Understorey Plant Functional Traits of Platycladus orientalis Depends on Crown Closure and Soil Properties in the Loess Plateau, China
Abstract
1. Introduction
2. Materials and Methods
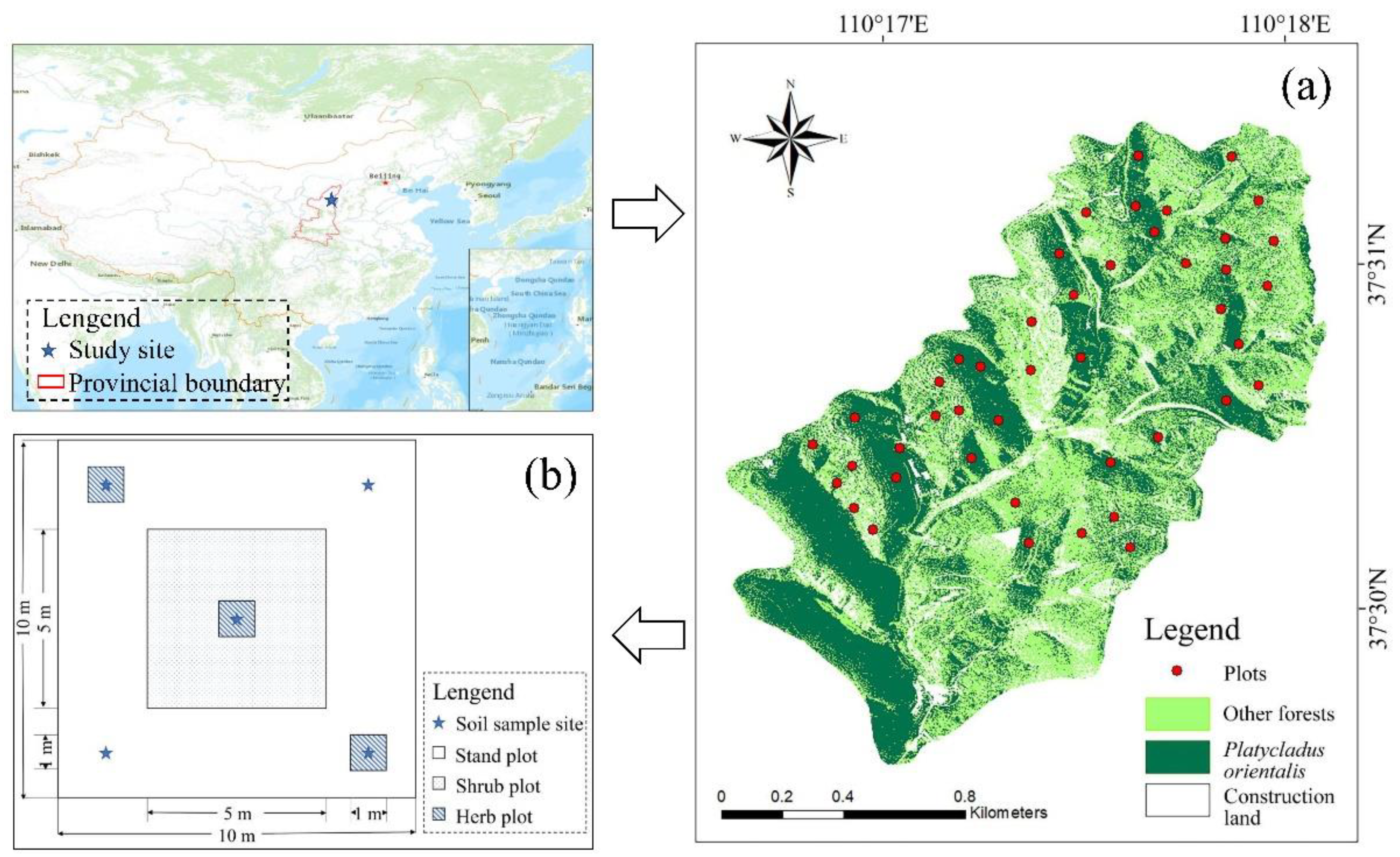
2.1. Study Area
2.2. Sample Design
2.3. Soil Properties
2.4. Plant Functional Traits
2.5. Statistical Analyses
3. Results
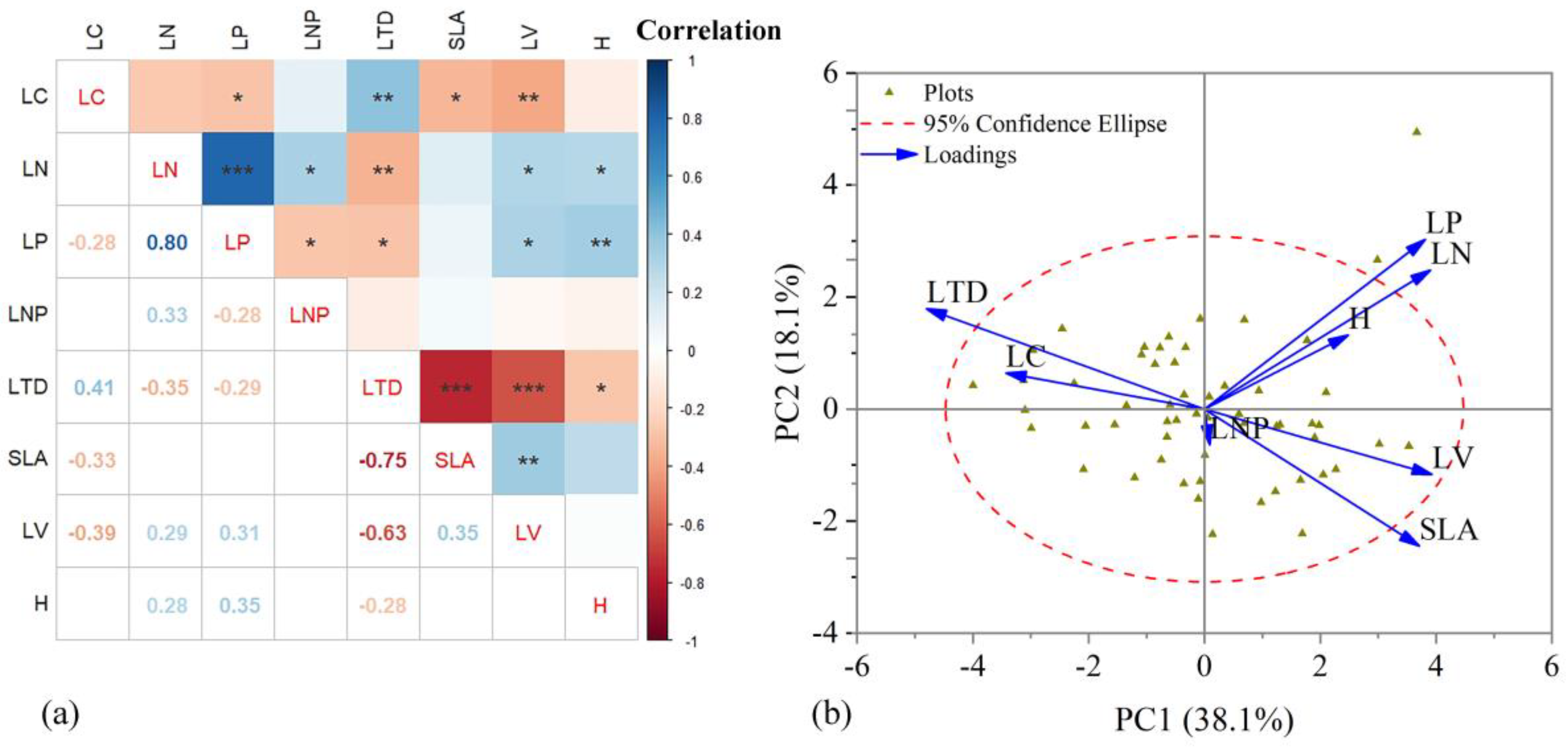
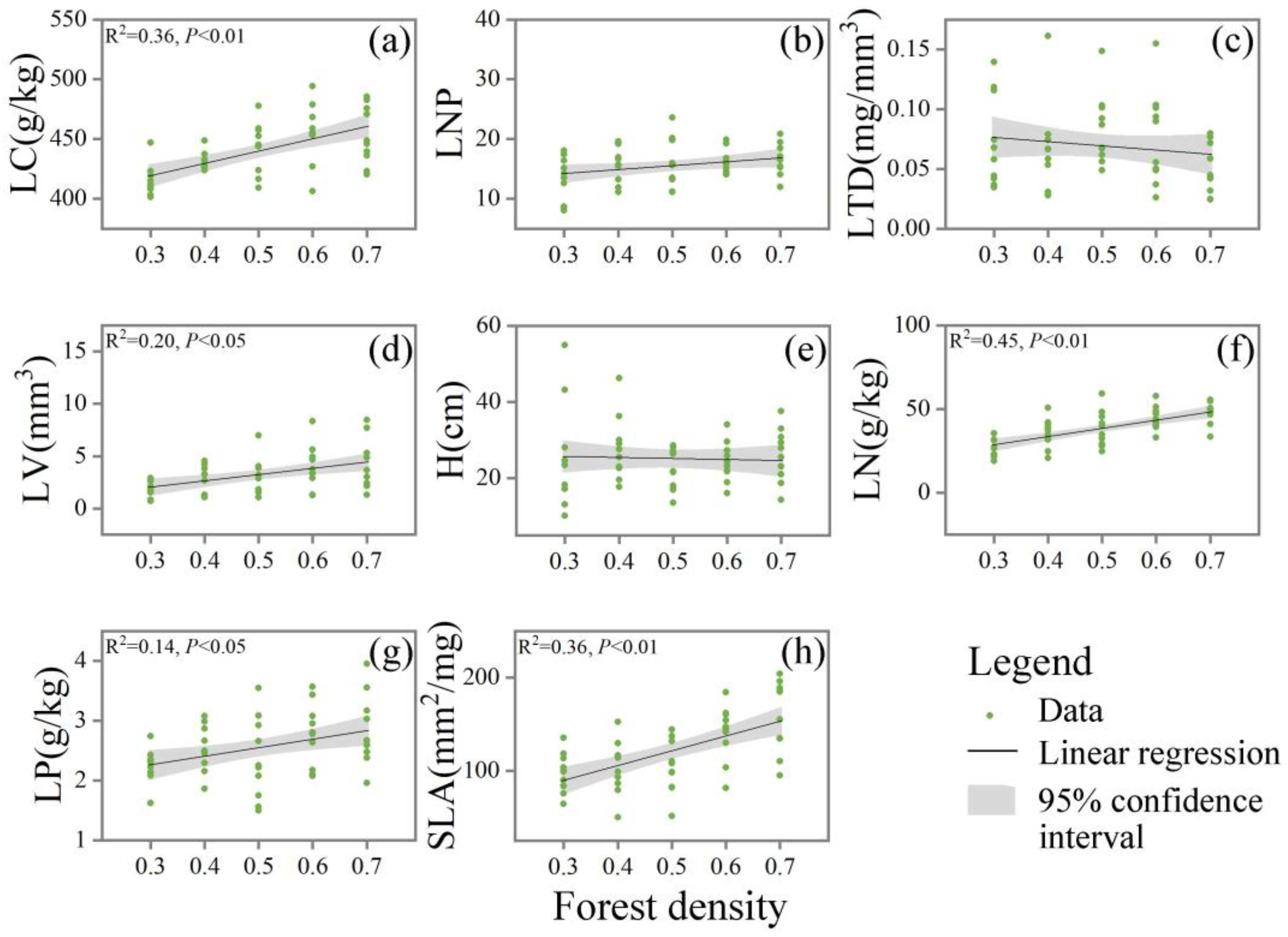
3.1. Relationships among Plant Functional Traits
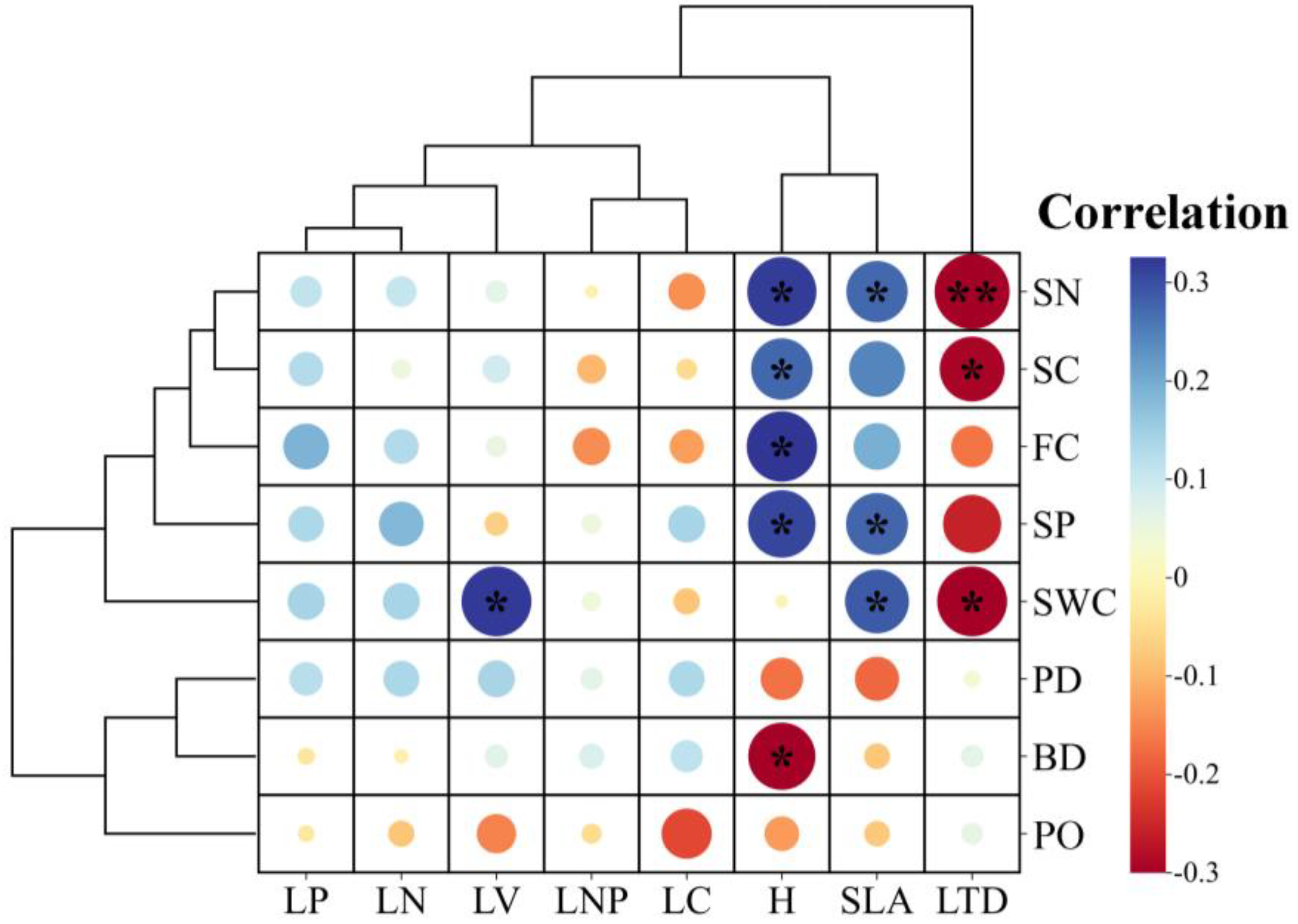
3.2. Relationships between Soil Properties and Plant Functional Traits
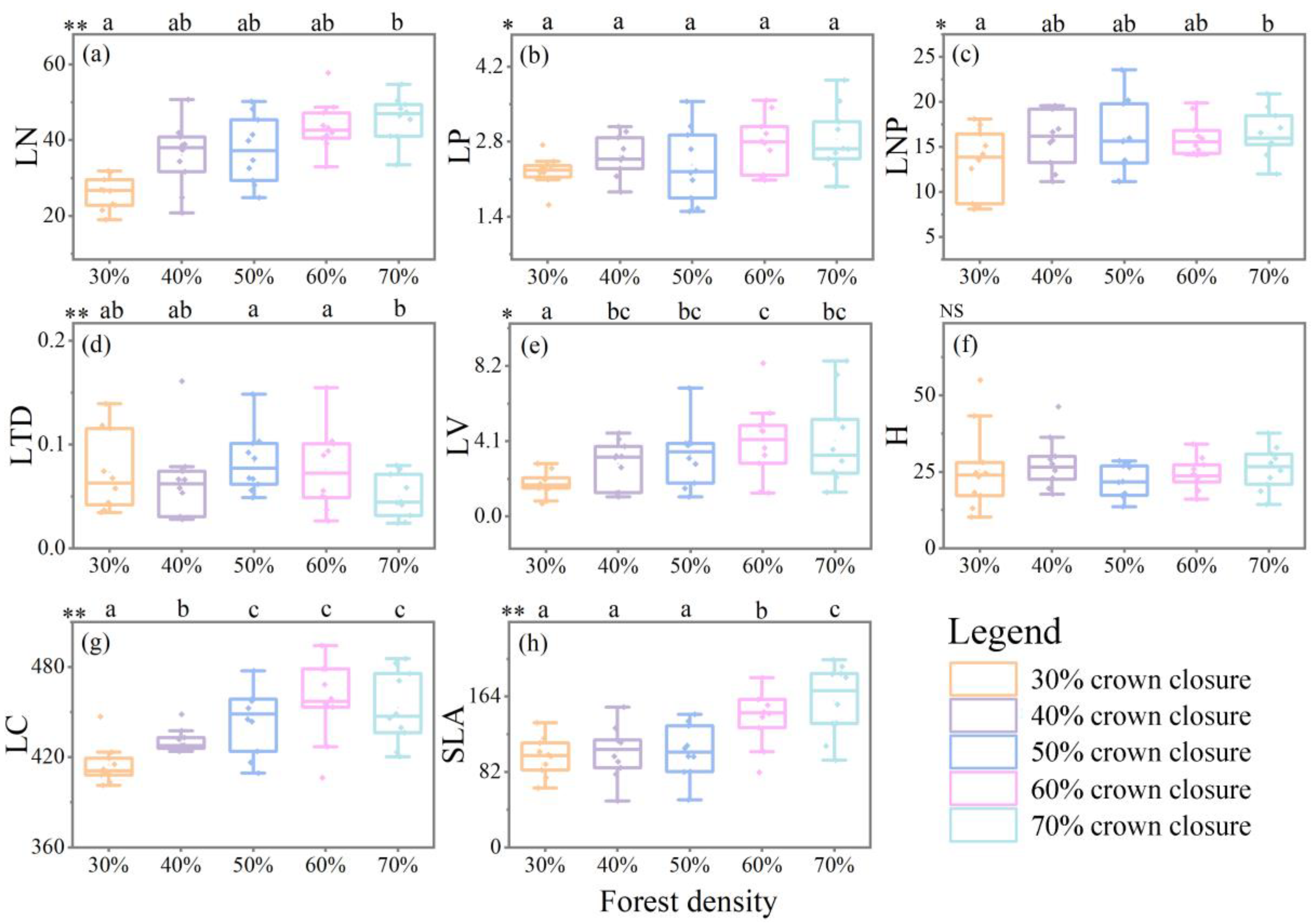
3.3. Plant Functional Traits under Different Types of Crown Closure of Platycladus orientalis (L.) Franco
3.4. The Effect of Crown Closure on Plant Functional Traits and Soil Properties
3.5. The Effect of Crown Closure and Soil Properties on Plant Functional Traits
4. Discussion
4.1. Trade-Offs and Synergies between Plant Functional Traits
4.2. Effect of Forest Crown Closure on Soil Properties
4.3. Forest Crown Closure Indirectly Affects Plant Functional Traits through Soil Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niinemets, Ü.; Keenan, T.F.; Hallik, L. A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytol. 2015, 205, 973–993. [Google Scholar] [CrossRef]
- Duan, G.; Wen, Z.; Xue, W.; Bu, Y.; Lu, J.; Wen, B.; Wang, B.; Chen, S. Agents affecting the plant functional traits in national soil and water conservation demonstration park (China). Plants 2022, 11, 2891. [Google Scholar] [CrossRef]
- Thuiller, W.; Albert, C.; Araújo, M.B.; Berry, P.M.; Cabeza, M.; Guisan, A.; Hickler, T.; Midgley, G.F.; Paterson, J.; Schurr, F.M. Predicting global change impacts on plant species’ distributions: Future challenges. Perspect. Plant Ecol. Evol. Syst. 2008, 9, 137–152. [Google Scholar] [CrossRef]
- Lavorel, S.; Garnier, E. Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the holy grail. Funct. Ecol. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus nutrition: Plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Singapore, 2018; pp. 171–190. [Google Scholar]
- Batool, T.; Ali, S.; Seleiman, M.F.; Naveed, N.H.; Ali, A.; Ahmed, K.; Abid, M.; Rizwan, M.; Shahid, M.R.; Alotaibi, M. Plant growth promoting rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Sci. Rep. 2020, 10, 16975. [Google Scholar] [CrossRef]
- Ding, J.; Jiao, X.; Bai, P.; Hu, Y.; Zhang, J.; Li, J. Effect of vapor pressure deficit on the photosynthesis, growth, and nutrient absorption of tomato seedlings. Sci. Hortic. 2022, 293, 110736. [Google Scholar] [CrossRef]
- Zhang, D.; Jiao, X.; Du, Q.; Song, X.; Li, J. Reducing the excessive evaporative demand improved photosynthesis capacity at low costs of irrigation via regulating water driving force and moderating plant water stress of two tomato cultivars. Agric. Water Manag. 2018, 199, 22–33. [Google Scholar] [CrossRef]
- Morales, F.; Pavlovič, A.; Abadía, A.; Abadía, J. Photosynthesis in poor nutrient soils, in compacted soils, and under drought. In The Leaf: A Platform for Performing Photosynthesis; Springer: Cham, Swizterland, 2018; pp. 371–399. [Google Scholar]
- Alemu, M.M. Effect of tree shade on coffee crop production. J. Sustain. Dev. 2015, 8, 66. [Google Scholar] [CrossRef]
- Sarr, D.A.; Hibbs, D.E.; Huston, M.A. A hierarchical perspective of plant diversity. Q. Rev. Biol. 2005, 80, 187–212. [Google Scholar] [CrossRef]
- Dwyer, J.M.; Hobbs, R.J.; Mayfield, M.M. Specific leaf area responses to environmental gradients through space and time. Ecology 2014, 95, 399–410. [Google Scholar] [CrossRef]
- Gray, E.F.; Wright, I.J.; Falster, D.S.; Eller, A.S.; Lehmann, C.; Bradford, M.G.; Cernusak, L.A. Leaf: Wood allometry and functional traits together explain substantial growth rate variation in rainforest trees. AoB Plants 2019, 11, plz024. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Clare, S.; Kitajima, K. Physical defence traits enhance seedling survival of neotropical tree species. Funct. Ecol. 2007, 21, 1044–1054. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, C.-Z.; Kang, M.-P.; Li, X.-Y. The relationship of the main root-shoot morphological characteristics and biomass allocation of Saussurea salsa under different habitat conditions in Sugan lake wetland on the northern margin of the Qinghai-Tibet Plateau. Ecol. Indic. 2021, 128, 107836. [Google Scholar] [CrossRef]
- Garnier, E.; Cortez, J.; Billès, G.; Navas, M.-L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A. Plant functional markers capture ecosystem properties during secondary succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- de la Riva, E.G.; Tosto, A.; Pérez-Ramos, I.M.; Navarro-Fernández, C.M.; Olmo, M.; Anten, N.P.; Marañón, T.; Villar, R. A plant economics spectrum in Mediterranean forests along environmental gradients: Is there coordination among leaf, stem and root traits? J. Veg. Sci. 2016, 27, 187–199. [Google Scholar] [CrossRef]
- Funk, J.L.; Larson, J.E.; Ames, G.M.; Butterfield, B.J.; Cavender-Bares, J.; Firn, J.; Laughlin, D.C.; Sutton-Grier, A.E.; Williams, L.; Wright, J. Revisiting the Holy Grail: Using plant functional traits to understand ecological processes. Biol. Rev. 2017, 92, 1156–1173. [Google Scholar] [CrossRef]
- Duan, G.; Zhou, R.; Wang, L.; Zheng, C.; Liu, Y.; Chai, X.; Zhou, C.; Wen, Z. Effects of different soil and water conservation measures on plant diversity and productivity in Loess Plateau. J. Environ. Manag. 2023, 348, 119330. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Wang, L.; Liu, Y.; Wang, C.; Mao, X. Assessing the effects of China’s three-north shelter forest program over 40 years. Sci. Total Environ. 2023, 857, 159354. [Google Scholar] [CrossRef]
- Lozano-Baez, S.E.; Cooper, M.; Meli, P.; Ferraz, S.F.; Rodrigues, R.R.; Sauer, T.J. Land restoration by tree planting in the tropics and subtropics improves soil infiltration, but some critical gaps still hinder conclusive results. For. Ecol. Manag. 2019, 444, 89–95. [Google Scholar] [CrossRef]
- Pei, Y.; Huang, L.; Zhang, Y.; Pan, Y. Water use pattern and transpiration of mongolian pine plantations in relation to stand age on northern loess plateau of china. Agric. For. Meteorol. 2023, 330, 109320. [Google Scholar] [CrossRef]
- Tianjiao, F.; Tianxing, W.; Keesstra, S.D.; Jianjun, Z.; Huaxing, B.; Ruoshui, W.; Ping, W. Long-term effects of vegetation restoration on hydrological regulation functions and the implications to afforestation on the Loess Plateau. Agric. For. Meteorol. 2023, 330, 109313. [Google Scholar] [CrossRef]
- Gong, C.; Tan, Q.; Xu, M.; Liu, G. Mixed-species plantations can alleviate water stress on the loess plateau. For. Ecol. Manag. 2020, 458, 117767. [Google Scholar] [CrossRef]
- Li, G.; Du, S.; Wen, Z. Mapping the climatic suitable habitat of oriental arborvitae (Platycladus orientalis) for introduction and cultivation at a global scale. Sci. Rep. 2016, 6, 30009. [Google Scholar] [CrossRef] [PubMed]
- Stanturf, J.A.; Palik, B.J.; Dumroese, R.K. Contemporary forest restoration: A review emphasizing function. For. Ecol. Manag. 2014, 331, 292–323. [Google Scholar] [CrossRef]
- Huang, X.; Chen, J.; Li, S.; Su, J. Selective logging effects on plant functional traits depend on soil enzyme activity and nutrient cycling in a Pinus yunnanensis forest. For. Ecol. Manag. 2023, 545, 121284. [Google Scholar] [CrossRef]
- Bu, W.; Zang, R.; Ding, Y.; Zhang, J.; Ruan, Y. Relationships between plant functional traits at the community level and environmental factors during succession in a tropical lowland rainforest on Hainan Island, South China. Biodivers. Sci. 2013, 21, 278. [Google Scholar]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Laughlin, D.C. Applying trait-based models to achieve functional targets for theory-driven ecological restoration. Ecol. Lett. 2014, 17, 771–784. [Google Scholar] [CrossRef]
- Reich, P.B.; Wright, I.J.; Cavender-Bares, J.; Craine, J.; Oleksyn, J.; Westoby, M.; Walters, M. The evolution of plant functional variation: Traits, spectra, and strategies. Int. J. Plant Sci. 2003, 164, S143–S164. [Google Scholar] [CrossRef]
- Oguchi, R.; Onoda, Y.; Terashima, I.; Tholen, D. Leaf anatomy and function. In The Leaf: A Platform for Performing Photosynthesis; Springer: Cham, Swizterland, 2018; pp. 97–139. [Google Scholar]
- Lyu, H.; Li, Y.; Wang, Y.; Wang, P.; Shang, Y.; Yang, X.; Wang, F.; Yu, A. Drive soil nitrogen transformation and improve crop nitrogen absorption and utilization-a review of green manure applications. Front. Plant Sci. 2024, 14, 1305600. [Google Scholar] [CrossRef]
- Konôpka, B.; Pajtík, J.; Marušák, R.; Bošeľa, M.; Lukac, M. Specific leaf area and leaf area index in developing stands of Fagus sylvatica L. and Picea abies Karst. For. Ecol. Manag. 2016, 364, 52–59. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, Y.; Yi, X.; Zhang, X.; Zhang, W. Cotton responds to different plant population densities by adjusting specific leaf area to optimize canopy photosynthetic use efficiency of light and nitrogen. Field Crops Res. 2016, 188, 10–16. [Google Scholar] [CrossRef]
- Karthika, K.; Rashmi, I.; Parvathi, M. Biological functions, uptake and transport of essential nutrients in relation to plant growth. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Singapore, 2018; pp. 1–49. [Google Scholar]
- Hou, G.; Bi, H.; Wang, N.; Cui, Y.; Ma, X.; Zhao, D.; Wang, S. Optimizing the stand density of Robinia pseudoacacia L. Forests of the loess plateau, China, based on response to soil water and soil nutrient. Forests 2019, 10, 663. [Google Scholar] [CrossRef]
- Ola, A.; Schmidt, S.; Lovelock, C.E. The effect of heterogeneous soil bulk density on root growth of field-grown mangrove species. Plant Soil 2018, 432, 91–105. [Google Scholar] [CrossRef]
- Lu, Y.; Si, B.; Li, H.; Biswas, A. Elucidating controls of the variability of deep soil bulk density. Geoderma 2019, 348, 146–157. [Google Scholar] [CrossRef]
- Le Bissonnais, Y.; Prieto, I.; Roumet, C.; Nespoulous, J.; Metayer, J.; Huon, S.; Villatoro, M.; Stokes, A. Soil aggregate stability in Mediterranean and tropical agro-ecosystems: Effect of plant roots and soil characteristics. Plant Soil 2018, 424, 303–317. [Google Scholar] [CrossRef]
- de Almeida, W.S.; Panachuki, E.; de Oliveira, P.T.S.; da Silva Menezes, R.; Sobrinho, T.A.; de Carvalho, D.F. Effect of soil tillage and vegetal cover on soil water infiltration. Soil Tillage Res. 2018, 175, 130–138. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Vose, J.M.; Sun, G.; Ford, C.R.; Bredemeier, M.; Otsuki, K.; Wei, X.; Zhang, Z.; Zhang, L. Forest ecohydrological research in the 21st century: What are the critical needs? Ecohydrology 2011, 4, 146–158. [Google Scholar] [CrossRef]
- Bayala, J.; Prieto, I. Water acquisition, sharing and redistribution by roots: Applications to agroforestry systems. Plant Soil 2020, 453, 17–28. [Google Scholar] [CrossRef]
- Quideau, S.; Chadwick, O.; Benesi, A.; Graham, R.; Anderson, M. A direct link between forest vegetation type and soil organic matter composition. Geoderma 2001, 104, 41–60. [Google Scholar] [CrossRef]
- Baldrian, P. Forest microbiome: Diversity, complexity and dynamics. FEMS Microbiol. Rev. 2017, 41, 109–130. [Google Scholar] [CrossRef] [PubMed]
- Poirier, V.; Roumet, C.; Munson, A.D. The root of the matter: Linking root traits and soil organic matter stabilization processes. Soil Biol. Biochem. 2018, 120, 246–259. [Google Scholar] [CrossRef]
- Bhattacharya, S.S.; Kim, K.-H.; Das, S.; Uchimiya, M.; Jeon, B.H.; Kwon, E.; Szulejko, J.E. A review on the role of organic inputs in maintaining the soil carbon pool of the terrestrial ecosystem. J. Environ. Manag. 2016, 167, 214–227. [Google Scholar] [CrossRef]
- Farley, K.A.; Kelly, E.F.; Hofstede, R.G. Soil organic carbon and water retention after conversion of grasslands to pine plantations in the Ecuadorian Andes. Ecosystems 2004, 7, 729–739. [Google Scholar] [CrossRef]
- Wang, L.; Deng, D.; Feng, Q.; Xu, Z.; Pan, H.; Li, H. Changes in litter input exert divergent effects on the soil microbial community and function in stands of different densities. Sci. Total Environ. 2022, 845, 157297. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Song, H.; Jia, Y.; Liu, J. Agents affecting the productivity of pine plantations on the Loess Plateau in China: A study based on structural equation modeling. Forests 2020, 11, 1328. [Google Scholar] [CrossRef]
- Raz-Yaseef, N.; Rotenberg, E.; Yakir, D. Effects of spatial variations in soil evaporation caused by tree shading on water flux partitioning in a semi-arid pine forest. Agric. For. Meteorol. 2010, 150, 454–462. [Google Scholar] [CrossRef]
- Doheny-Adams, T.; Hunt, L.; Franks, P.J.; Beerling, D.J.; Gray, J.E. Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 547–555. [Google Scholar] [CrossRef]
- Nyawade, S.O.; Gachene, C.K.; Karanja, N.N.; Gitari, H.I.; Schulte-Geldermann, E.; Parker, M.L. Controlling soil erosion in smallholder potato farming systems using legume intercrops. Geoderma Reg. 2019, 17, e00225. [Google Scholar] [CrossRef]
- McDonald, M.; Healey, J.; Stevens, P. The effects of secondary forest clearance and subsequent land-use on erosion losses and soil properties in the Blue Mountains of Jamaica. Agric. Ecosyst. Environ. 2002, 92, 1–19. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Tsamir, M.; Gottlieb, S.; Preisler, Y.; Rotenberg, E.; Tatarinov, F.; Yakir, D.; Tague, C.; Klein, T. Stand density effects on carbon and water fluxes in a semi-arid forest, from leaf to stand-scale. For. Ecol. Manag. 2019, 453, 117573. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, G.; Liu, L.; Wen, Z.; Tang, Y.; Wang, B. Understorey Plant Functional Traits of Platycladus orientalis Depends on Crown Closure and Soil Properties in the Loess Plateau, China. Forests 2024, 15, 1042. https://doi.org/10.3390/f15061042
Duan G, Liu L, Wen Z, Tang Y, Wang B. Understorey Plant Functional Traits of Platycladus orientalis Depends on Crown Closure and Soil Properties in the Loess Plateau, China. Forests. 2024; 15(6):1042. https://doi.org/10.3390/f15061042
Chicago/Turabian StyleDuan, Gaohui, Lifeng Liu, Zhongming Wen, Yu Tang, and Boheng Wang. 2024. "Understorey Plant Functional Traits of Platycladus orientalis Depends on Crown Closure and Soil Properties in the Loess Plateau, China" Forests 15, no. 6: 1042. https://doi.org/10.3390/f15061042
APA StyleDuan, G., Liu, L., Wen, Z., Tang, Y., & Wang, B. (2024). Understorey Plant Functional Traits of Platycladus orientalis Depends on Crown Closure and Soil Properties in the Loess Plateau, China. Forests, 15(6), 1042. https://doi.org/10.3390/f15061042






