere and Bulk Soil of a Larix Principis-Rupprechtii Plantation in North China. Forests 2023, 14, 1315. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic Stoichiometry of Microbial Organic Nutrient Acquisition in Soil and Sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Chen, J.; Lu, X.; Doughty, R.; Zhao, F.; Zhong, Z.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Responses of Soil Total Microbial Biomass and Community Compositions to Rainfall Reductions. Soil Biol. Biochem. 2018, 116, 4–10. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, S.; Shen, H.; Zhao, M.; Xu, L.; Xing, A.; Fang, J. Soil Extracellular Enzyme Activity and Stoichiometry in China’s Forests. Funct. Ecol. 2020, 34, 1461–1471. [Google Scholar] [CrossRef]
- Yao, B.; Wang, X.; Li, Y.; Lian, J.; Li, Y.; Luo, Y.; Li, Y. Soil Extracellular Enzyme Activity Reflects the Change of Nitrogen to Phosphorus Limitation of Microorganisms during Vegetation Restoration in Semi-Arid Sandy Land of Northern China. Front. Environ. Sci. 2023, 11, 1298027. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of Soil Enzyme Activity at Global Scale: Stoichiometry of Soil Enzyme Activity. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, G.; Li, P.; Li, Q.; Xue, S. Ecoenzymatic Stoichiometry and Microbial Nutrient Limitation during Secondary Succession of Natural Grassland on the Loess Plateau, China. Soil Tillage Res. 2020, 200, 104605. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Yu, J.; Wang, K.; Li, J.; Cui, Y.; Shangguan, Z.; Deng, L. Soil Enzyme Activity and Stoichiometry in Response to Precipitation Changes in Terrestrial Ecosystems. Soil Biol. Biochem. 2024, 191, 109321. [Google Scholar] [CrossRef]
- Abay, P.; Gong, L.; Luo, Y.; Zhu, H.; Ding, Z. Soil Extracellular Enzyme Stoichiometry Reveals the Nutrient Limitations in Soil Microbial Metabolism under Different Carbon Input Manipulations. Sci. Total Environ. 2024, 913, 169793. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Follstad Shah, J.J. Ecoenzymatic Stoichiometry and Ecological Theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 313–343. [Google Scholar] [CrossRef]
- Luo, H.; Yu, J.; Li, R.; Gu, J.D.; Luo, L.; Zhang, Y.; He, Y.; Xiao, Y.; Deng, S.; Zhang, Y.; et al. Microbial Biomass C:N:P as a Better Indicator than Soil and Ecoenzymatic C:N:P for Microbial Nutrient Limitation and C Dynamics in Zoige Plateau Peatland Soils. Int. Biodeterior. 2022, 175, 105492. [Google Scholar]
- Xu, Z.; Yu, G.; Zhang, X.; He, N.; Wang, Q.; Wang, S.; Wang, R.; Zhao, N.; Jia, Y.; Wang, C. Soil Enzyme Activity and Stoichiometry in Forest Ecosystems along the North-South Transect in Eastern China (NSTEC). Soil Biol. Biochem. 2017, 104, 152–163. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.; Gao, D.; Wang, X.; Liu, W.; Deng, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Ecoenzymatic Stoichiometry and Nutrient Dynamics along a Revegetation Chronosequence in the Soils of Abandoned Land and Robinia Pseudoacacia Plantation on the Loess Plateau, China. Soil Biol. Biochem. 2019, 134, 1–14. [Google Scholar] [CrossRef]
- Yang, F.; Ding, L.; Zhang, Y.; Wang, P.; Liu, J.; Zou, C.; Chen, C. Discussion on the Conversion Method of Soil Enzyme Stoichiometry from Different Vegetation Types in Guizhou Grassland, Southwest China. J. Phys. Conf. Ser. 2020, 1549, 022035. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector Analysis of Ecoenzyme Activities Reveal Constraints on Coupled C, N and P Dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Zhong, Z.; Huang, Z.; Wen, X.; Bian, F.; Yang, C. Determining Changes in Microbial Nutrient Limitations in Bamboo Soils under Different Management Practices via Enzyme Stoichiometry. CATENA 2023, 223, 106939. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhang, Q.; Lin, K.; Zhou, J. Enzyme stoichiometry evidence revealed that five years nitrogen addition exacerbated the carbon and phosphorus limitation of soil microorganisms in a Phyllostachys pubescens. Chin. J. Appl. Ecol. 2021, 32, 521–528. [Google Scholar] [CrossRef]
- Su, W. Fertilization Theory and Practice for Phyllostachys edulis Stand Based on Growth and Nutrient Accumulation Rules. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2012. [Google Scholar]
- Su, W.; Fan, S.; Zhao, J.; Cai, C. Effects of Various Fertilization Placements on the Fate of Urea-15N in Moso Bamboo Forests. For. Ecol. Manag. 2019, 453, 117632. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, W.; Bai, S.H.; Niu, Y.; Hu, D.; Ji, H.; Xu, Z. Minor Increases in Phyllostachys Edulis (Moso Bamboo) Biomass despite Evident Alterations of Soil Bacterial Community Structure after Phosphorus Fertilization Alone: Based on Field Studies at Different Altitudes. For. Ecol. Manag. 2019, 451, 117561. [Google Scholar] [CrossRef]
- Tietz, A.; Kirschner, A.; Langergraber, G.; Sleytr, K.; Haberl, R. Characterisation of Microbial Biocoenosis in Vertical Subsurface Flow Constructed Wetlands. Sci. Total Environ. 2007, 380, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Riaz, M.; Ming, C.; Li, Y.; Wang, X.; Jiang, C. Assessing the Difference of Biochar and Aged Biochar to Improve Soil Fertility and Cabbage (Brassica oleracea Var. Capitata) Productivity. J. Soils Sediments 2023, 23, 606–618. [Google Scholar] [CrossRef]
- Xia, H.; Riaz, M.; Tang, X.; Yan, L.; El-Desouki, Z.; Li, Y.; Wang, X.; Cuncang, J. Insight into Mechanisms of Biochar-Fertilizer Induced of Microbial Community and Microbiology of Nitrogen Cycle in Acidic Soil. J. Environ. Manag. 2023, 336, 117602. [Google Scholar] [CrossRef] [PubMed]
- Marklein, A.R.; Houlton, B.Z. Nitrogen Inputs Accelerate Phosphorus Cycling Rates across a Wide Variety of Terrestrial Ecosystems. New Phytol. 2012, 193, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Jian, S.; Li, J.; Chen, J.; Wang, G.; Mayes, M.A.; Dzantor, K.E.; Hui, D.; Luo, Y. Soil Extracellular Enzyme Activities, Soil Carbon and Nitrogen Storage under Nitrogen Fertilization: A Meta-Analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.; Li, J.; Zhou, X.; Cao, J.; Wang, R.; Wang, Y.; Shelton, S.; Jin, Z.; Walker, L.M.; et al. Costimulation of Soil Glycosidase Activity and Soil Respiration by Nitrogen Addition. Glob. Chang. Biol. 2017, 23, 1328–1337. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Z.; Liu, Y.; Zheng, H.; Li, H. Response of soil enzyme activity and stoichiometric ratio to simulated nitrogen deposition in subalpine coniferous forests of western Sichuan. Chin. J. Appl. Environ. Biol. 2019, 25, 791–800. [Google Scholar] [CrossRef]
- Kou, Z.; Li, C.; Chang, S.; Miao, Y.; Zhang, W.; Li, Q.; Dang, T.; Wang, Y. Effects of Nitrogen and Phosphorus Additions on Soil Microbial Community Structure and Ecological Processes in the Farmland of Chinese Loess Plateau. J. Arid. Land 2023, 15, 960–974. [Google Scholar] [CrossRef]
- Carrara, J.E.; Walter, C.A.; Hawkins, J.S.; Peterjohn, W.T.; Averill, C.; Brzostek, E.R. Interactions among Plants, Bacteria, and Fungi Reduce Extracellular Enzyme Activities under Long-term N Fertilization. Glob. Chang. Biol. 2018, 24, 2721–2734. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, X.; Wang, C.; Bai, E. Effect of nitrogen and phosphorus addition on soil enzyme activities: A meta-analysis. Chin. J. Appl. Ecol. 2018, 29, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Sui, B. Effect of Potassium Fertilizer Application on Microbial Community in Tobacco-Growing Soil. Master’s Thesis, Yunnan Agricultural University, Kunming, China, 2023. [Google Scholar]
- Jia, X.; Zhong, Y.; Liu, J.; Zhu, G.; Shangguan, Z.; Yan, W. Effects of Nitrogen Enrichment on Soil Microbial Characteristics: From Biomass to Enzyme Activities. Geoderma 2020, 366, 114256. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.; van Groenigen, K.J.; Hungate, B.A.; Cao, J.; Zhou, X.; Wang, R. A Keystone Microbial Enzyme for Nitrogen Control of Soil Carbon Storage. Sci. Adv. 2018, 4, eaaq1689. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, D.; Zhao, J.; Zhang, W.; Xiao, K.; Wang, K. Nitrogen Addition Aggravates Microbial Carbon Limitation: Evidence from Ecoenzymatic Stoichiometry. Geoderma 2018, 329, 61–64. [Google Scholar] [CrossRef]
- Tu, L.; Chen, G.; Peng, Y.; Hu, H.; Hu, T.; Zhang, J.; Li, X.; Liu, L.; Tang, Y. Soil Biochemical Responses to Nitrogen Addition in a Bamboo Forest. PLoS ONE 2014, 9, e102315. [Google Scholar] [CrossRef]
- Merchant, S.S.; Helmann, J.D. Elemental Economy: Microbial Strategies for Optimizing Growth in the Face of Nutrient Limitation. Adv. Microb. Physiol. 2012, 60, 91–210. [Google Scholar] [PubMed]
- Sinsabaugh, R.L.; Follstad Shah, J.J. Ecoenzymatic Stoichiometry of Recalcitrant Organic Matter Decomposition: The Growth Rate Hypothesis in Reverse. Biogeochemistry 2011, 102, 31–43. [Google Scholar] [CrossRef]
- Deng, Q.; Hui, D.; Dennis, S.; Reddy, K.C. Responses of Terrestrial Ecosystem Phosphorus Cycling to Nitrogen Addition: A Meta-analysis. Glob. Ecol. Biogeogr. 2017, 26, 713–728. [Google Scholar] [CrossRef]
- Averill, C.; Waring, B. Nitrogen Limitation of Decomposition and Decay: How Can It Occur? Glob. Chang. Biol. 2018, 24, 1417–1427. [Google Scholar] [CrossRef]
- Peng, X.; Wang, W. Stoichiometry of Soil Extracellular Enzyme Activity along a Climatic Transect in Temperate Grasslands of Northern China. Soil Biol. Biochem. 2016, 98, 74–84. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, C.; Wang, Y.; Cheng, H.; An, S.; Chang, S.X. Soil Extracellular Enzyme Stoichiometry Reflects the Shift from P- to N-Limitation of Microorganisms with Grassland Restoration. Soil Biol. Biochem. 2020, 149, 107928. [Google Scholar] [CrossRef]
- Gai, X.; Zhong, Z.; Zhang, X.; Bian, F.; Yang, C. Effects of Chicken Farming on Soil Organic Carbon Fractions and Fungal Communities in a Lei Bamboo (Phyllostachys praecox) Forest in Subtropical China. For. Ecol. Manag. 2021, 479, 118603. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, W.; Li, Q.; Guo, Z.; Li, Y.; Zhao, Z.; Zhai, J.; Liu, G.; Xue, S. Ecoenzymatic Stoichiometry and Nutrient Limitation under a Natural Secondary Succession of Vegetation on the Loess Plateau, China. Land Degrad. Dev. 2021, 32, 399–409. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Lu, X.; Zhu, Y.; Han, X.; Yan, J.; Yan, L.; Zou, W. Impact of Combined Organic Amendments and Chemical Fertilizers on Soil Microbial Limitations, Soil Quality, and Soybean Yield. Plant Soil 2024, 1–18. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Y.; Duan, C.; Wang, X.; Zhang, X.; Ju, W.; Chen, H.; Yue, S.; Wang, Y.; Li, S.; et al. Ecoenzymatic Stoichiometry Reveals Microbial Phosphorus Limitation Decreases the Nitrogen Cycling Potential of Soils in Semi-Arid Agricultural Ecosystems. Soil Tillage Res. 2020, 197, 104463. [Google Scholar] [CrossRef]
- Liu, C.; Wang, B.; Zhu, Y.; Qu, T.; Xue, Z.; Li, X.; Zhou, Z.; An, S. Eco-Enzymatic Stoichiometry and Microbial Non-Homeostatic Regulation Depend on Relative Resource Availability during Litter Decomposition. Ecol. Indic. 2022, 145, 109729. [Google Scholar] [CrossRef]
- Song, S.; Wang, X.; He, C.; Chi, Y. Effects of Utilization Methods on C, N, P Rate and Enzyme Activity of Artificial Grassland in Karst Desertification Area. Agronomy 2023, 13, 1368. [Google Scholar] [CrossRef]
- Jing, X.; Chen, X.; Xiao, W.; Lin, L.; Wang, C.; He, J.-S.; Zhu, B. Soil Enzymatic Responses to Multiple Environmental Drivers in the Tibetan Grasslands: Insights from Two Manipulative Field Experiments and a Meta-Analysis. Pedobiologia 2018, 71, 50–58. [Google Scholar] [CrossRef]
- Li, J.; Sang, C.; Yang, J.; Qu, L.; Xia, Z.; Sun, H.; Jiang, P.; Wang, X.; He, H.; Wang, C. Stoichiometric Imbalance and Microbial Community Regulate Microbial Elements Use Efficiencies under Nitrogen Addition. Soil Biol. Biochem. 2021, 156, 108207. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Q.; Hu, X.; Fang, X.-M.; Wang, S.; Chen, F.-S. Divergent Responses of Soil Microbial Community to Long-Term Nitrogen and Phosphorus Additions in a Subtropical Chinese Fir Plantation. CATENA 2024, 242, 108132. [Google Scholar] [CrossRef]
- Cui, Y.; Bing, H.; Moorhead, D.L.; Delgado-Baquerizo, M.; Ye, L.; Yu, J.; Zhang, S.; Wang, X.; Peng, S.; Guo, X.; et al. Ecoenzymatic Stoichiometry Reveals Widespread Soil Phosphorus Limitation to Microbial Metabolism across Chinese Forests. Commun. Earth Environ. 2022, 3, 184. [Google Scholar] [CrossRef]
- Cui, Y.; Moorhead, D.L.; Peng, S.; Sinsabaugh, R.L.; Peñuelas, J. Predicting Microbial Nutrient Limitations from a Stoichiometry-Based Threshold Framework. Innov. Geosci. 2024, 2, 100048. [Google Scholar] [CrossRef]
- Cui, Y.; Bing, H.; Fang, L.; Jiang, M.; Shen, G.; Yu, J.; Wang, X.; Zhu, H.; Wu, Y.; Zhang, X. Extracellular Enzyme Stoichiometry Reveals the Carbon and Phosphorus Limitations of Microbial Metabolisms in the Rhizosphere and Bulk Soils in Alpine Ecosystems. Plant Soil 2021, 458, 7–20. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Hämmerle, I.; Fuchslueger, L.; Hofhansl, F.; Knoltsch, A.; Schnecker, J.; Takriti, M.; Watzka, M.; Wild, B.; et al. Adjustment of Microbial Nitrogen Use Efficiency to Carbon: Nitrogen Imbalances Regulates Soil Nitrogen Cycling. Nat. Commun. 2014, 5, 3694. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Sun, T.; Zhu, L.; Wanek, W.; Cheng, Q.; Wang, X.; Zhou, J.; Liu, X.; Ma, Q.; Wu, L.; et al. Microbial Adaption to Stoichiometric Imbalances Regulated the Size of Soil Mineral-Associated Organic Carbon Pool under Continuous Organic Amendments. Geoderma 2024, 445, 116883. [Google Scholar] [CrossRef]
- Cai, M.; Zhang, Y.; Zhao, G.; Zhao, B.; Cong, N.; Zhu, J.; Zheng, Z.; Wu, W.; Duan, X. Excessive Climate Warming Exacerbates Nitrogen Limitation on Microbial Metabolism in an Alpine Meadow of the Tibetan Plateau: Evidence from Soil Ecoenzymatic Stoichiometry. Sci. Total Environ. 2024, 930, 172731. [Google Scholar] [CrossRef]

| Treatment | SOC g/kg | TN g/kg | TP g/kg | AP mg/kg | pH | MBC mg/kg | MBN mg/kg | MBP mg/kg | C:Ns | C:Ps | N:Ps | C:Nm | C:Pm | N:Pm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CK | 16.94 ± 0.85 ab | 1.25 ± 0.11 a | 0.39 ± 0.02 b | 7.63 ± 0.53 bc | 4.59 ± 0.03 a | 376.29 ± 39.94 a | 55.05 ± 1.64 a | 3.51 ± 0.23 c | 13.56 ± 0.09 a | 43.77 ± 0.58 a | 3.27 ± 0.46 a | 6.89 ± 0.92 a | 108.27 ± 14.58 a | 15.81 ± 1.17 a |
| N0 | 19.23 ± 0.54 a | 1.44 ± 0.08 a | 0.67 ± 0.05 a | 8.8 ± 1.01 b | 4.57 ± 0.03 a | 415.3 ± 3.97 ab | 61.59 ± 2.28 a | 3.59 ± 0.33 c | 13.4 ± 0.94 a | 29.33 ± 2.75 b | 2.21 ± 0.28 b | 6.76 ± 0.26 a | 117.84 ± 11.53 a | 17.37 ± 1.2 a |
| N1 | 13.62 ± 1.58 b | 1.13 ± 0.04 a | 0.74 ± 0.07 a | 5.81 ± 0.46 c | 4.58 ± 0.03 a | 117.58 ± 4.01 b | 21.53 ± 0.48 b | 2.64 ± 0.03 d | 12.12 ± 1.63 a | 18.94 ± 3.49 d | 1.55 ± 0.1 b | 5.47 ± 0.3 b | 44.57 ± 1.86 b | 8.16 ± 0.19 b |
| N2 | 14.48 ± 1.06 b | 1.23 ± 0.14 a | 0.71 ± 0.01 a | 9.48 ± 0.83 b | 4.68 ± 0.07 a | 162.14 ± 10.51 c | 33.98 ± 0.25 c | 4.41 ± 0.06 b | 11.98 ± 0.9 a | 20.28 ± 1.11 cd | 1.71 ± 0.16 b | 4.77 ± 0.31 b | 36.69 ± 1.97 b | 7.7 ± 0.09 b |
| N3 | 17.68 ± 2.29 ab | 1.41 ± 0.1 a | 0.64 ± 0.01 a | 14.34 ± 0.99 a | 4.61 ± 0.09 a | 332.72 ± 8.81 c | 59.45 ± 1.51 a | 6.04 ± 0.2 a | 12.47 ± 1 a | 27.55 ± 3.26 bc | 2.2 ± 0.17 b | 5.6 ± 0.01 b | 55.12 ± 0.69 b | 9.85 ± 0.14 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, H.; Su, W.; Zhou, Y.; Wang, Z.; Long, Y.; Sun, Y.; Fan, S. Enzyme Activity Stoichiometry Suggests That Fertilization, Especially Nitrogen Fertilization, Alleviates Nutrient Limitation of Soil Microorganisms in Moso Bamboo Forests. Forests 2024, 15, 1040. https://doi.org/10.3390/f15061040
Chu H, Su W, Zhou Y, Wang Z, Long Y, Sun Y, Fan S. Enzyme Activity Stoichiometry Suggests That Fertilization, Especially Nitrogen Fertilization, Alleviates Nutrient Limitation of Soil Microorganisms in Moso Bamboo Forests. Forests. 2024; 15(6):1040. https://doi.org/10.3390/f15061040
Chicago/Turabian StyleChu, Haoyu, Wenhui Su, Yaqi Zhou, Ziye Wang, Yongmei Long, Yutong Sun, and Shaohui Fan. 2024. "Enzyme Activity Stoichiometry Suggests That Fertilization, Especially Nitrogen Fertilization, Alleviates Nutrient Limitation of Soil Microorganisms in Moso Bamboo Forests" Forests 15, no. 6: 1040. https://doi.org/10.3390/f15061040
APA StyleChu, H., Su, W., Zhou, Y., Wang, Z., Long, Y., Sun, Y., & Fan, S. (2024). Enzyme Activity Stoichiometry Suggests That Fertilization, Especially Nitrogen Fertilization, Alleviates Nutrient Limitation of Soil Microorganisms in Moso Bamboo Forests. Forests, 15(6), 1040. https://doi.org/10.3390/f15061040






